

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328726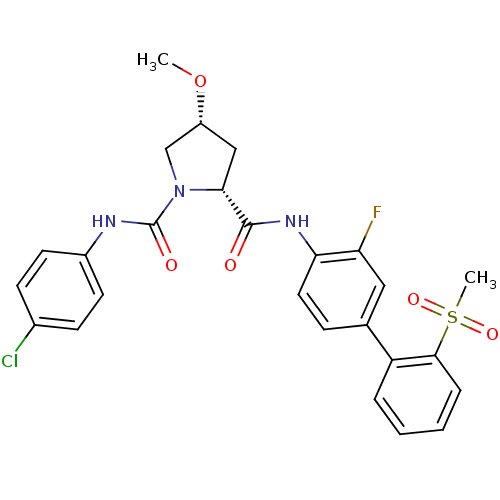 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249132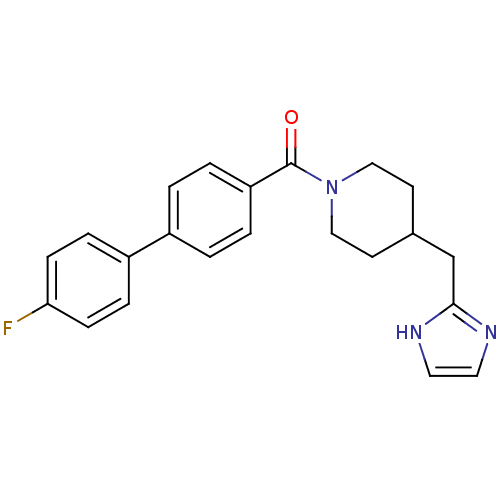 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(4'-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249483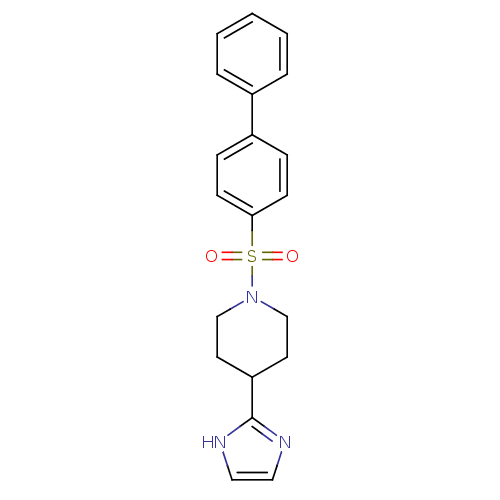 (1-(biphenyl-4-ylsulfonyl)-4-(1H-imidazol-2-yl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328728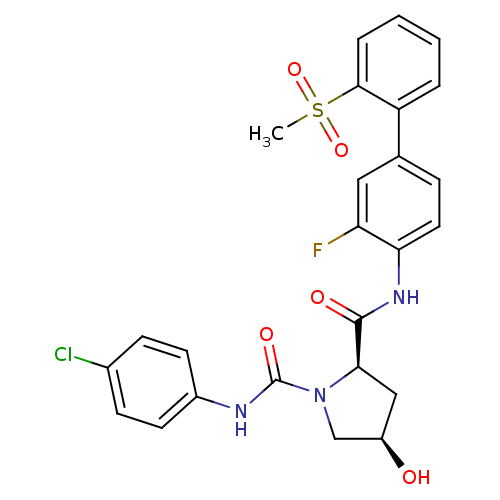 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249464 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249131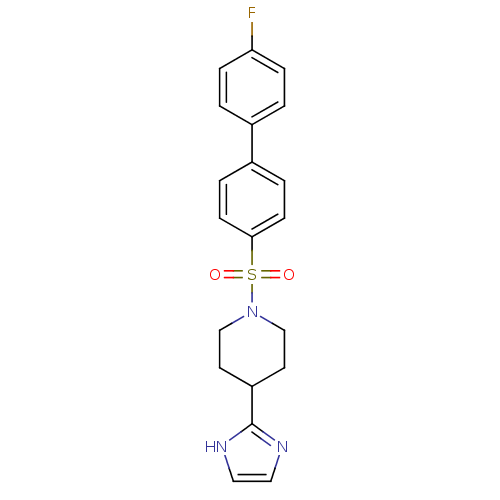 (1-(4'-fluorobiphenyl-4-ylsulfonyl)-4-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249134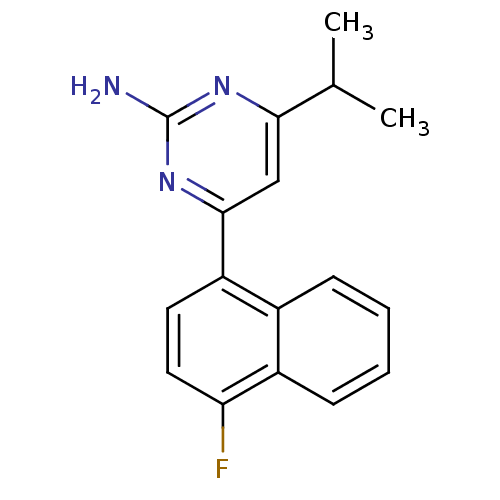 (4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81692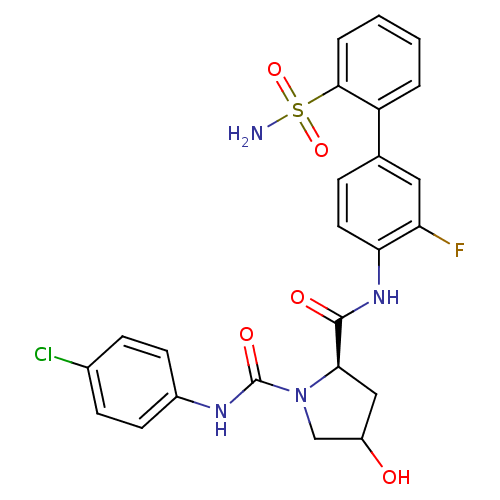 (4-Substituted Pyrrolidine Ring, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81698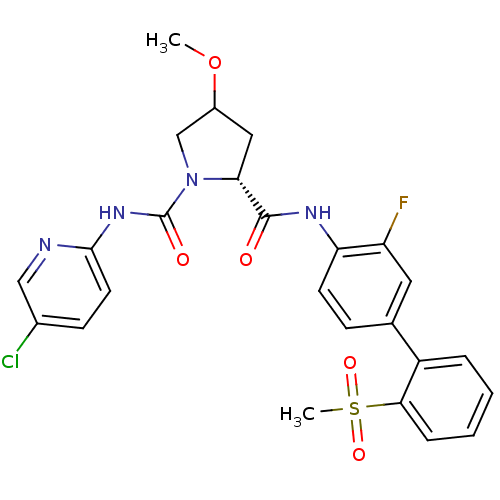 (4-Substituted Pyrrolidine Ring, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249486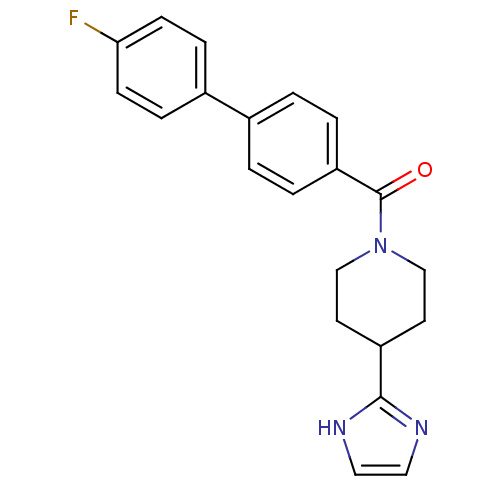 ((4-(1H-imidazol-2-yl)piperidin-1-yl)(4'-fluorobiph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81693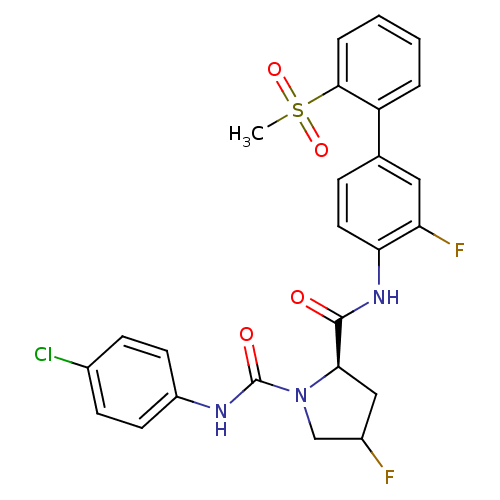 (4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328726 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249176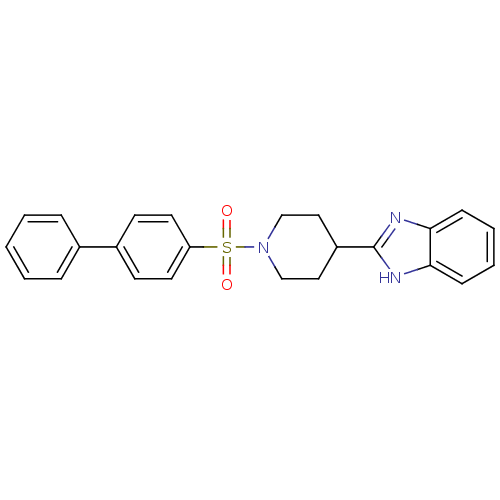 (2-(1-(biphenyl-4-ylsulfonyl)piperidin-4-yl)-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249482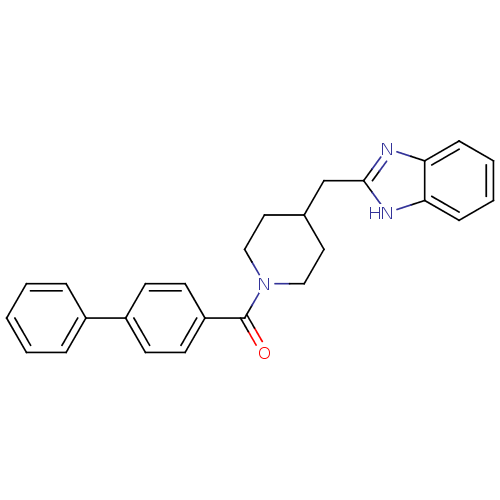 ((4-((1H-benzo[d]imidazol-2-yl)methyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249462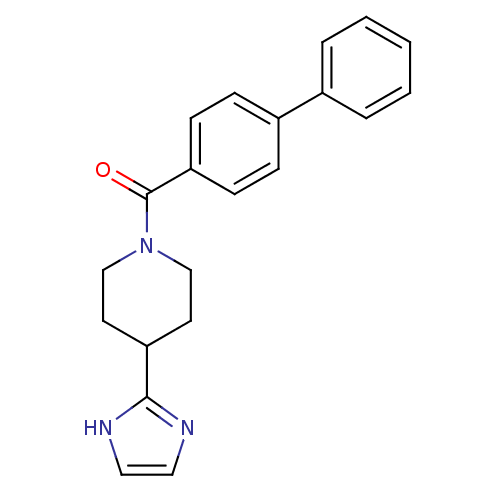 ((4-(1H-imidazol-2-yl)piperidin-1-yl)(biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249126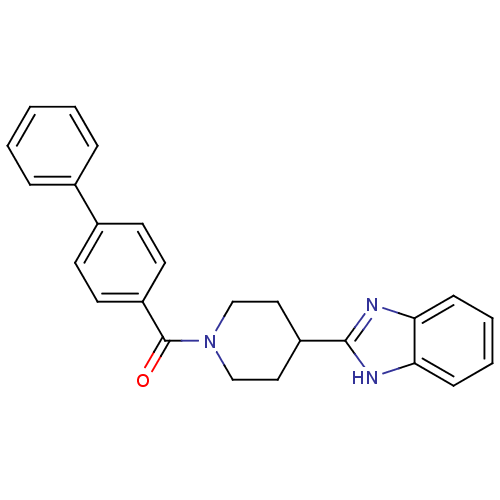 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249128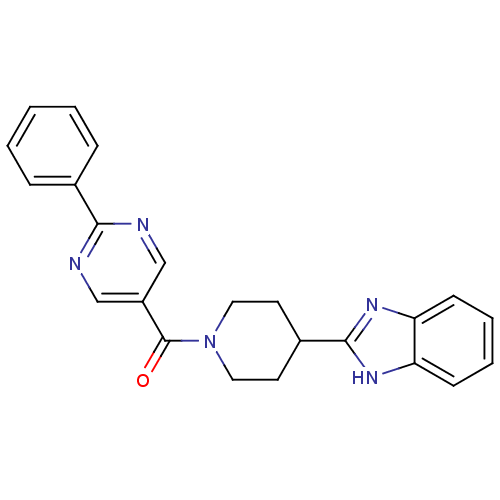 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81695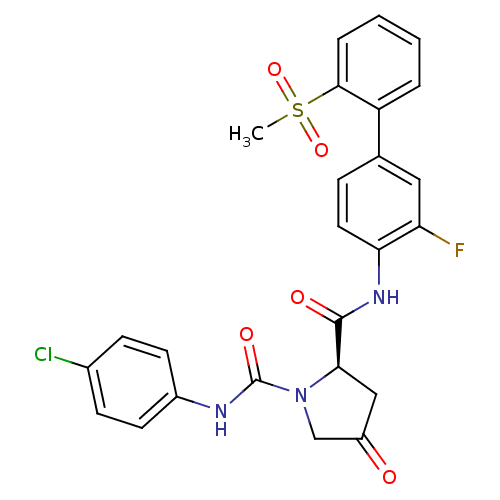 (4-Substituted Pyrrolidine Ring, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249427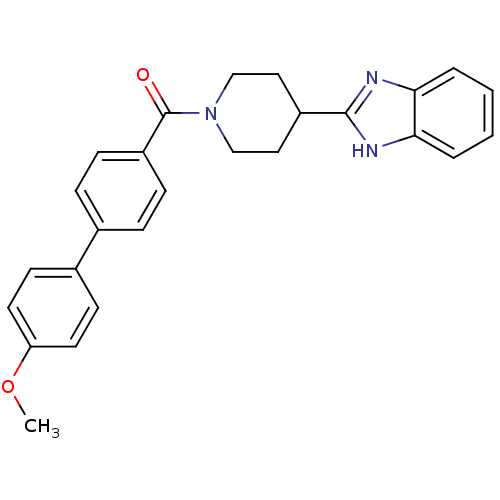 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4'-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249180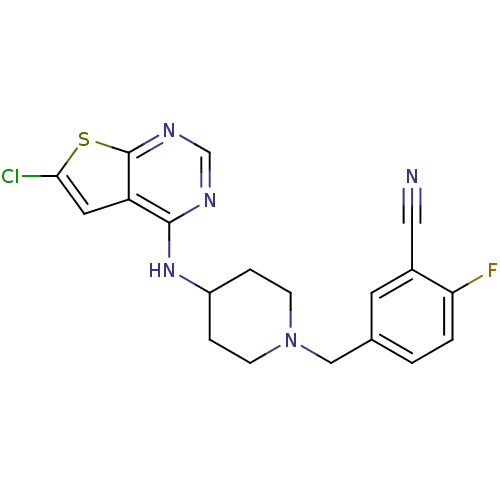 (5-((4-(6-chlorothieno[2,3-d]pyrimidin-4-ylamino)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249129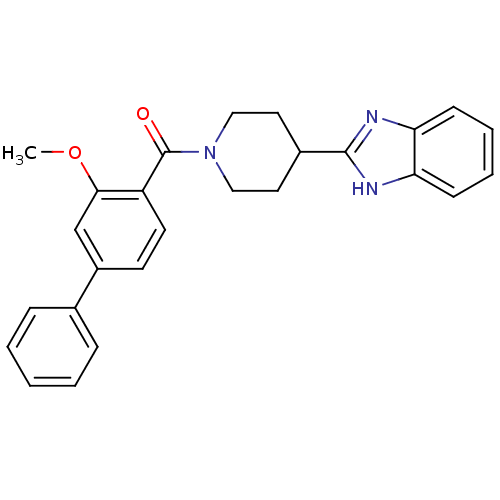 