

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3624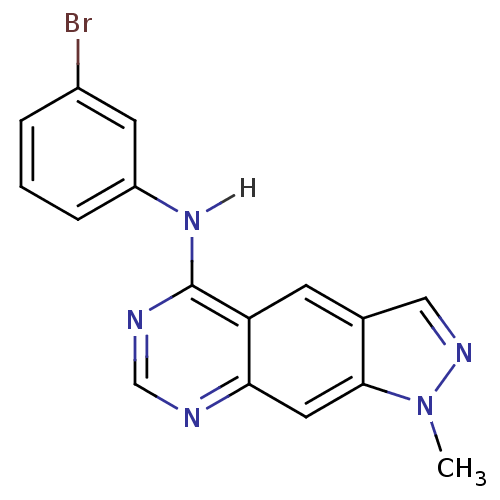 (5-[(3-Bromophenyl)amino]-1-methylpyrazolo[4,3-g]-q...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583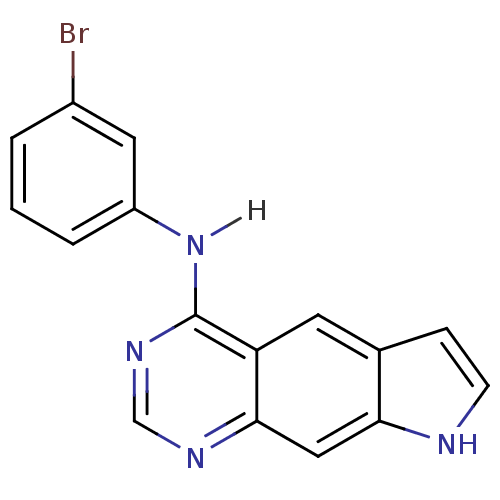 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3582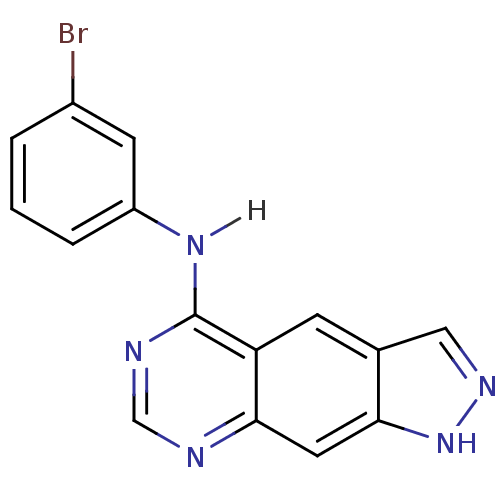 (5-[(3-Bromophenyl)amino]-1H-pyrazolo[4,3-g]quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6266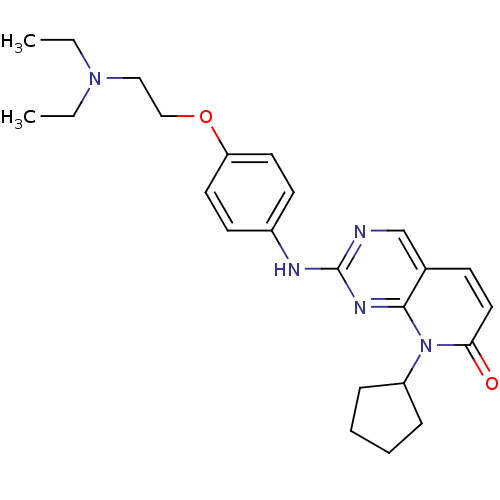 (8-Cyclopentyl-2-[4-(2-diethylaminoethoxy)phenylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3642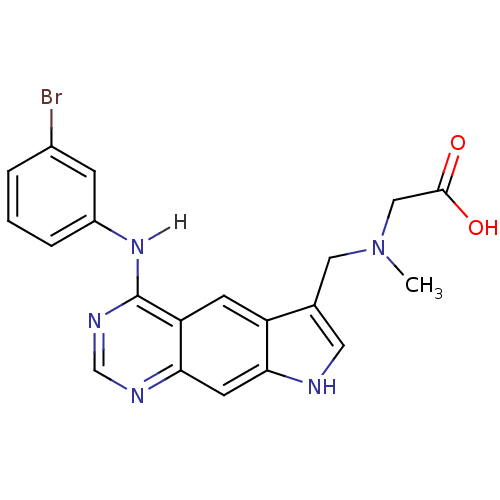 (2-[({4-[(3-bromophenyl)amino]-8H-pyrrolo[3,2-g]qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3630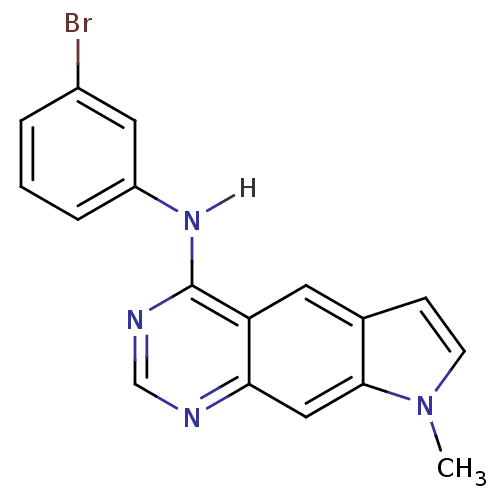 (5-[(3-Bromophenyl)amino]-1-methylpyrrolo[3,2-g]-qu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3631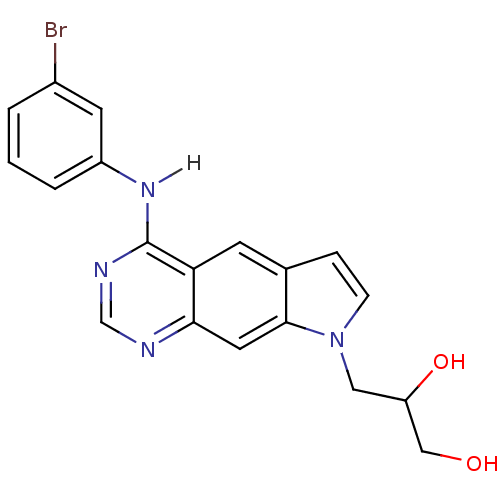 (3-{4-[(3-bromophenyl)amino]-8H-pyrrolo[3,2-g]quina...