

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11542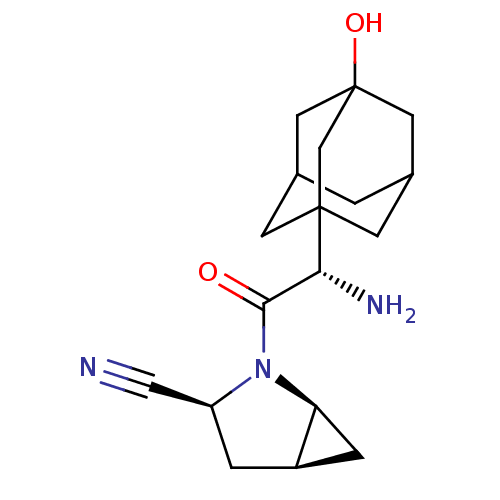 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11541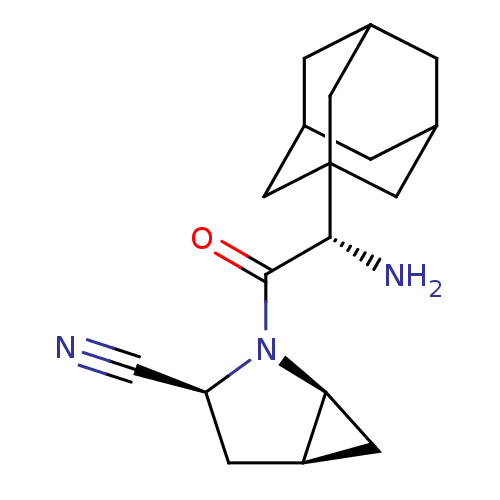 ((1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacety...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11530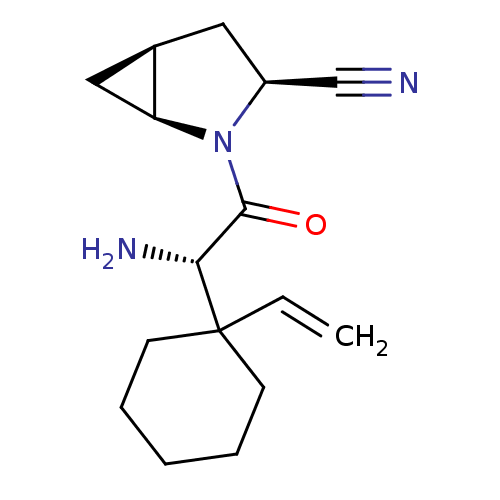 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11544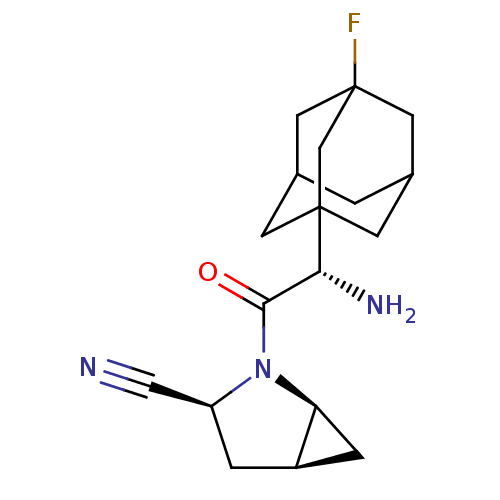 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11543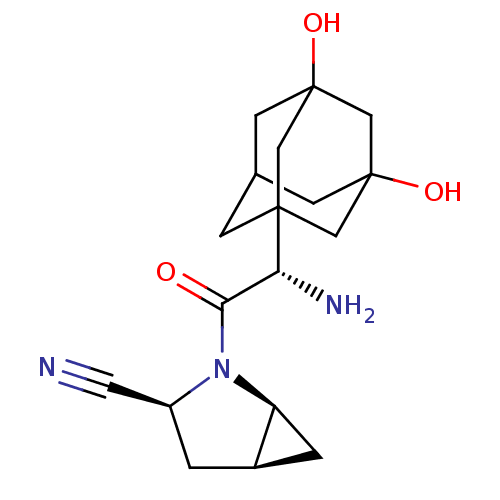 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11529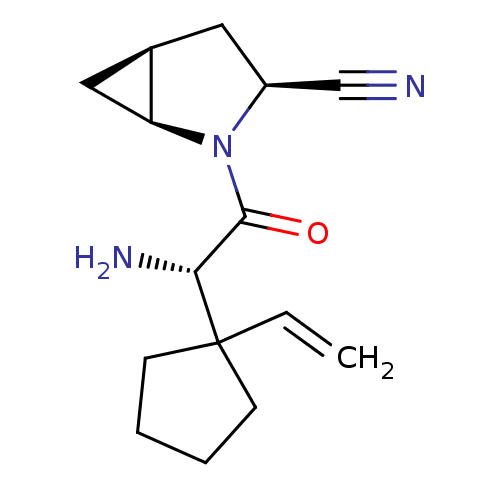 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11535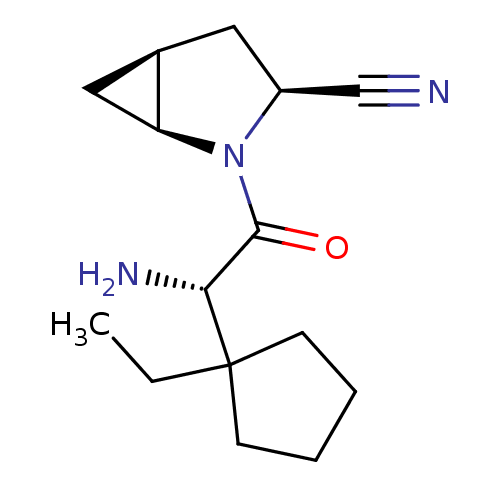 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11533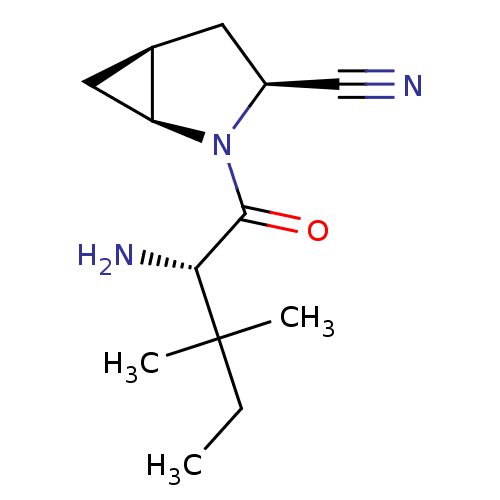 