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(3-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81697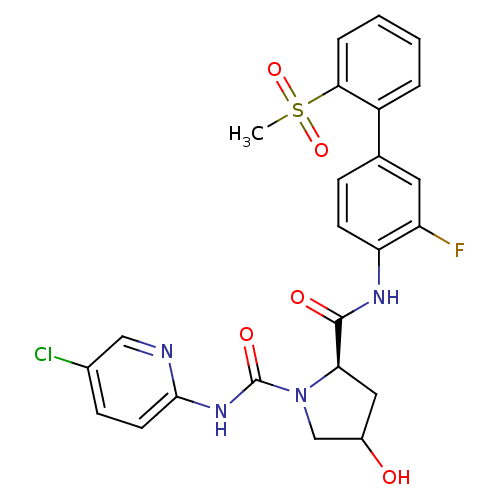 (4-Substituted Pyrrolidine Ring, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81696 (4-Substituted Pyrrolidine Ring, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249130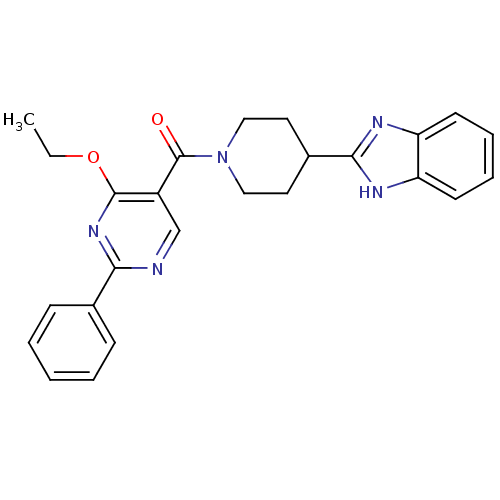 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328728 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249484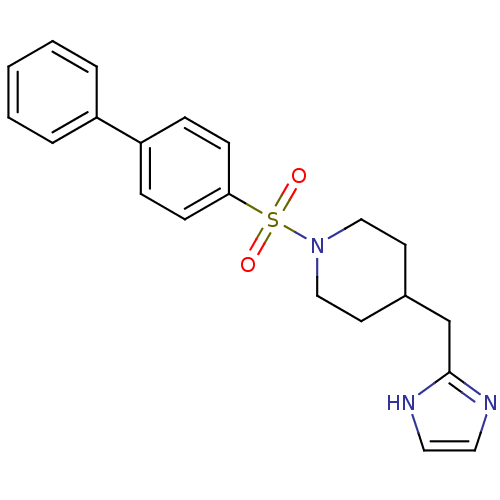 (4-((1H-imidazol-2-yl)methyl)-1-(biphenyl-4-ylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81693 (4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249132 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(4'-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81699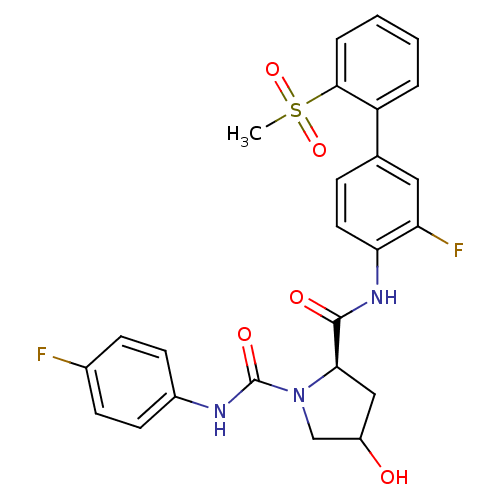 (4-Substituted Pyrrolidine