 Found 96 hits with Last Name = 'tevar' and Initial = 's'
Found 96 hits with Last Name = 'tevar' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449244
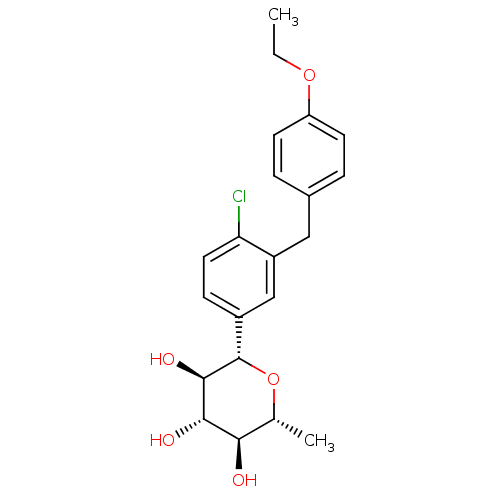
(CHEMBL3125150)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](C)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H25ClO5/c1-3-26-16-7-4-13(5-8-16)10-15-11-14(6-9-17(15)22)21-20(25)19(24)18(23)12(2)27-21/h4-9,11-12,18-21,23-25H,3,10H2,1-2H3/t12-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20880
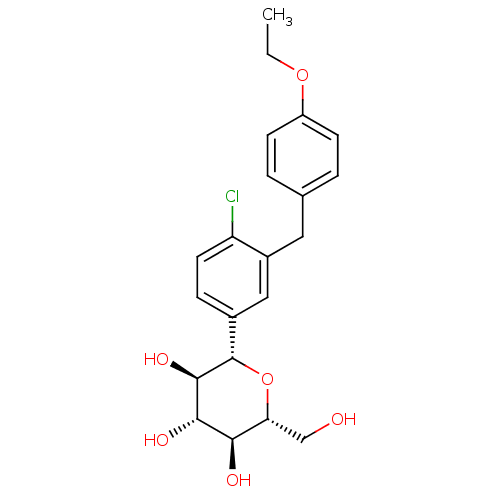
((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50329198
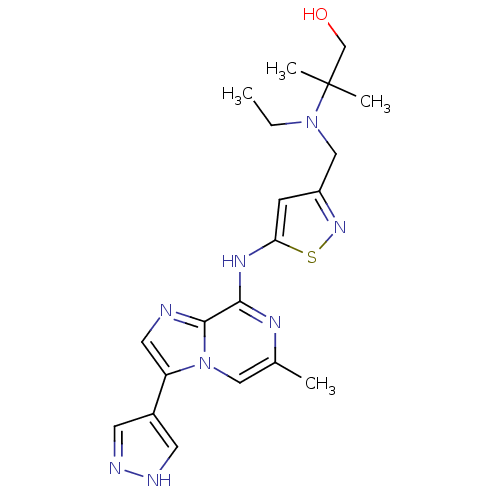
(2-(ethyl((5-(6-methyl-3-(1H-pyrazol-4-yl)imidazo[1...)Show SMILES CCN(Cc1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1)C(C)(C)CO Show InChI InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRK4 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50329197
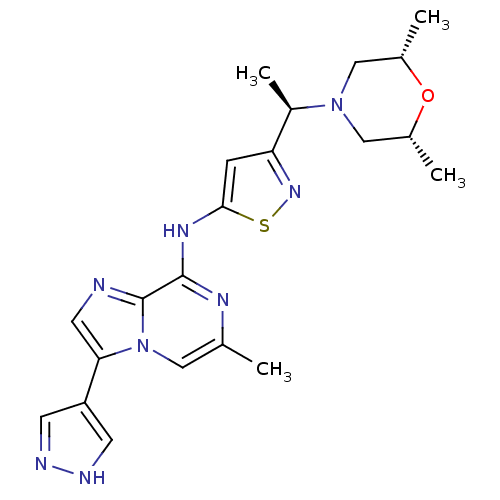
(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type IV
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Camk4 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CSNK1d |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TSSK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PLK3 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IKK-beta |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cMET |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50329198

(2-(ethyl((5-(6-methyl-3-(1H-pyrazol-4-yl)imidazo[1...)Show SMILES CCN(Cc1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1)C(C)(C)CO Show InChI InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50329201