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.10 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11538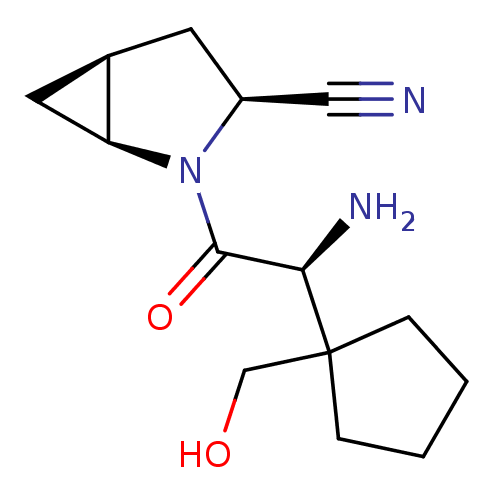 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11539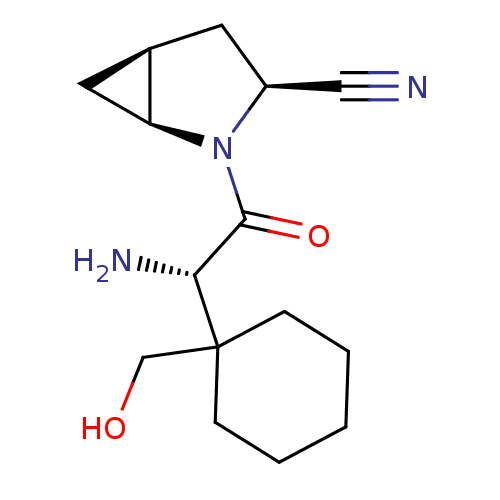 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11532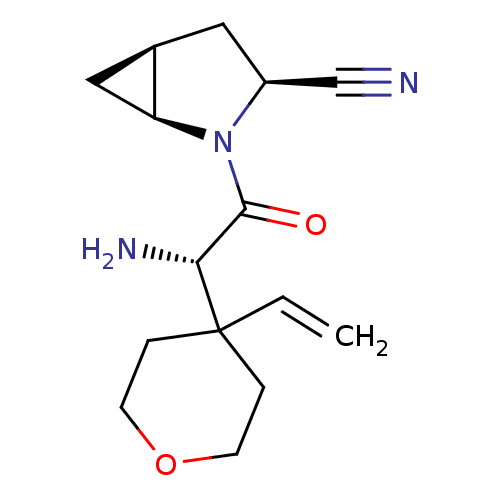 ((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11531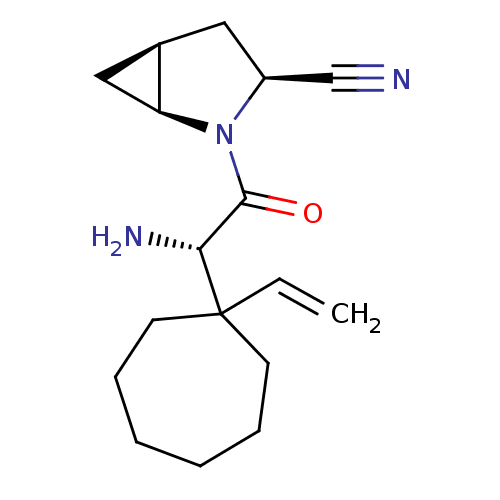 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcycloheptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11528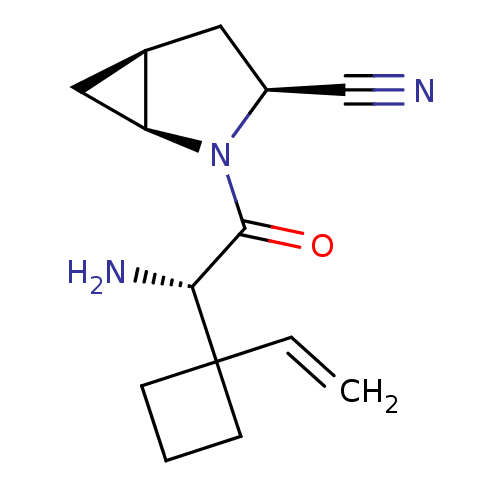 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclobutyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11536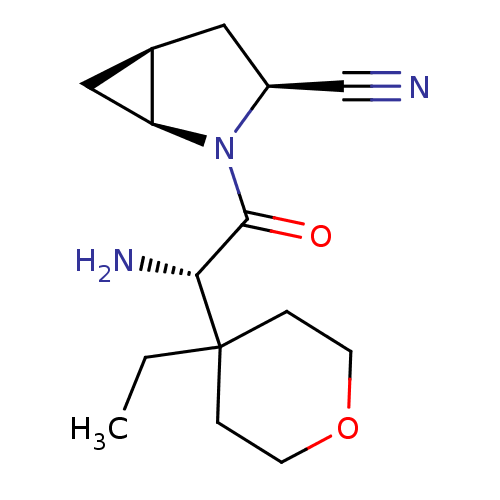 ((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethyloxan-4-yl)ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11527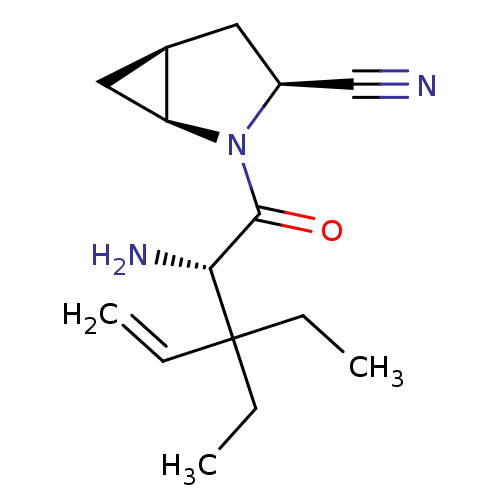 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpent-4-enoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11534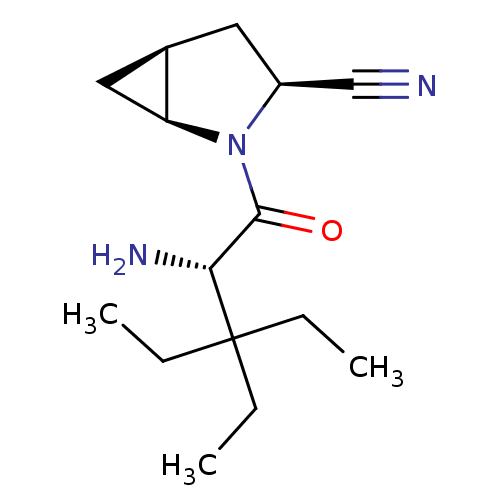 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpentanoyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11537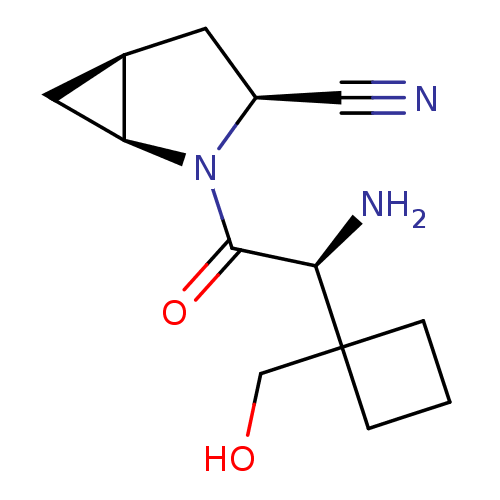 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11525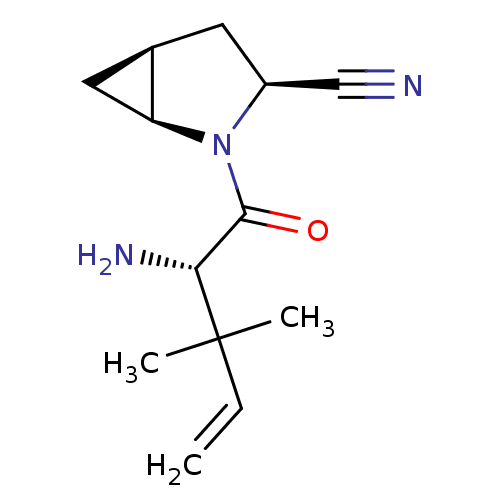 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpent-4-enoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11540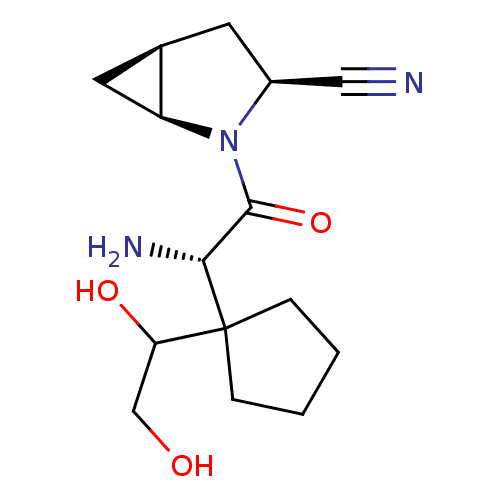 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(1,2-dihydroxyethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 143 