Ring, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249459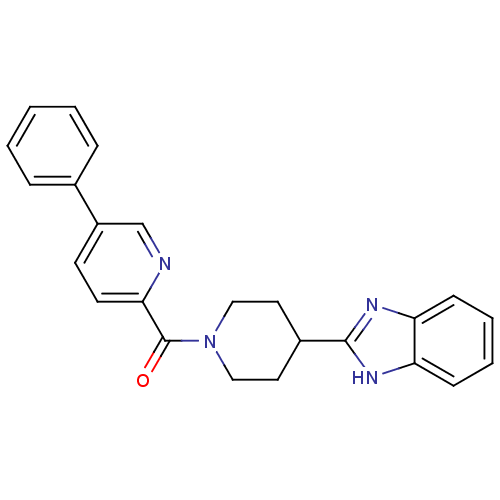 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(5-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249464 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249173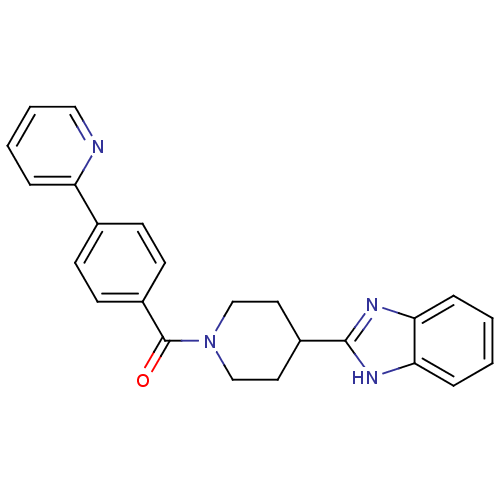 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-(py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328725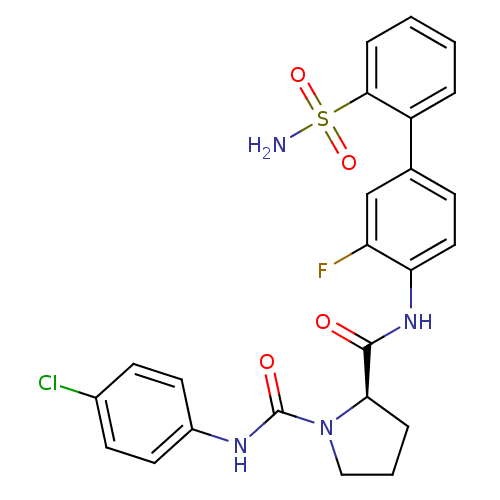 ((R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-sulfamoylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249134 (4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249175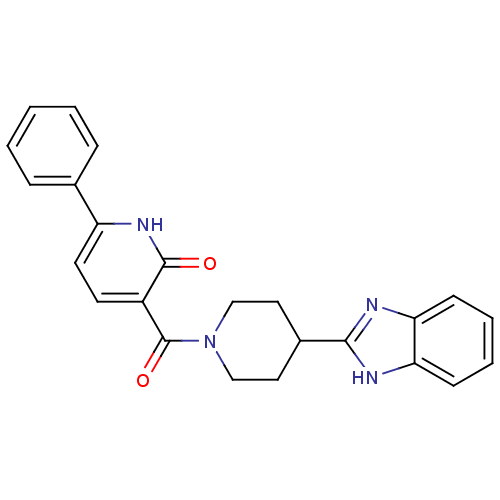 (3-(4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carbon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249131 (1-(4'-fluorobiphenyl-4-ylsulfonyl)-4-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249424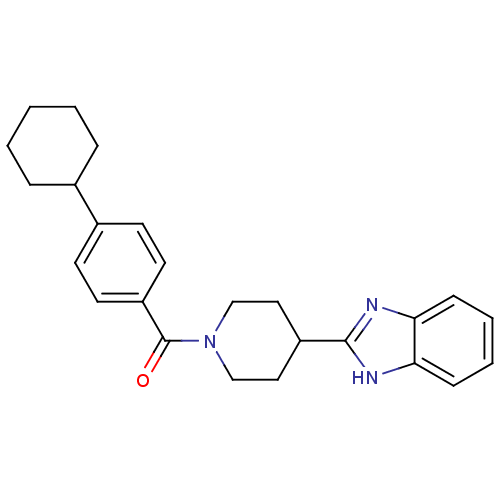 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249483 (1-(biphenyl-4-ylsulfonyl)-4-(1H-imidazol-2-yl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81681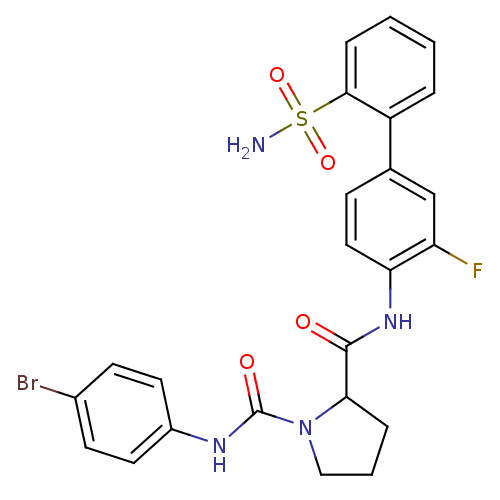 (P1 Phenyl Ring, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249486 ((4-(1H-imidazol-2-yl)piperidin-1-yl)(4'-fluorobiph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328725 ((R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-sulfamoylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249425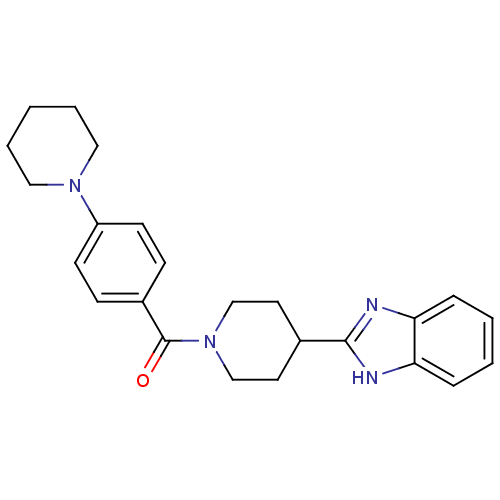 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-(pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249177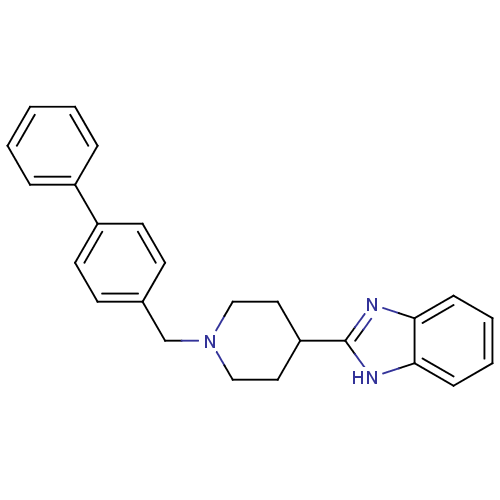 (2-(1-(biphenyl-4-ylmethyl)piperidin-4-yl)-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249462 ((4-(1H-imidazol-2-yl)piperidin-1-yl)(biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249460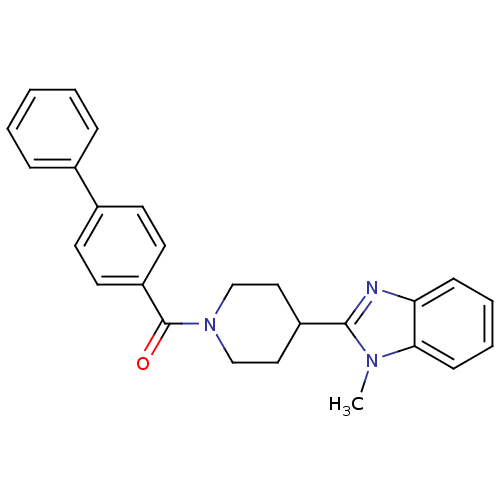 (CHEMBL472416 | biphenyl-4-yl(4-(1-methyl-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249456 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4'-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249174 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-(py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249179 ((3-(1H-benzo[d]imidazol-2-yl)azetidin-1-yl)(biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249180 (5-((4-(6-chlorothieno[2,3-d]pyrimidin-4-ylamino)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249482 ((4-((1H-benzo[d]imidazol-2-yl)methyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |