 Found 839 hits with Last Name = 'tomczuk' and Initial = 'be'
Found 839 hits with Last Name = 'tomczuk' and Initial = 'be' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50123504
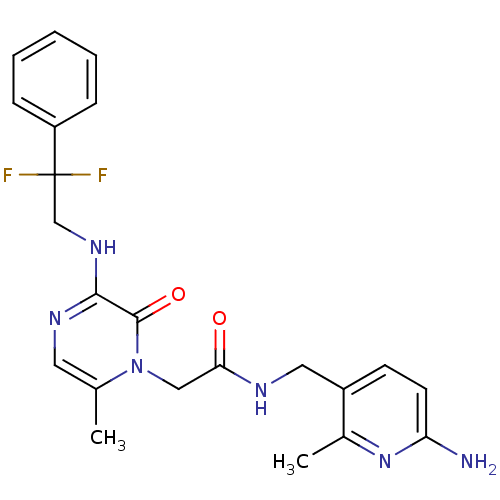
(CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24F2N6O2/c1-14-10-27-20(28-13-22(23,24)17-6-4-3-5-7-17)21(32)30(14)12-19(31)26-11-16-8-9-18(25)29-15(16)2/h3-10H,11-13H2,1-2H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50031790

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(17-19-11-5-2-6-12-19)27-24(30)20(18-23(29)28-32)13-7-4-10-16-33-21-14-8-3-9-15-21/h2-3,5-6,8-9,11-12,14-15,20,22,32H,4,7,10,13,16-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377618
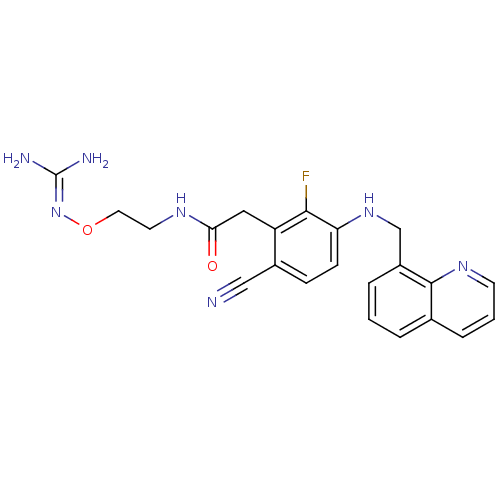
(CHEMBL254353)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]-c2cccc3cccnc23)ccc1C#N Show InChI InChI=1S/C22H22FN7O2/c23-20-17(11-19(31)27-9-10-32-30-22(25)26)15(12-24)6-7-18(20)29-13-16-4-1-3-14-5-2-8-28-21(14)16/h1-8,29H,9-11,13H2,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377625
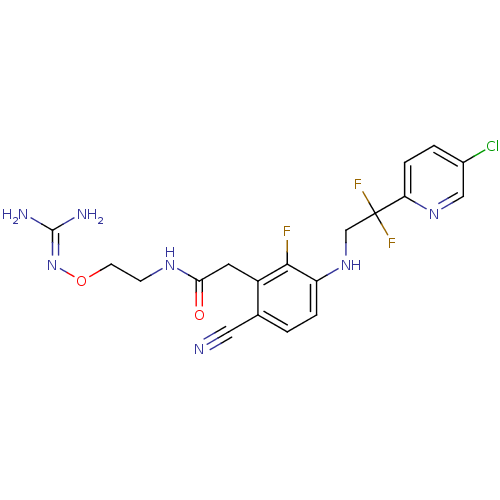
(CHEMBL254557)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccc(Cl)cn2)ccc1C#N Show InChI InChI=1S/C19H19ClF3N7O2/c20-12-2-4-15(28-9-12)19(22,23)10-29-14-3-1-11(8-24)13(17(14)21)7-16(31)27-5-6-32-30-18(25)26/h1-4,9,29H,5-7,10H2,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377615
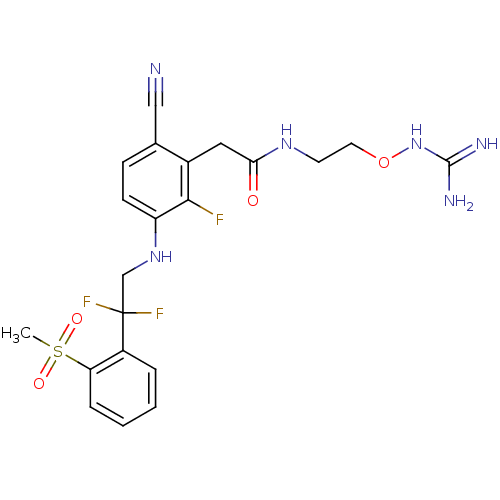
(CHEMBL254962)Show SMILES CS(=O)(=O)c1ccccc1C(F)(F)CNc1ccc(C#N)c(CC(=O)NCCONC(N)=N)c1F Show InChI InChI=1S/C21H23F3N6O4S/c1-35(32,33)17-5-3-2-4-15(17)21(23,24)12-29-16-7-6-13(11-25)14(19(16)22)10-18(31)28-8-9-34-30-20(26)27/h2-7,29H,8-10,12H2,1H3,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377623
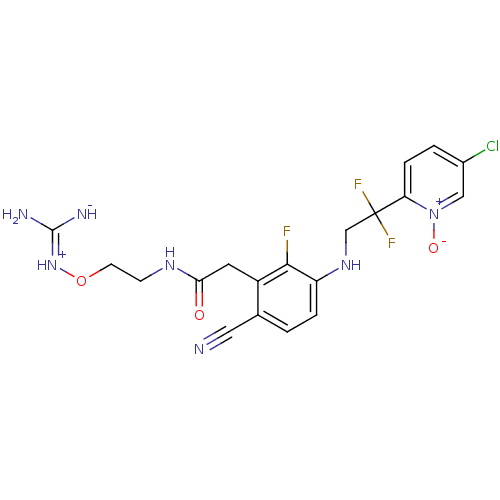
(CHEMBL254759)Show SMILES N\C([NH-])=[NH+]\OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccc(Cl)c[n+]2[O-])ccc1C#N Show InChI InChI=1S/C19H19ClF3N7O3/c20-12-2-4-15(30(32)9-12)19(22,23)10-28-14-3-1-11(8-24)13(17(14)21)7-16(31)27-5-6-33-29-18(25)26/h1-4,9,28H,5-7,10H2,(H5,25,26,27,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377611
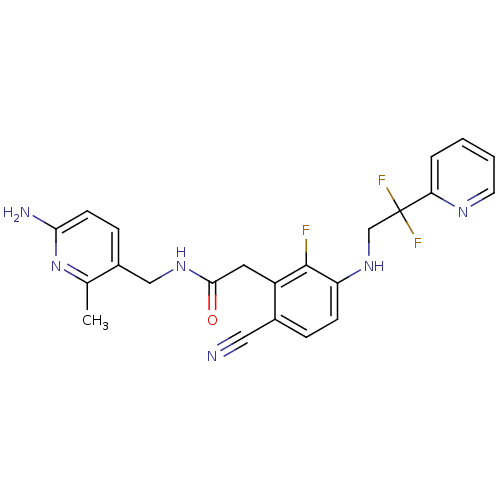
(CHEMBL258018)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C23H21F3N6O/c1-14-16(6-8-20(28)32-14)12-30-21(33)10-17-15(11-27)5-7-18(22(17)24)31-13-23(25,26)19-4-2-3-9-29-19/h2-9,31H,10,12-13H2,1H3,(H2,28,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377619