(3-((2,2-dimethylpiperidin-1-yl)methyl)-N-(6-methyl...)Show SMILES Cc1cn2c(cnc2c(Nc2cc(CN3CCCCC3(C)C)ns2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C21H26N8S/c1-14-12-29-17(15-9-23-24-10-15)11-22-20(29)19(25-14)26-18-8-16(27-30-18)13-28-7-5-4-6-21(28,2)3/h8-12H,4-7,13H2,1-3H3,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50329196
(3-(1-((2S,6R)-2,6-dimethylmorpholino)cyclopropyl)-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C1(CC1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C22H26N8OS/c1-13-10-30-17(16-7-24-25-8-16)9-23-21(30)20(26-13)27-19-6-18(28-32-19)22(4-5-22)29-11-14(2)31-15(3)12-29/h6-10,14-15H,4-5,11-12H2,1-3H3,(H,24,25)(H,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50329196

(3-(1-((2S,6R)-2,6-dimethylmorpholino)cyclopropyl)-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C1(CC1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C22H26N8OS/c1-13-10-30-17(16-7-24-25-8-16)9-23-21(30)20(26-13)27-19-6-18(28-32-19)22(4-5-22)29-11-14(2)31-15(3)12-29/h6-10,14-15H,4-5,11-12H2,1-3H3,(H,24,25)(H,26,27)/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20875
(1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...)Show SMILES OC[C@H]1O[C@@H](Oc2cc(O)cc(O)c2C(=O)CCc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C21H24O10/c22-9-16-18(27)19(28)20(29)21(31-16)30-15-8-12(24)7-14(26)17(15)13(25)6-3-10-1-4-11(23)5-2-10/h1-2,4-5,7-8,16,18-24,26-29H,3,6,9H2/t16-,18-,19+,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM20875

(1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...)Show SMILES OC[C@H]1O[C@@H](Oc2cc(O)cc(O)c2C(=O)CCc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C21H24O10/c22-9-16-18(27)19(28)20(29)21(31-16)30-15-8-12(24)7-14(26)17(15)13(25)6-3-10-1-4-11(23)5-2-10/h1-2,4-5,7-8,16,18-24,26-29H,3,6,9H2/t16-,18-,19+,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50093599
(CHEMBL3585803)Show SMILES CCCCc1nc(Cl)c(C(=O)N[C@@H](Cc2ccccc2)C(O)=O)n1C |r| Show InChI InChI=1S/C18H22ClN3O3/c1-3-4-10-14-21-16(19)15(22(14)2)17(23)20-13(18(24)25)11-12-8-6-5-7-9-12/h5-9,13H,3-4,10-11H2,1-2H3,(H,20,23)(H,24,25)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method |
Bioorg Med Chem 23: 3526-33 (2015)
Article DOI: 10.1016/j.bmc.2015.04.024
BindingDB Entry DOI: 10.7270/Q2DB83M4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021153
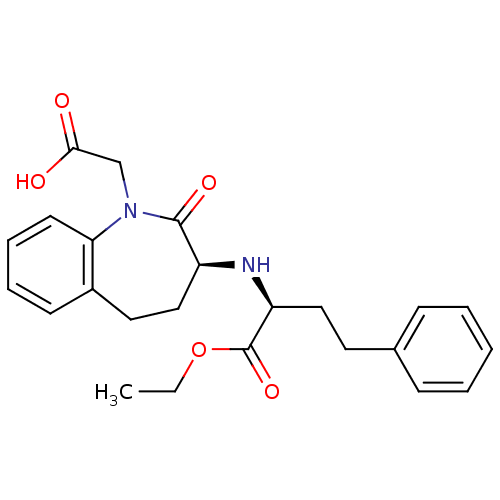
(1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method |
Bioorg Med Chem 23: 3526-33 (2015)
Article DOI: 10.1016/j.bmc.2015.04.024
BindingDB Entry DOI: 10.7270/Q2DB83M4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50368166
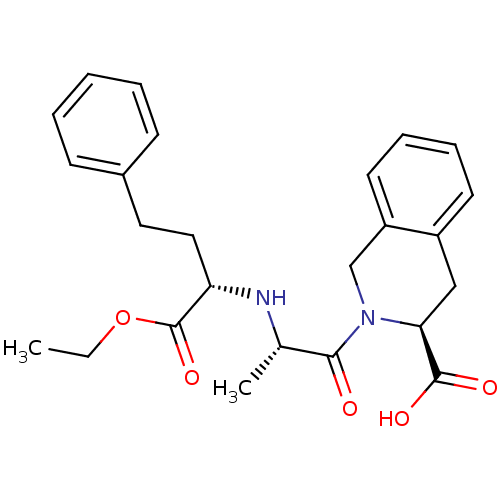
(Accupril | QUINAPRIL)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H30N2O5/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30)/t17-,21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method |
Bioorg Med Chem 23: 3526-33 (2015)
Article DOI: 10.1016/j.bmc.2015.04.024
BindingDB Entry DOI: 10.7270/Q2DB83M4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50017129
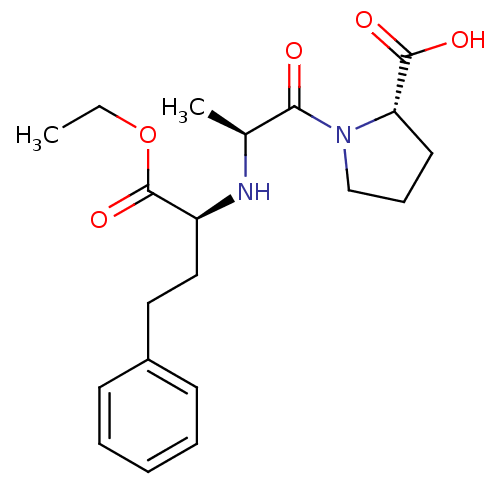
((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method |
Bioorg Med Chem 23: 3526-33 (2015)
Article DOI: 10.1016/j.bmc.2015.04.024
BindingDB Entry DOI: 10.7270/Q2DB83M4 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449247
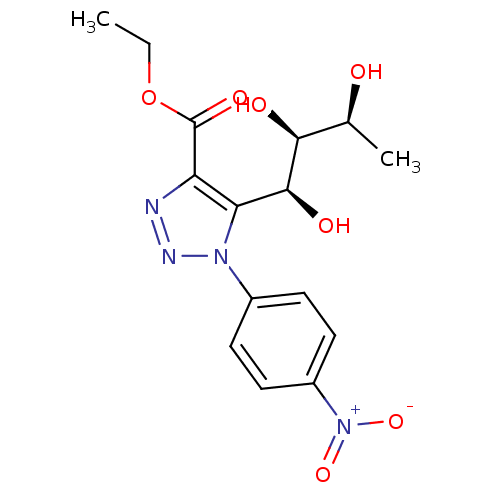
(CHEMBL3125147)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C15H18N4O7/c1-3-26-15(23)11-12(14(22)13(21)8(2)20)18(17-16-11)9-4-6-10(7-5-9)19(24)25/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449242
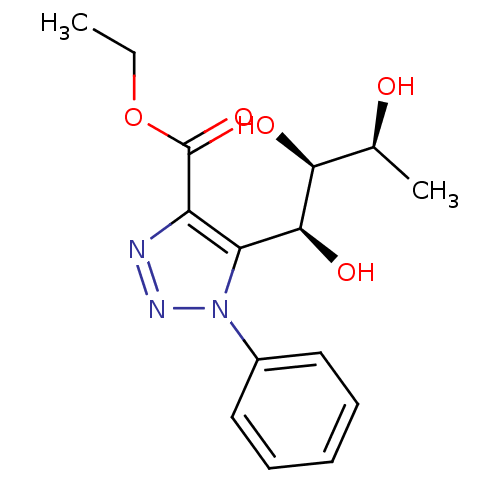
(CHEMBL3125151)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O5/c1-3-23-15(22)11-12(14(21)13(20)9(2)19)18(17-16-11)10-7-5-4-6-8-10/h4-9,13-14,19-21H,3H2,1-2H3/t9-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50329197

(3-((R)-1-((2S,6R)-2,6-dimethylmorpholino)ethyl)-N-...)Show SMILES C[C@@H](N1C[C@H](C)O[C@H](C)C1)c1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-9-29-18(16-6-23-24-7-16)8-22-21(29)20(25-12)26-19-5-17(27-31-19)15(4)28-10-13(2)30-14(3)11-28/h5-9,13-15H,10-11H2,1-4H3,(H,23,24)(H,25,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449239
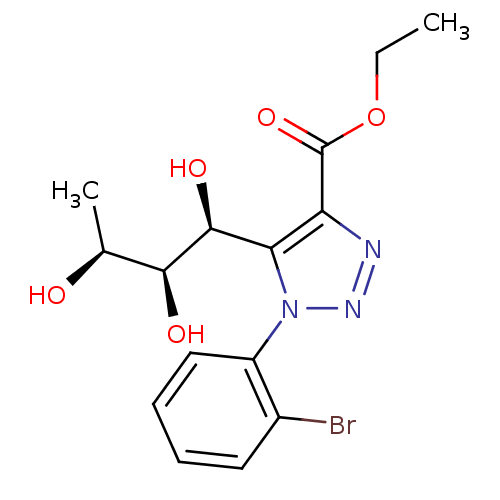
(CHEMBL3125155)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccccc1Br |r| Show InChI InChI=1S/C15H18BrN3O5/c1-3-24-15(23)11-12(14(22)13(21)8(2)20)19(18-17-11)10-7-5-4-6-9(10)16/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449240
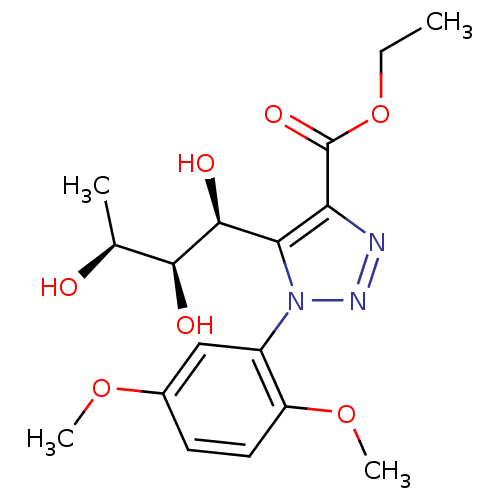
(CHEMBL3125154)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1cc(OC)ccc1OC |r| Show InChI InChI=1S/C17H23N3O7/c1-5-27-17(24)13-14(16(23)15(22)9(2)21)20(19-18-13)11-8-10(25-3)6-7-12(11)26-4/h6-9,15-16,21-23H,5H2,1-4H3/t9-,15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449252