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392983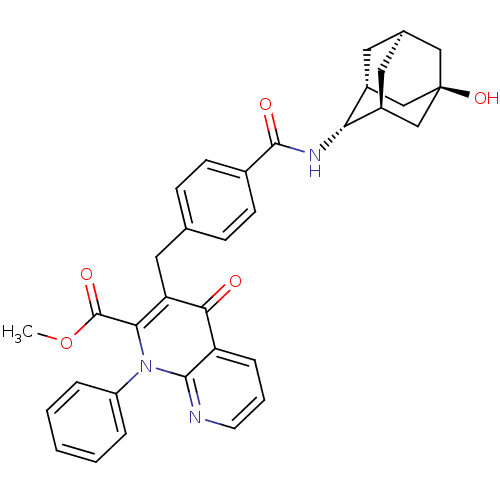 (CHEMBL2152383) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392984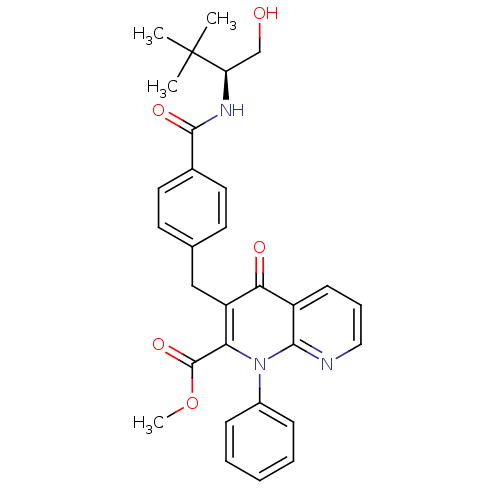 (CHEMBL2152384) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392990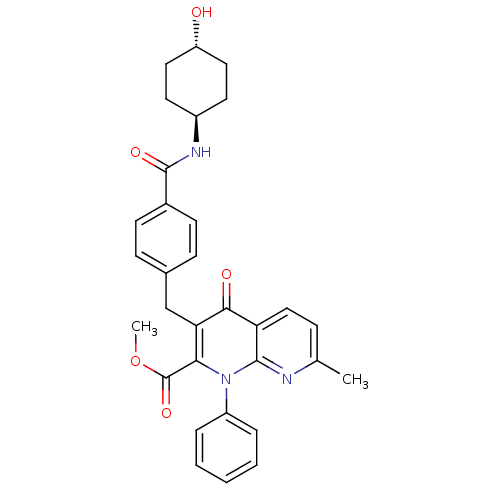 (CHEMBL2152390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305572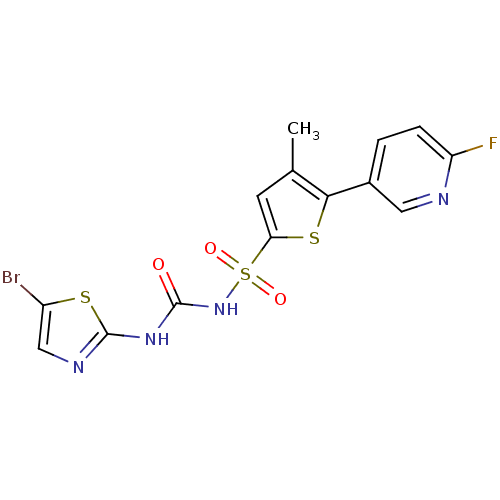 (CHEMBL590111 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305562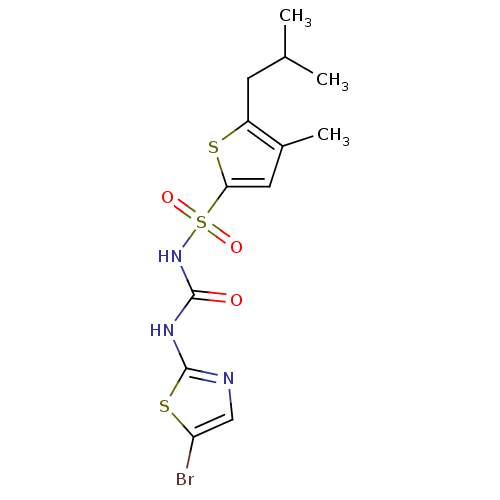 (CHEMBL590359 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305562 (CHEMBL590359 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305595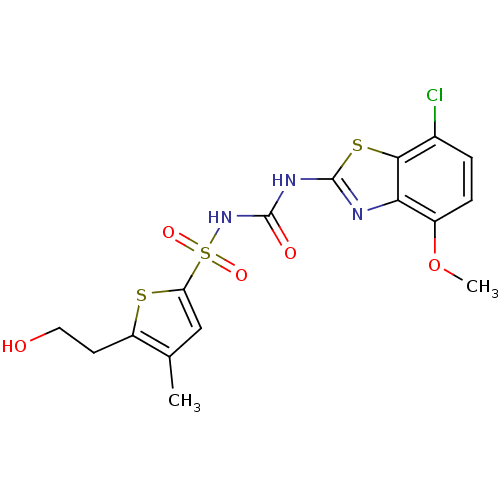 (CHEMBL591317 | N-(7-chloro-4-methoxybenzo[d]thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305567 (CHEMBL589863 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305569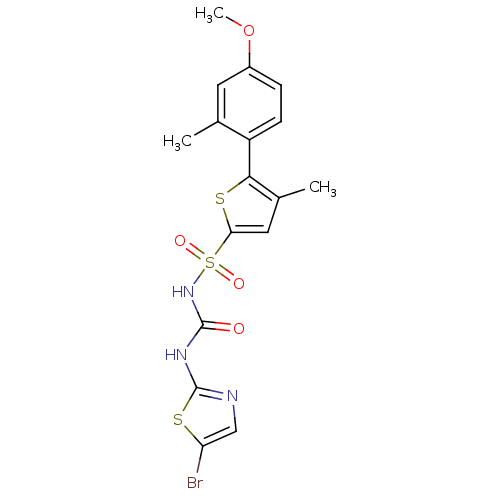 (CHEMBL590597 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392982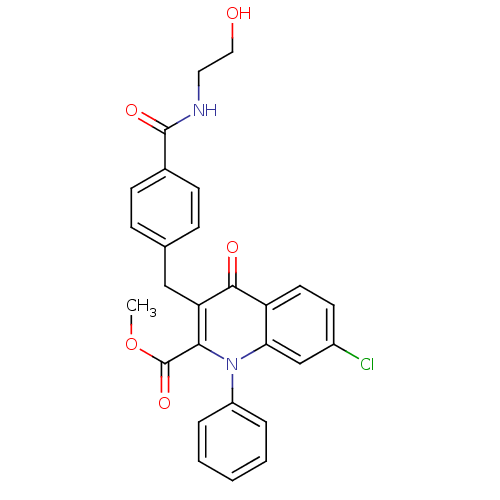 (CHEMBL2152382) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392986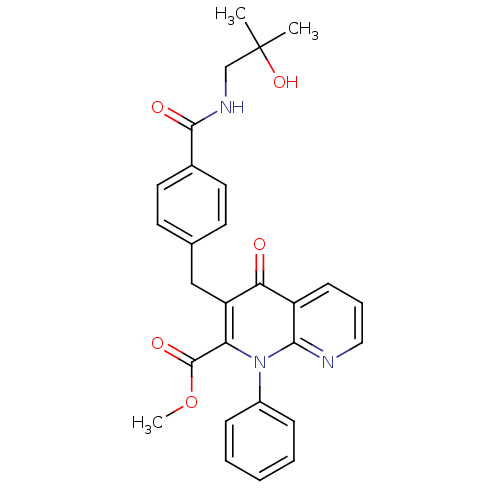 (CHEMBL2152386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392989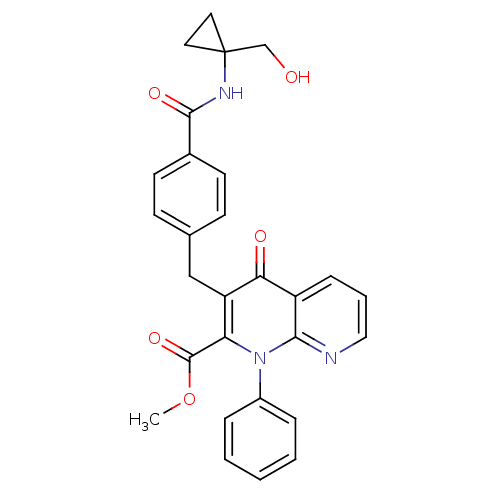 (CHEMBL2152389) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392988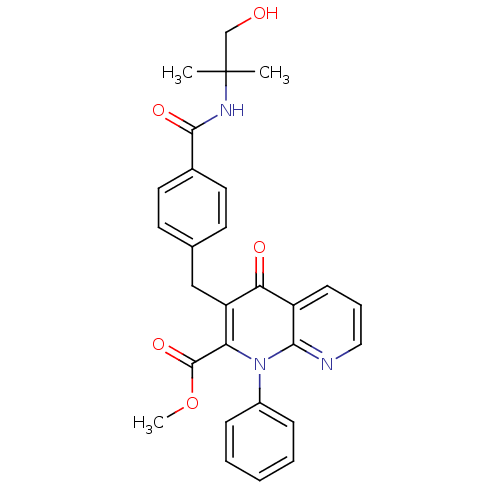 (CHEMBL2152388) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392985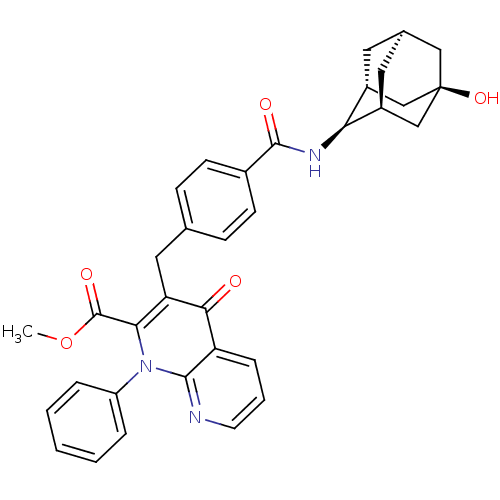 (CHEMBL2152385) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392992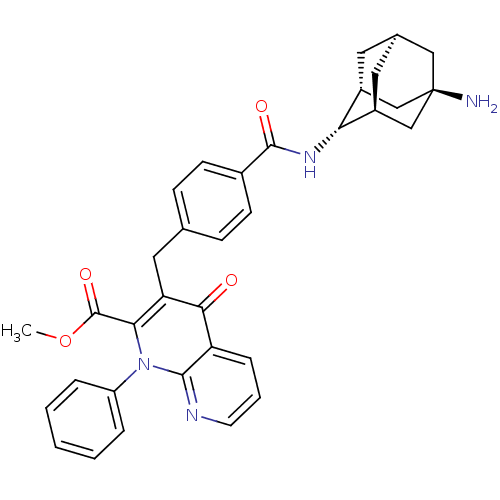 (CHEMBL2152392) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305573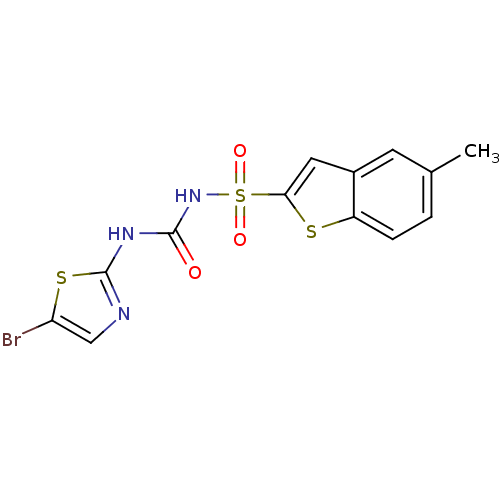 (CHEMBL590839 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305571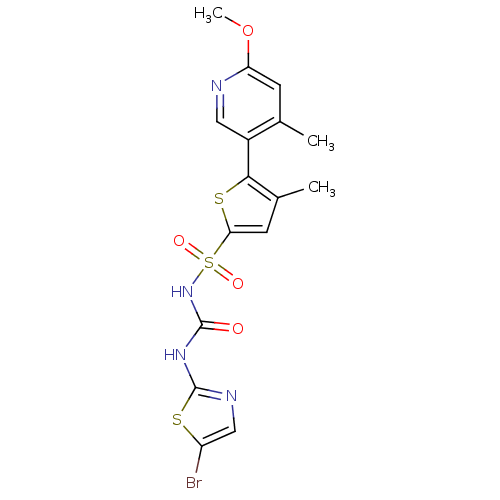 (CHEMBL601691 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50392987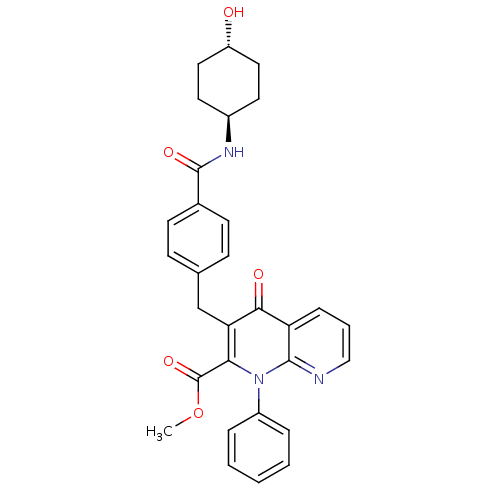 (CHEMBL2152387) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of JNK1 after 30 mins by TR-FRET assay | ACS Med Chem Lett 3: 764-768 (2012) Article DOI: 10.1021/ml300175c BindingDB Entry DOI: 10.7270/Q22808P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305572 (CHEMBL590111 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305565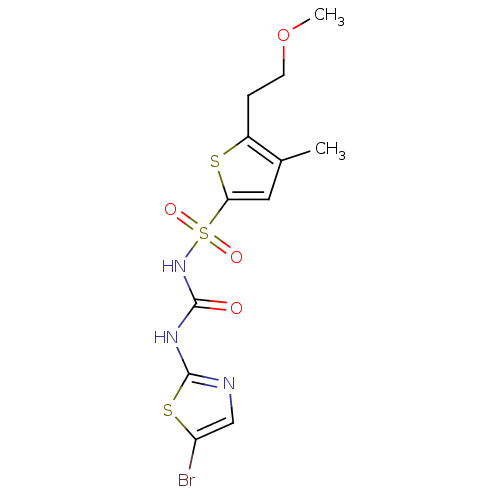 (CHEMBL590361 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305571 (CHEMBL601691 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305561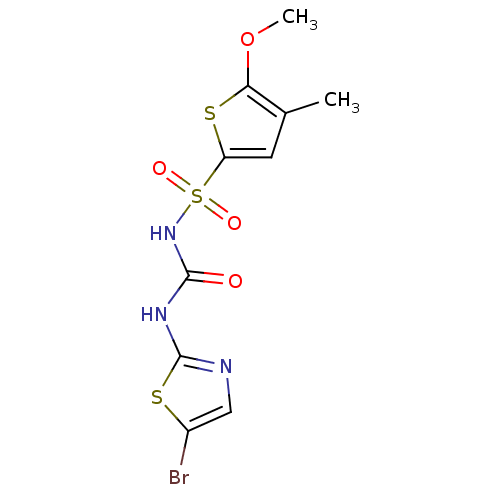 (CHEMBL593282 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305575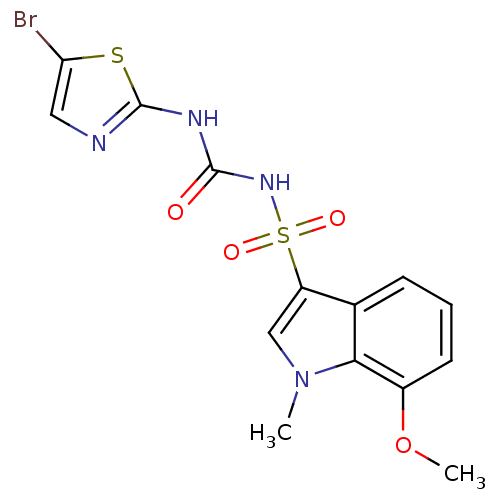 (CHEMBL590113 | N-(5-bromothiazol-2-ylcarbamoyl)-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305570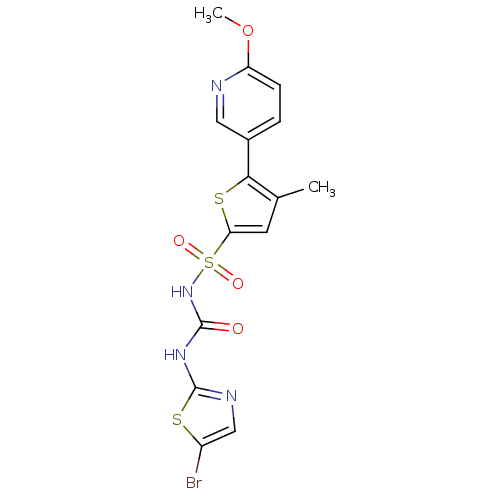 (CHEMBL589865 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305569 (CHEMBL590597 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305580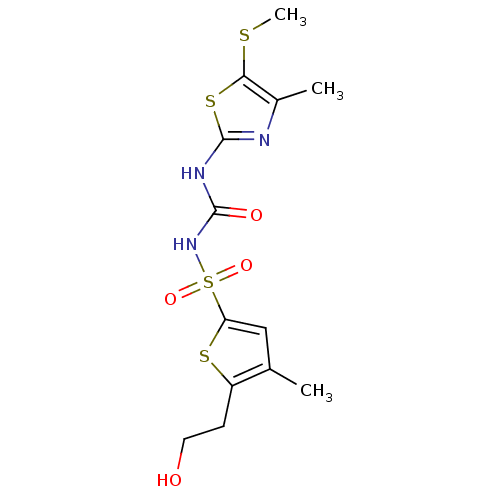 (5-(2-hydroxyethyl)-4-methyl-N-(4-methyl-5-(methylt...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305560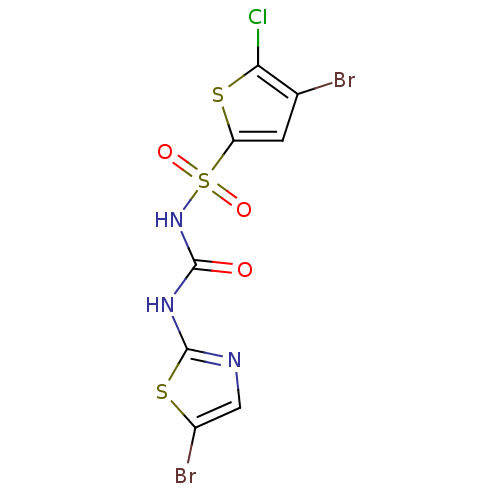 (4-bromo-N-(5-bromothiazol-2-ylcarbamoyl)-5-chlorot...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver using fructose-2,6phosphate as a substrate | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50344569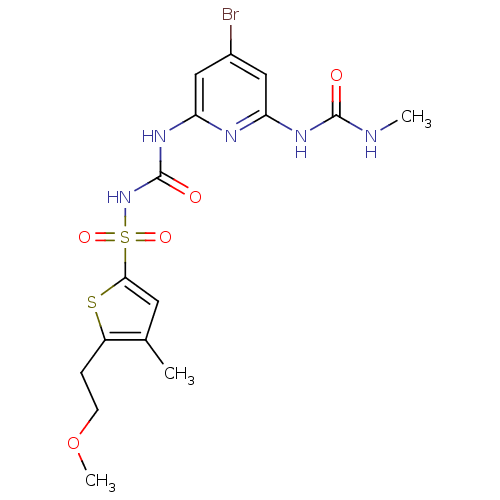 (CHEMBL1778447 | N-(4-bromo-6-(3-methylureido)pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase | Bioorg Med Chem Lett 21: 3237-42 (2011) Article DOI: 10.1016/j.bmcl.2011.04.044 BindingDB Entry DOI: 10.7270/Q2C24WS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305581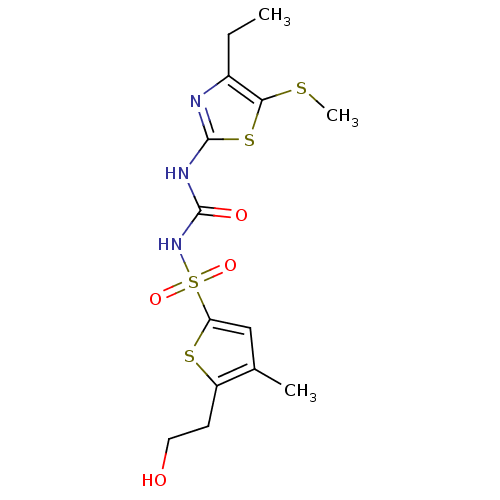 (CHEMBL590363 | N-(4-ethyl-5-(methylthio)thiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305567 (CHEMBL589863 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50305565 (CHEMBL590361 | N-(5-bromothiazol-2-ylcarbamoyl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of FBPase in human liver | Bioorg Med Chem Lett 20: 594-9 (2010) Article DOI: 10.1016/j.bmcl.2009.11.093 BindingDB Entry DOI: 10.7270/Q27H1JPX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 461 total ) | Next | Last >> |