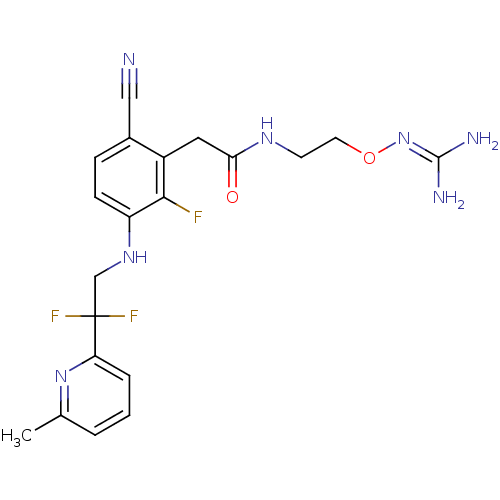
(CHEMBL402758)Show SMILES [#6]-c1cccc(n1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-3-2-4-16(29-12)20(22,23)11-28-15-6-5-13(10-24)14(18(15)21)9-17(31)27-7-8-32-30-19(25)26/h2-6,28H,7-9,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031783
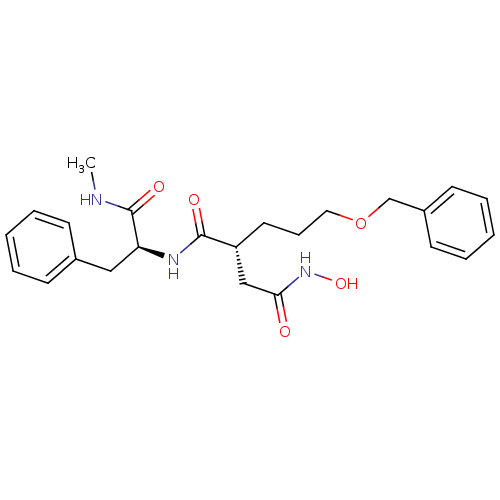
((R)-2-(3-(benzyloxy)propyl)-N4-hydroxy-N1-((S)-1-(...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCOCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C24H31N3O5/c1-25-24(30)21(15-18-9-4-2-5-10-18)26-23(29)20(16-22(28)27-31)13-8-14-32-17-19-11-6-3-7-12-19/h2-7,9-12,20-21,31H,8,13-17H2,1H3,(H,25,30)(H,26,29)(H,27,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase, matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 343-348 (1995)
Article DOI: 10.1016/0960-894X(95)00032-O
BindingDB Entry DOI: 10.7270/Q2JH3M44 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031795
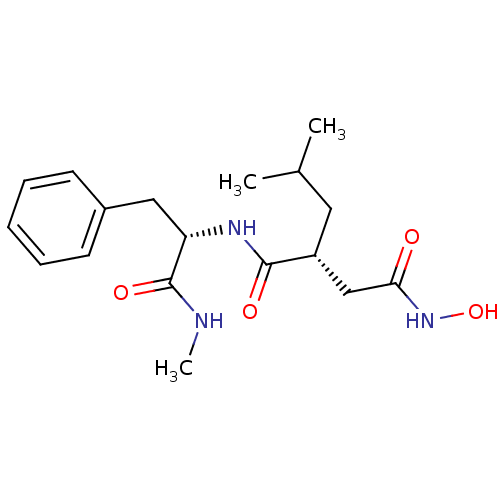
((N-(2-HYDROXAMATEMETHYLENE-4-METHYL-PENTOYL)PHENYL...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C18H27N3O4/c1-12(2)9-14(11-16(22)21-25)17(23)20-15(18(24)19-3)10-13-7-5-4-6-8-13/h4-8,12,14-15,25H,9-11H2,1-3H3,(H,19,24)(H,20,23)(H,21,22)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377620
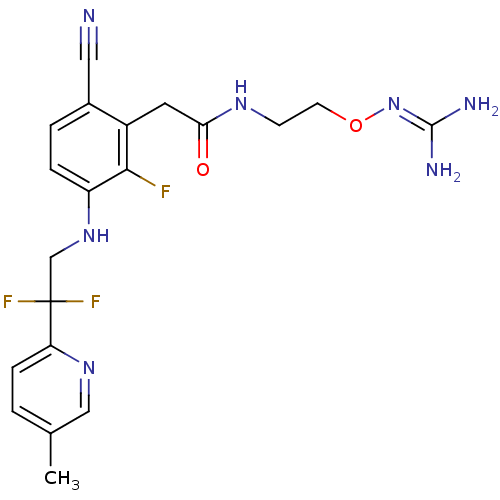
(CHEMBL254784)Show SMILES [#6]-c1ccc(nc1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-2-5-16(28-10-12)20(22,23)11-29-15-4-3-13(9-24)14(18(15)21)8-17(31)27-6-7-32-30-19(25)26/h2-5,10,29H,6-8,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377622
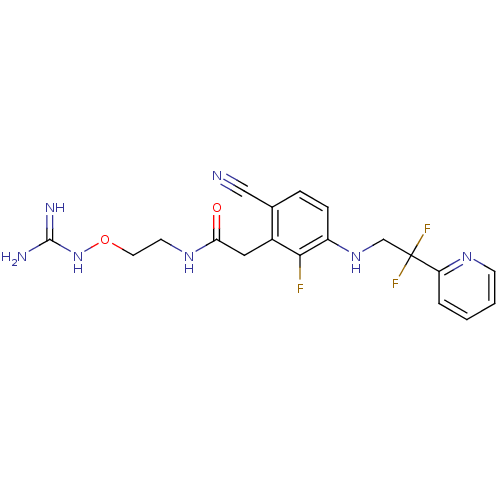
(CHEMBL257543)Show SMILES NC(=N)NOCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C19H20F3N7O2/c20-17-13(9-16(30)27-7-8-31-29-18(24)25)12(10-23)4-5-14(17)28-11-19(21,22)15-3-1-2-6-26-15/h1-6,28H,7-9,11H2,(H,27,30)(H4,24,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50031784
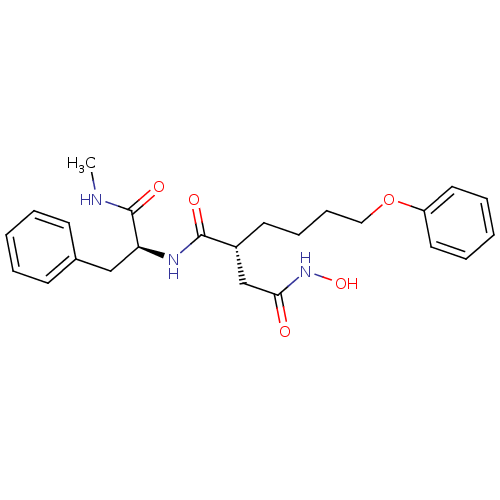
((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C24H31N3O5/c1-25-24(30)21(16-18-10-4-2-5-11-18)26-23(29)19(17-22(28)27-31)12-8-9-15-32-20-13-6-3-7-14-20/h2-7,10-11,13-14,19,21,31H,8-9,12,15-17H2,1H3,(H,25,30)(H,26,29)(H,27,28)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312651