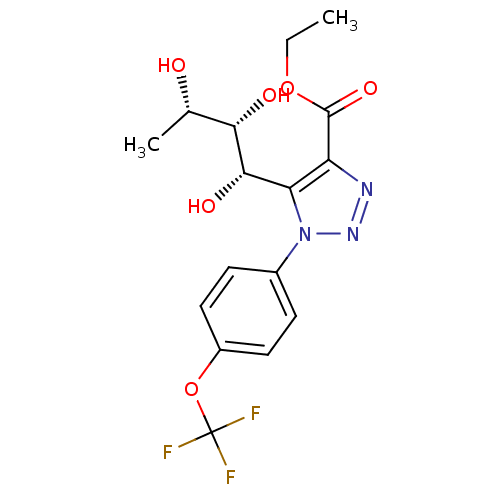
(CHEMBL3125142)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C16H18F3N3O6/c1-3-27-15(26)11-12(14(25)13(24)8(2)23)22(21-20-11)9-4-6-10(7-5-9)28-16(17,18)19/h4-8,13-14,23-25H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449251
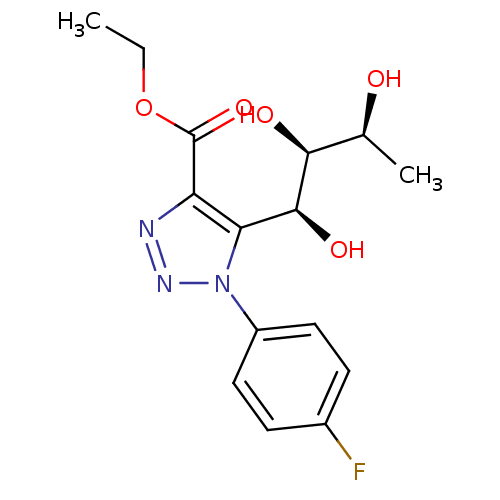
(CHEMBL3125143)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C15H18FN3O5/c1-3-24-15(23)11-12(14(22)13(21)8(2)20)19(18-17-11)10-6-4-9(16)5-7-10/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449243
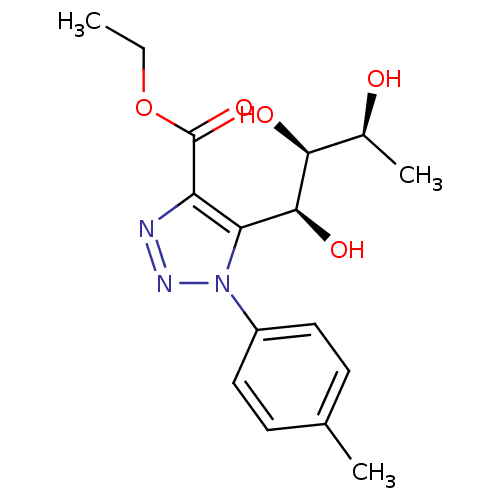
(CHEMBL3125152)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(C)cc1 |r| Show InChI InChI=1S/C16H21N3O5/c1-4-24-16(23)12-13(15(22)14(21)10(3)20)19(18-17-12)11-7-5-9(2)6-8-11/h5-8,10,14-15,20-22H,4H2,1-3H3/t10-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449248
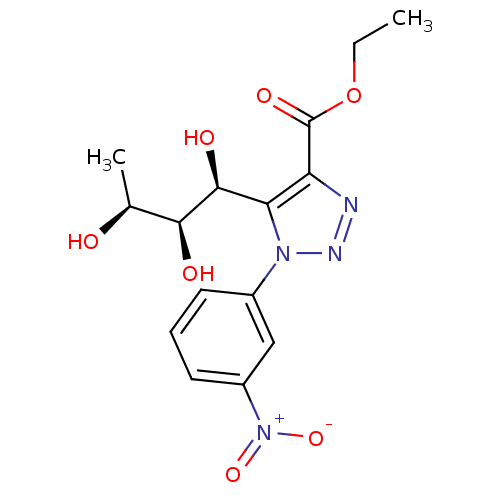
(CHEMBL3125146)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C15H18N4O7/c1-3-26-15(23)11-12(14(22)13(21)8(2)20)18(17-16-11)9-5-4-6-10(7-9)19(24)25/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50329198

(2-(ethyl((5-(6-methyl-3-(1H-pyrazol-4-yl)imidazo[1...)Show SMILES CCN(Cc1cc(Nc2nc(C)cn3c(cnc23)-c2cn[nH]c2)sn1)C(C)(C)CO Show InChI InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 |
Bioorg Med Chem Lett 20: 6739-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.140
BindingDB Entry DOI: 10.7270/Q2K074HX |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449253
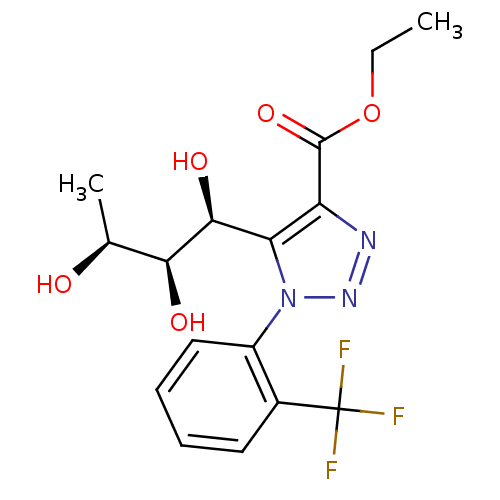
(CHEMBL3125141)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C16H18F3N3O5/c1-3-27-15(26)11-12(14(25)13(24)8(2)23)22(21-20-11)10-7-5-4-6-9(10)16(17,18)19/h4-8,13-14,23-25H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449250
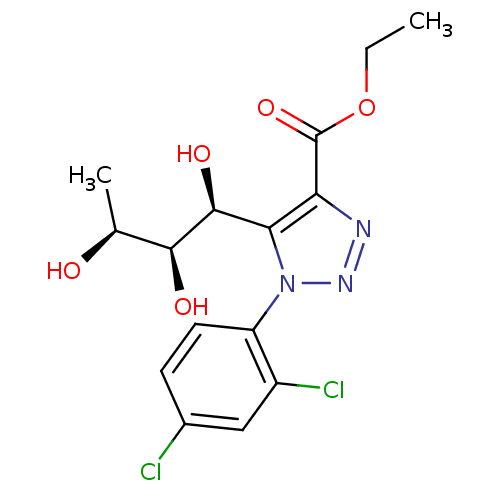
(CHEMBL3125144)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C15H17Cl2N3O5/c1-3-25-15(24)11-12(14(23)13(22)7(2)21)20(19-18-11)10-5-4-8(16)6-9(10)17/h4-7,13-14,21-23H,3H2,1-2H3/t7-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021153

(1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins |
Bioorg Med Chem 21: 4485-93 (2013)
Article DOI: 10.1016/j.bmc.2013.05.031
BindingDB Entry DOI: 10.7270/Q2P55PXT |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449241
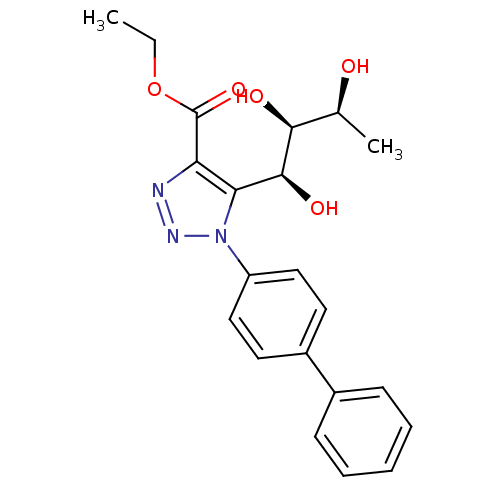
(CHEMBL3125153)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C21H23N3O5/c1-3-29-21(28)17-18(20(27)19(26)13(2)25)24(23-22-17)16-11-9-15(10-12-16)14-7-5-4-6-8-14/h4-13,19-20,25-27H,3H2,1-2H3/t13-,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50449247

(CHEMBL3125147)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C15H18N4O7/c1-3-26-15(23)11-12(14(22)13(21)8(2)20)18(17-16-11)9-4-6-10(7-5-9)19(24)25/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449245
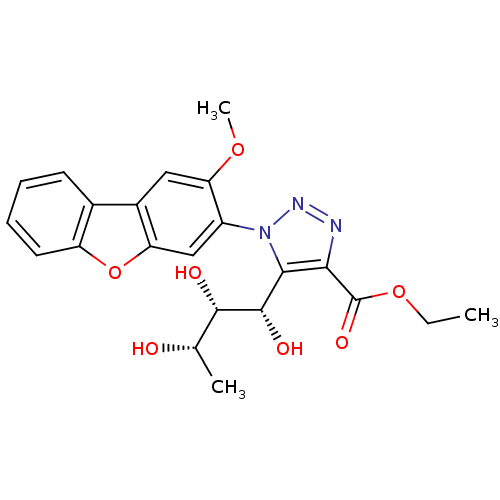
(CHEMBL3125149)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1cc2oc3ccccc3c2cc1OC |r| Show InChI InChI=1S/C22H23N3O7/c1-4-31-22(29)18-19(21(28)20(27)11(2)26)25(24-23-18)14-10-16-13(9-17(14)30-3)12-7-5-6-8-15(12)32-16/h5-11,20-21,26-28H,4H2,1-3H3/t11-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50084681
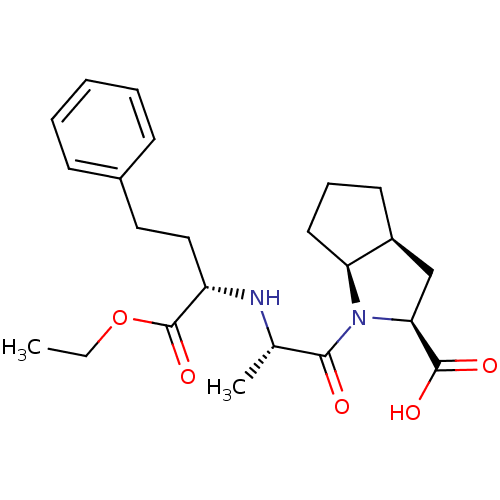
(1-[2-(1-Ethoxycarbonyl-3-phenyl-propylamino)-propi...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1[C@H]2CCC[C@H]2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hippuryl-histidyl-leucine hydrolysis after 30 mins by colorimetric method |
Bioorg Med Chem 23: 3526-33 (2015)
Article DOI: 10.1016/j.bmc.2015.04.024
BindingDB Entry DOI: 10.7270/Q2DB83M4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50084681

(1-[2-(1-Ethoxycarbonyl-3-phenyl-propylamino)-propi...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1[C@H]2CCC[C@H]2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins |
Bioorg Med Chem 21: 4485-93 (2013)
Article DOI: 10.1016/j.bmc.2013.05.031
BindingDB Entry DOI: 10.7270/Q2P55PXT |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50449249
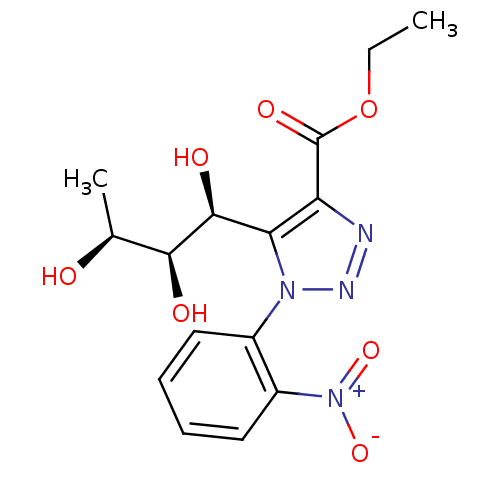
(CHEMBL3125145)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccccc1[N+]([O-])=O |r| Show InChI InChI=1S/C15H18N4O7/c1-3-26-15(23)11-12(14(22)13(21)8(2)20)18(17-16-11)9-6-4-5-7-10(9)19(24)25/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50367879
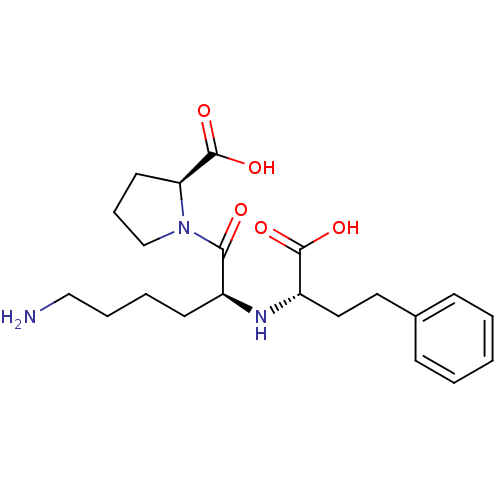
(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins |
Bioorg Med Chem 21: 4485-93 (2013)
Article DOI: 10.1016/j.bmc.2013.05.031
BindingDB Entry DOI: 10.7270/Q2P55PXT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50438379
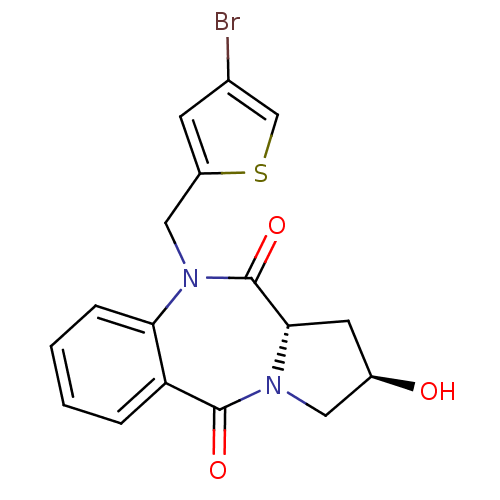
(CHEMBL2413588)Show SMILES O[C@@H]1C[C@@H]2N(C1)C(=O)c1ccccc1N(Cc1cc(Br)cs1)C2=O |r| Show InChI InChI=1S/C17H15BrN2O3S/c18-10-5-12(24-9-10)8-20-14-4-2-1-3-13(14)16(22)19-7-11(21)6-15(19)17(20)23/h1-5,9,11,15,21H,6-8H2/t11-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung ACE assessed as hydrolysis of hippuryl-histidyl-leucine to hippuric acid and histidyl-leucine after 30 mins |
Bioorg Med Chem 21: 4485-93 (2013)
Article DOI: 10.1016/j.bmc.2013.05.031
BindingDB Entry DOI: 10.7270/Q2P55PXT |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449254
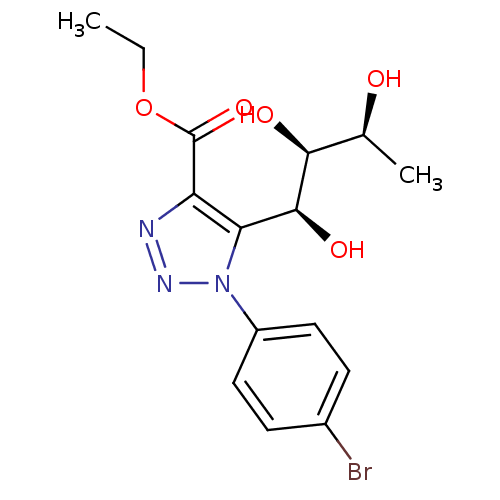
(CHEMBL3125156)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccc(Br)cc1 |r| Show InChI InChI=1S/C15H18BrN3O5/c1-3-24-15(23)11-12(14(22)13(21)8(2)20)19(18-17-11)10-6-4-9(16)5-7-10/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50449242

(CHEMBL3125151)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O5/c1-3-23-15(22)11-12(14(21)13(20)9(2)19)18(17-16-11)10-7-5-4-6-8-10/h4-9,13-14,19-21H,3H2,1-2H3/t9-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50449239

(CHEMBL3125155)Show SMILES CCOC(=O)c1nnn(c1[C@H](O)[C@@H](O)[C@H](C)O)-c1ccccc1Br |r| Show InChI InChI=1S/C15H18BrN3O5/c1-3-24-15(23)11-12(14(22)13(21)8(2)20)19(18-17-11)10-7-5-4-6-9(10)16/h4-8,13-14,20-22H,3H2,1-2H3/t8-,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) expressed in HEK293 cells using 2-NBDG as substrate incubated for 30 mins prior to substrate addition measured a... |
Bioorg Med Chem Lett 24: 1528-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.077
BindingDB Entry DOI: 10.7270/Q2PG1T6N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data