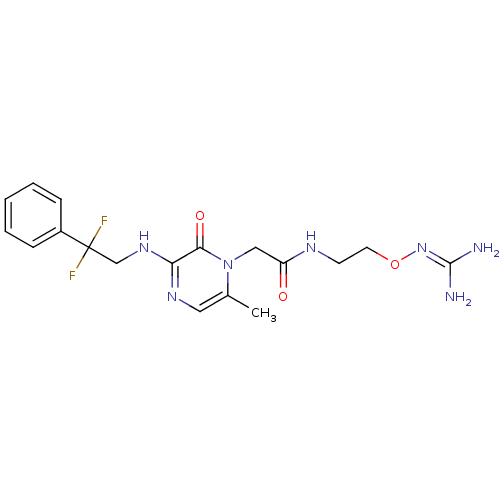
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]C(F)(F)c2ccccc2)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C18H23F2N7O3/c1-12-9-24-15(25-11-18(19,20)13-5-3-2-4-6-13)16(29)27(12)10-14(28)23-7-8-30-26-17(21)22/h2-6,9H,7-8,10-11H2,1H3,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50286354
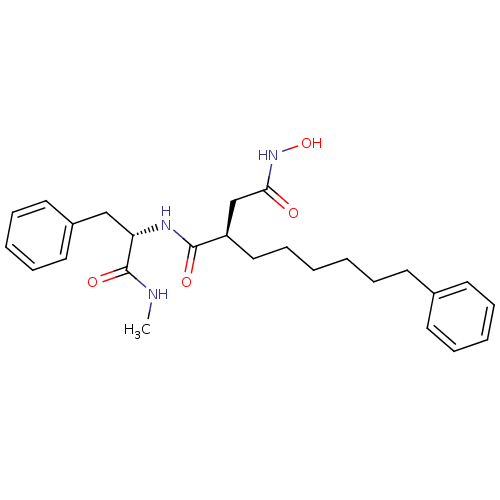
((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C26H35N3O4/c1-27-26(32)23(18-21-15-9-5-10-16-21)28-25(31)22(19-24(30)29-33)17-11-3-2-6-12-20-13-7-4-8-14-20/h4-5,7-10,13-16,22-23,33H,2-3,6,11-12,17-19H2,1H3,(H,27,32)(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377614
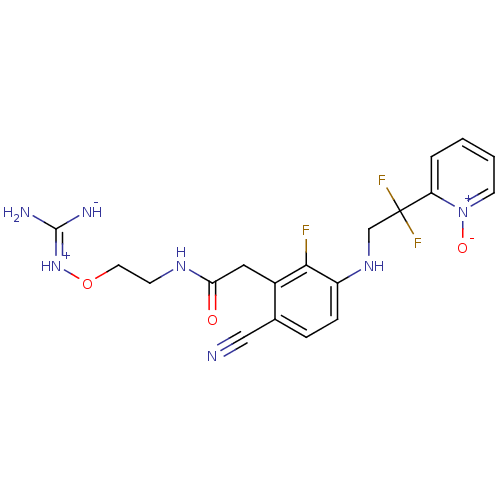
(CHEMBL401655)Show SMILES N\C([NH-])=[NH+]\OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2cccc[n+]2[O-])ccc1C#N Show InChI InChI=1S/C19H20F3N7O3/c20-17-13(9-16(30)26-6-8-32-28-18(24)25)12(10-23)4-5-14(17)27-11-19(21,22)15-3-1-2-7-29(15)31/h1-5,7,27H,6,8-9,11H2,(H5,24,25,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50031795

((N-(2-HYDROXAMATEMETHYLENE-4-METHYL-PENTOYL)PHENYL...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C18H27N3O4/c1-12(2)9-14(11-16(22)21-25)17(23)20-15(18(24)19-3)10-13-7-5-4-6-8-13/h4-8,12,14-15,25H,9-11H2,1-3H3,(H,19,24)(H,20,23)(H,21,22)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312653
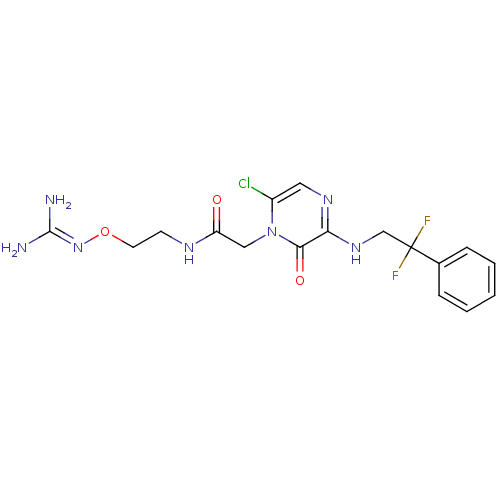
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-n1c(Cl)cnc(-[#7]-[#6]C(F)(F)c2ccccc2)c1=O Show InChI InChI=1S/C17H20ClF2N7O3/c18-12-8-24-14(25-10-17(19,20)11-4-2-1-3-5-11)15(29)27(12)9-13(28)23-6-7-30-26-16(21)22/h1-5,8H,6-7,9-10H2,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377617
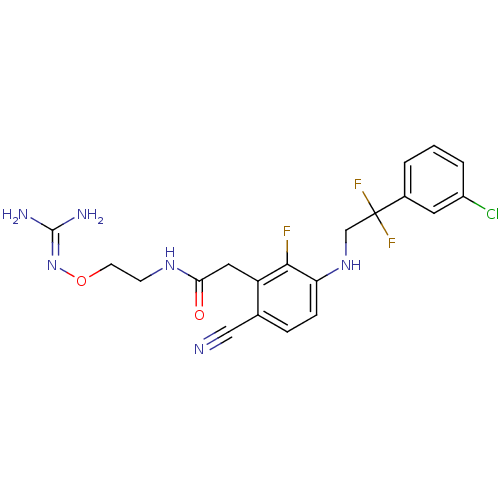
(CHEMBL403359)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2cccc(Cl)c2)ccc1C#N Show InChI InChI=1S/C20H20ClF3N6O2/c21-14-3-1-2-13(8-14)20(23,24)11-29-16-5-4-12(10-25)15(18(16)22)9-17(31)28-6-7-32-30-19(26)27/h1-5,8,29H,6-7,9,11H2,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377624
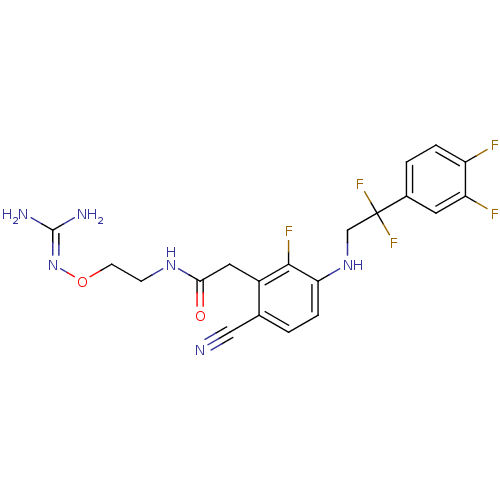
(CHEMBL403310)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccc(F)c(F)c2)ccc1C#N Show InChI InChI=1S/C20H19F5N6O2/c21-14-3-2-12(7-15(14)22)20(24,25)10-30-16-4-1-11(9-26)13(18(16)23)8-17(32)29-5-6-33-31-19(27)28/h1-4,7,30H,5-6,8,10H2,(H,29,32)(H4,27,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031795

((N-(2-HYDROXAMATEMETHYLENE-4-METHYL-PENTOYL)PHENYL...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C18H27N3O4/c1-12(2)9-14(11-16(22)21-25)17(23)20-15(18(24)19-3)10-13-7-5-4-6-8-13/h4-8,12,14-15,25H,9-11H2,1-3H3,(H,19,24)(H,20,23)(H,21,22)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase(HNC), MMP-8 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031774
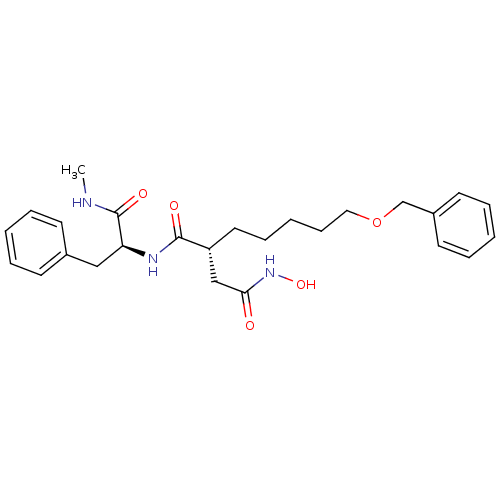
((R)-2-(5-(benzyloxy)pentyl)-N4-hydroxy-N1-((S)-1-(...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C26H35N3O5/c1-27-26(32)23(17-20-11-5-2-6-12-20)28-25(31)22(18-24(30)29-33)15-9-4-10-16-34-19-21-13-7-3-8-14-21/h2-3,5-8,11-14,22-23,33H,4,9-10,15-19H2,1H3,(H,27,32)(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase(HNC), MMP-8 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031784

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C24H31N3O5/c1-25-24(30)21(16-18-10-4-2-5-11-18)26-23(29)19(17-22(28)27-31)12-8-9-15-32-20-13-6-3-7-14-20/h2-7,10-11,13-14,19,21,31H,8-9,12,15-17H2,1H3,(H,25,30)(H,26,29)(H,27,28)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human fibroblast collagenase, matrix metalloproteinase-1 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031772
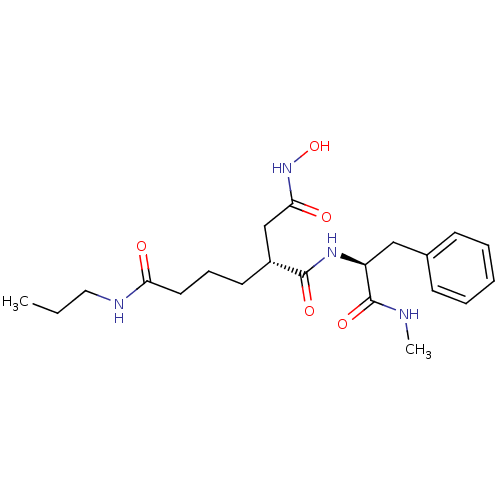
((R)-3-((S)-1-Methylcarbamoyl-2-phenyl-ethylcarbamo...)Show SMILES CCCNC(=O)CCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C21H32N4O5/c1-3-12-23-18(26)11-7-10-16(14-19(27)25-30)20(28)24-17(21(29)22-2)13-15-8-5-4-6-9-15/h4-6,8-9,16-17,30H,3,7,10-14H2,1-2H3,(H,22,29)(H,23,26)(H,24,28)(H,25,27)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase, matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 343-348 (1995)
Article DOI: 10.1016/0960-894X(95)00032-O
BindingDB Entry DOI: 10.7270/Q2JH3M44 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031795

((N-(2-HYDROXAMATEMETHYLENE-4-METHYL-PENTOYL)PHENYL...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C18H27N3O4/c1-12(2)9-14(11-16(22)21-25)17(23)20-15(18(24)19-3)10-13-7-5-4-6-8-13/h4-8,12,14-15,25H,9-11H2,1-3H3,(H,19,24)(H,20,23)(H,21,22)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration of compound against human fibroblast stromelysin (HFS) was determined |
Bioorg Med Chem Lett 5: 343-348 (1995)
Article DOI: 10.1016/0960-894X(95)00032-O
BindingDB Entry DOI: 10.7270/Q2JH3M44 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031778
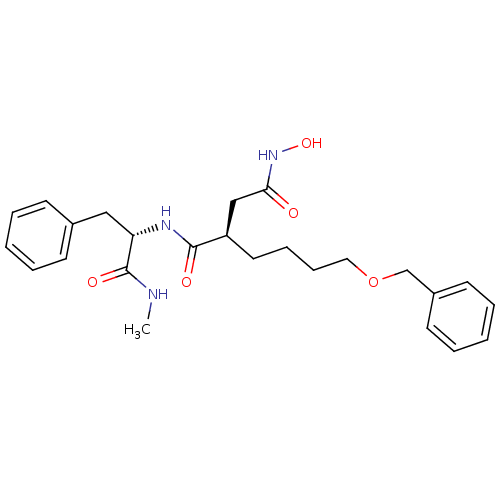
((R)-2-(4-(benzyloxy)butyl)-N4-hydroxy-N1-((S)-1-(m...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCOCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(16-19-10-4-2-5-11-19)27-24(30)21(17-23(29)28-32)14-8-9-15-33-18-20-12-6-3-7-13-20/h2-7,10-13,21-22,32H,8-9,14-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031772

((R)-3-((S)-1-Methylcarbamoyl-2-phenyl-ethylcarbamo...)Show SMILES CCCNC(=O)CCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C21H32N4O5/c1-3-12-23-18(26)11-7-10-16(14-19(27)25-30)20(28)24-17(21(29)22-2)13-15-8-5-4-6-9-15/h4-6,8-9,16-17,30H,3,7,10-14H2,1-2H3,(H,22,29)(H,23,26)(H,24,28)(H,25,27)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase(HNC), MMP-8 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50286354

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C26H35N3O4/c1-27-26(32)23(18-21-15-9-5-10-16-21)28-25(31)22(19-24(30)29-33)17-11-3-2-6-12-20-13-7-4-8-14-20/h4-5,7-10,13-16,22-23,33H,2-3,6,11-12,17-19H2,1H3,(H,27,32)(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031790

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(17-19-11-5-2-6-12-19)27-24(30)20(18-23(29)28-32)13-7-4-10-16-33-21-14-8-3-9-15-21/h2-3,5-6,8-9,11-12,14-15,20,22,32H,4,7,10,13,16-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031790

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(17-19-11-5-2-6-12-19)27-24(30)20(18-23(29)28-32)13-7-4-10-16-33-21-14-8-3-9-15-21/h2-3,5-6,8-9,11-12,14-15,20,22,32H,4,7,10,13,16-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase, matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 343-348 (1995)
Article DOI: 10.1016/0960-894X(95)00032-O
BindingDB Entry DOI: 10.7270/Q2JH3M44 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50031775
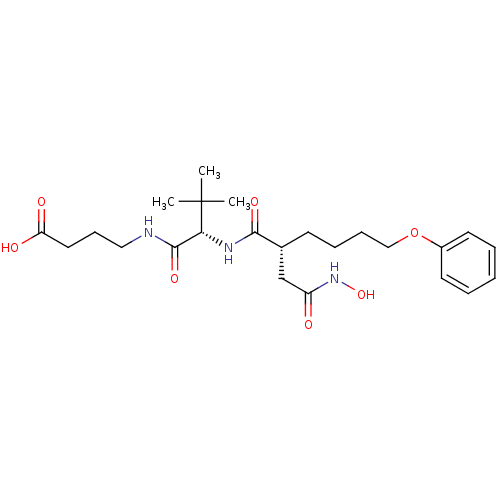
(4-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-6-phenoxy-h...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO)C(=O)NCCCC(O)=O Show InChI InChI=1S/C24H37N3O7/c1-24(2,3)21(23(32)25-14-9-13-20(29)30)26-22(31)17(16-19(28)27-33)10-7-8-15-34-18-11-5-4-6-12-18/h4-6,11-12,17,21,33H,7-10,13-16H2,1-3H3,(H,25,32)(H,26,31)(H,27,28)(H,29,30)/t17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibitory potency against human stromelysin, MMP-3 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031778

((R)-2-(4-(benzyloxy)butyl)-N4-hydroxy-N1-((S)-1-(m...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCOCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(16-19-10-4-2-5-11-19)27-24(30)21(17-23(29)28-32)14-8-9-15-33-18-20-12-6-3-7-13-20/h2-7,10-13,21-22,32H,8-9,14-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase(HNC), MMP-8 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031784

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C24H31N3O5/c1-25-24(30)21(16-18-10-4-2-5-11-18)26-23(29)19(17-22(28)27-31)12-8-9-15-32-20-13-6-3-7-14-20/h2-7,10-11,13-14,19,21,31H,8-9,12,15-17H2,1H3,(H,25,30)(H,26,29)(H,27,28)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase, matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 343-348 (1995)
Article DOI: 10.1016/0960-894X(95)00032-O
BindingDB Entry DOI: 10.7270/Q2JH3M44 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11123
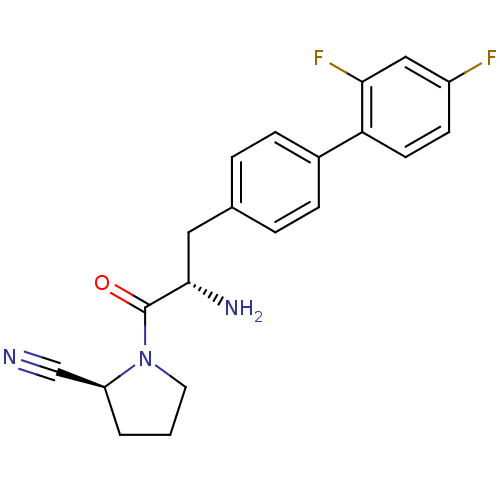
((2S)-1-[(2S)-2-amino-3-[4-(2,4-difluorophenyl)phen...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccc(F)cc1F)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H19F2N3O/c21-15-7-8-17(18(22)11-15)14-5-3-13(4-6-14)10-19(24)20(26)25-9-1-2-16(25)12-23/h3-8,11,16,19H,1-2,9-10,24H2/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... |
Bioorg Med Chem Lett 16: 123-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.037
BindingDB Entry DOI: 10.7270/Q2S180QJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312652
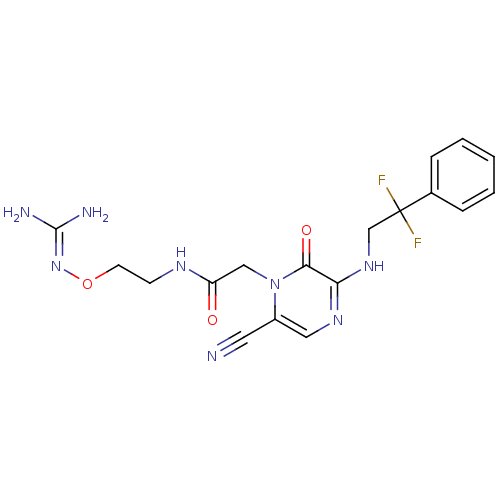
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-n1c(cnc(-[#7]-[#6]C(F)(F)c2ccccc2)c1=O)C#N Show InChI InChI=1S/C18H20F2N8O3/c19-18(20,12-4-2-1-3-5-12)11-26-15-16(30)28(13(8-21)9-25-15)10-14(29)24-6-7-31-27-17(22)23/h1-5,9H,6-7,10-11H2,(H,24,29)(H,25,26)(H4,22,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377607
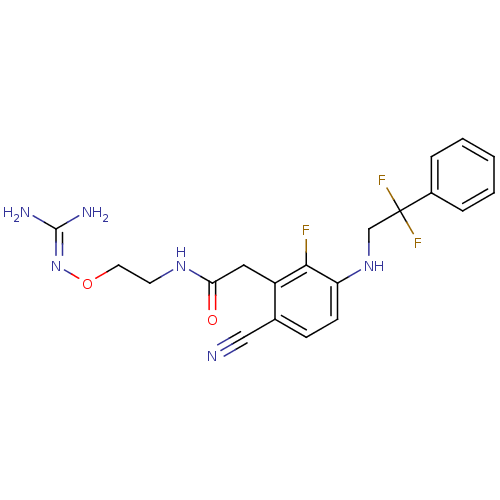
(CHEMBL404025)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccccc2)ccc1C#N Show InChI InChI=1S/C20H21F3N6O2/c21-18-15(10-17(30)27-8-9-31-29-19(25)26)13(11-24)6-7-16(18)28-12-20(22,23)14-4-2-1-3-5-14/h1-7,28H,8-10,12H2,(H,27,30)(H4,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
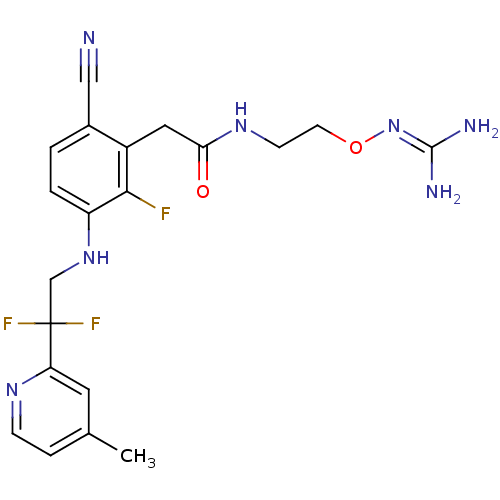
(Homo sapiens (Human)) | BDBM50377626
(CHEMBL254786)Show SMILES [#6]-c1ccnc(c1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-4-5-27-16(8-12)20(22,23)11-29-15-3-2-13(10-24)14(18(15)21)9-17(31)28-6-7-32-30-19(25)26/h2-5,8,29H,6-7,9,11H2,1H3,(H,28,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
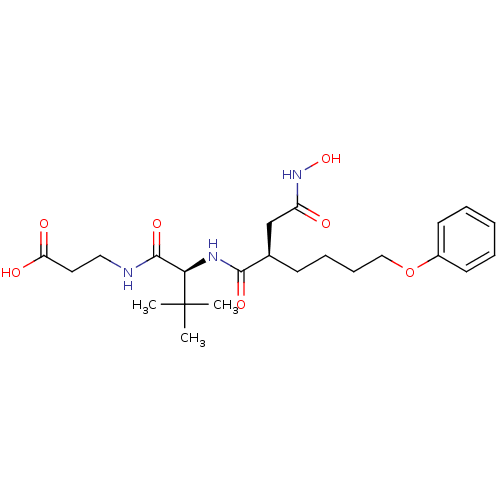
(Homo sapiens (Human)) | BDBM50031781
(3-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-6-phenoxy-h...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO)C(=O)NCCC(O)=O Show InChI InChI=1S/C23H35N3O7/c1-23(2,3)20(22(31)24-13-12-19(28)29)25-21(30)16(15-18(27)26-32)9-7-8-14-33-17-10-5-4-6-11-17/h4-6,10-11,16,20,32H,7-9,12-15H2,1-3H3,(H,24,31)(H,25,30)(H,26,27)(H,28,29)/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibitory potency against human stromelysin, MMP-3 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
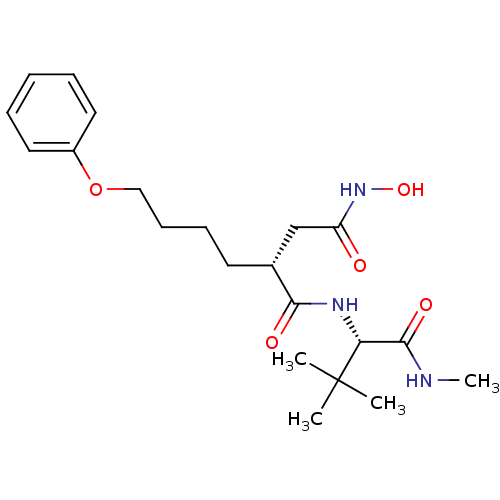
(Homo sapiens (Human)) | BDBM50031798
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCCOc1ccccc1)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C21H33N3O5/c1-21(2,3)18(20(27)22-4)23-19(26)15(14-17(25)24-28)10-8-9-13-29-16-11-6-5-7-12-16/h5-7,11-12,15,18,28H,8-10,13-14H2,1-4H3,(H,22,27)(H,23,26)(H,24,25)/t15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibitory potency against human fibroblast collagenase, MMP-1 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11121
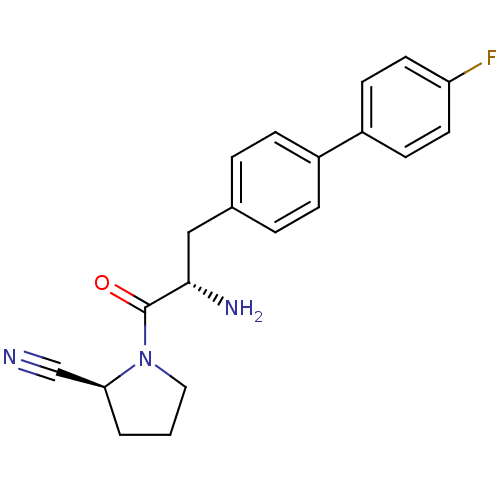
((2S)-1-[(2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]p...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccc(F)cc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H20FN3O/c21-17-9-7-16(8-10-17)15-5-3-14(4-6-15)12-19(23)20(25)24-11-1-2-18(24)13-22/h3-10,18-19H,1-2,11-12,23H2/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... |
Bioorg Med Chem Lett 16: 123-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.037
BindingDB Entry DOI: 10.7270/Q2S180QJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377616
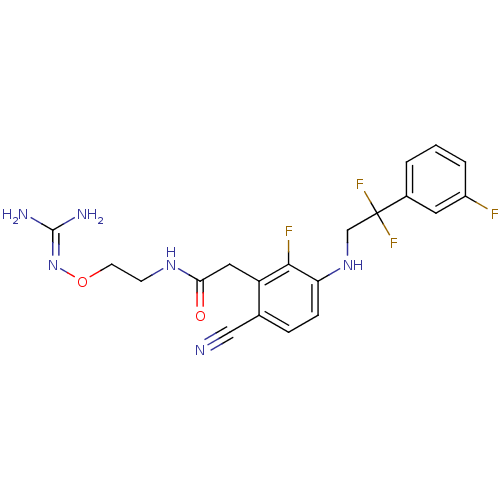
(CHEMBL258198)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2cccc(F)c2)ccc1C#N Show InChI InChI=1S/C20H20F4N6O2/c21-14-3-1-2-13(8-14)20(23,24)11-29-16-5-4-12(10-25)15(18(16)22)9-17(31)28-6-7-32-30-19(26)27/h1-5,8,29H,6-7,9,11H2,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50031778

((R)-2-(4-(benzyloxy)butyl)-N4-hydroxy-N1-((S)-1-(m...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCOCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(16-19-10-4-2-5-11-19)27-24(30)21(17-23(29)28-32)14-8-9-15-33-18-20-12-6-3-7-13-20/h2-7,10-13,21-22,32H,8-9,14-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50031770
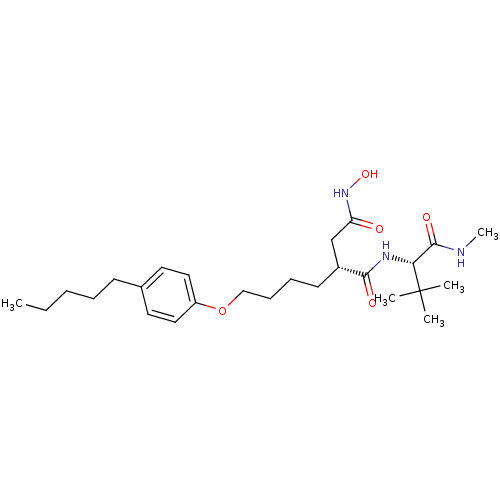
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCc1ccc(OCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C)cc1 Show InChI InChI=1S/C26H43N3O5/c1-6-7-8-11-19-13-15-21(16-14-19)34-17-10-9-12-20(18-22(30)29-33)24(31)28-23(25(32)27-5)26(2,3)4/h13-16,20,23,33H,6-12,17-18H2,1-5H3,(H,27,32)(H,28,31)(H,29,30)/t20-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 5: 349-352 (1995)
Article DOI: 10.1016/0960-894X(95)00033-P
BindingDB Entry DOI: 10.7270/Q2DR2VG9 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031788
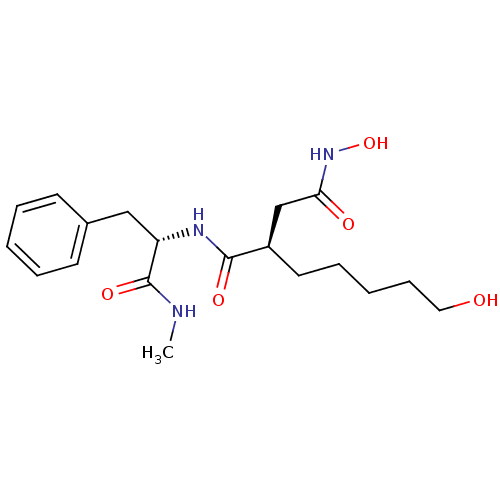
((R)-N*4*-Hydroxy-2-(5-hydroxy-pentyl)-N*1*-((S)-1-...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCO)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-20-19(26)16(12-14-8-4-2-5-9-14)21-18(25)15(13-17(24)22-27)10-6-3-7-11-23/h2,4-5,8-9,15-16,23,27H,3,6-7,10-13H2,1H3,(H,20,26)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase(HNC), MMP-8 |
J Med Chem 38: 2570-81 (1995)
BindingDB Entry DOI: 10.7270/Q2GM86B5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031788

((R)-N*4*-Hydroxy-2-(5-hydroxy-pentyl)-N*1*-((S)-1-...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCO)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-20-19(26)16(12-14-8-4-2-5-9-14)21-18(25)15(13-17(24)22-27)10-6-3-7-11-23/h2,4-5,8-9,15-16,23,27H,3,6-7,10-13H2,1H3,(H,20,26)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil collagenase, matrix metalloproteinase-8 |
Bioorg Med Chem Lett 5: 343-348 (1995)
Article DOI: 10.1016/0960-894X(95)00032-O
BindingDB Entry DOI: 10.7270/Q2JH3M44 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023
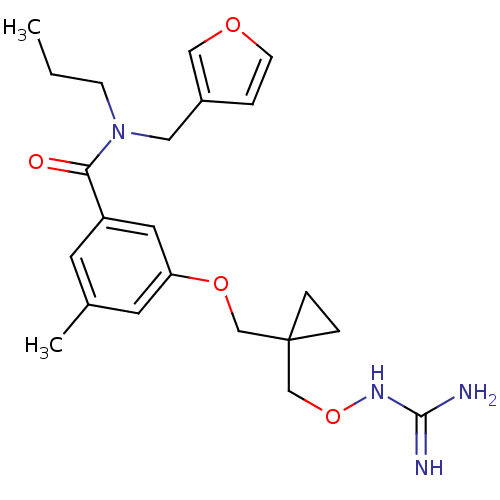
(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023

(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data