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3638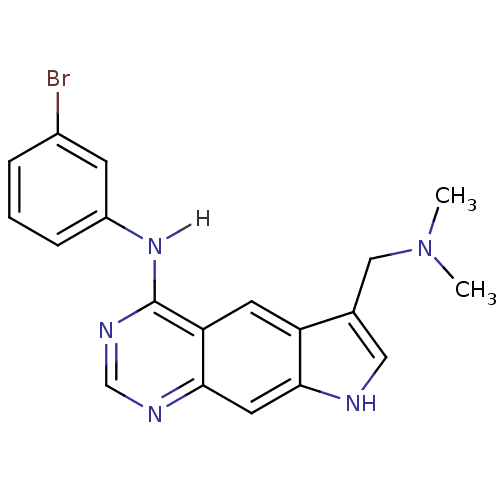 (5-[(3-Bromophenyl)amino]-3-[(N,N-dimethylamino)-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3641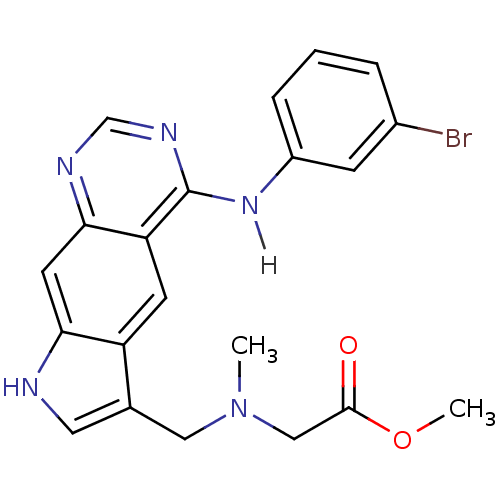 (5-[(3-Bromophenyl)amino]-3-[[N-(carboxymethyl)-N-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3637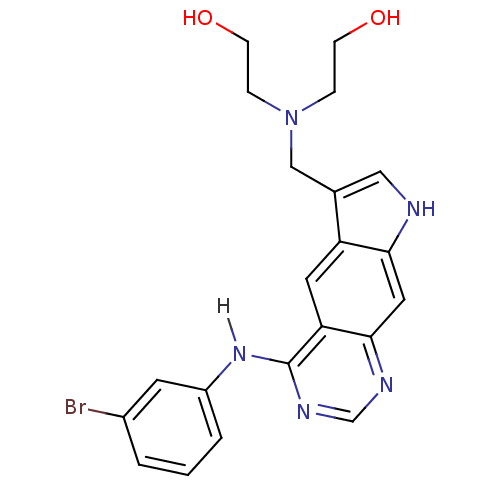 (2-[({4-[(3-bromophenyl)amino]-8H-pyrrolo[3,2-g]qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3627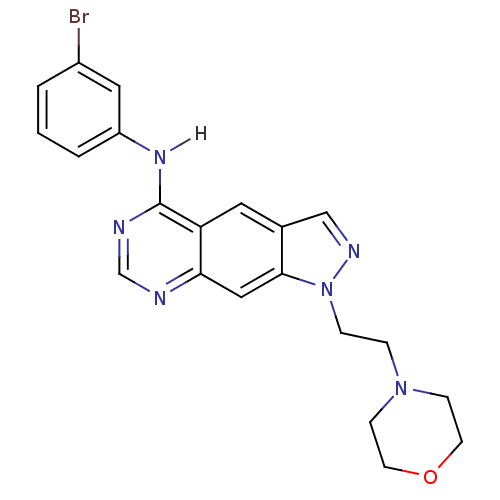 (5-[(3-Bromophenyl)amino]-1-[2-(4-morpholino)ethyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3634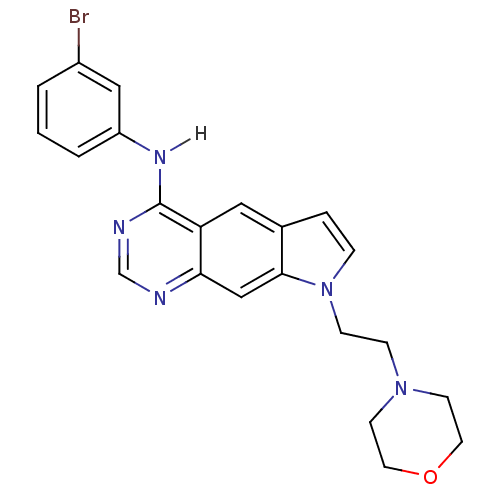 (5-[(3-Bromophenyl)amino]-1-[2-(4-morpholino)ethyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6268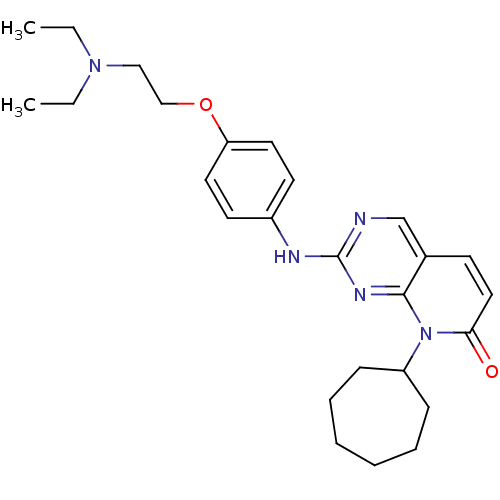 (8-Cycloheptyl-2-[4-(2-diethylaminoethoxy)phenylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6278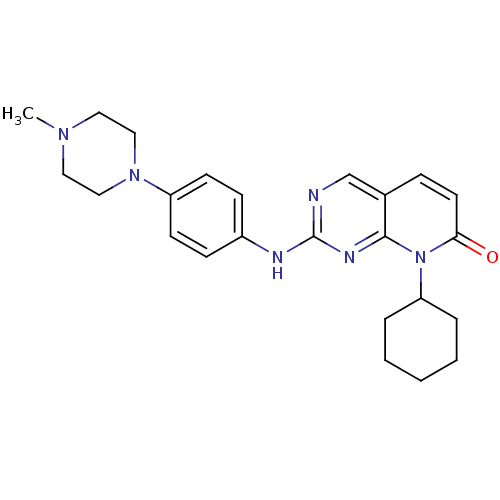 (8-Cyclohexyl-2-[4-(4-methylpiperazin-1-yl)phenylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3639 (5-[(3-Bromophenyl)amino]-3-[(4-morpholino)methyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3636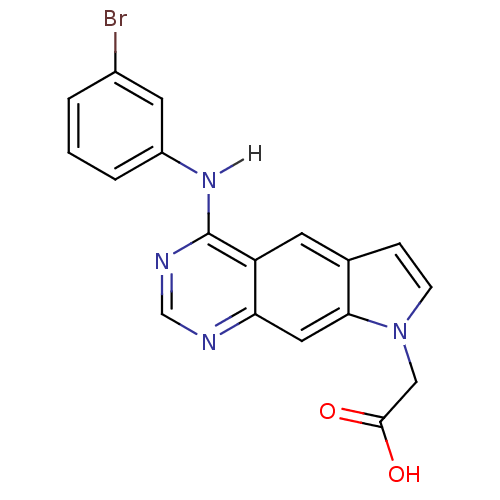 (2-{4-[(3-bromophenyl)amino]-8H-pyrrolo[3,2-g]quina...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6277 (8-Bicyclo[2.2.1]hept-2-yl-2-[4-(4-methylpiperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3640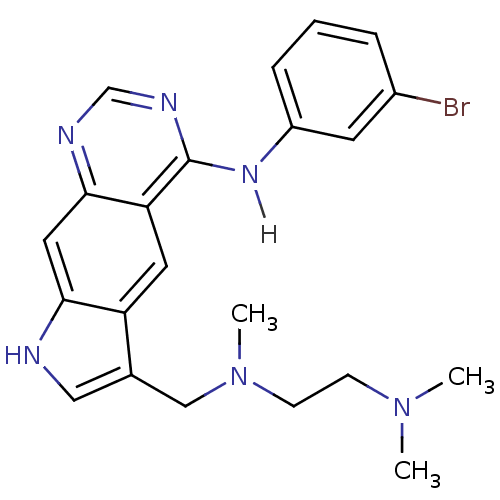 (5-[(3-Bromophenyl)amino]-3-[(N,N,N-trimethylethyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6280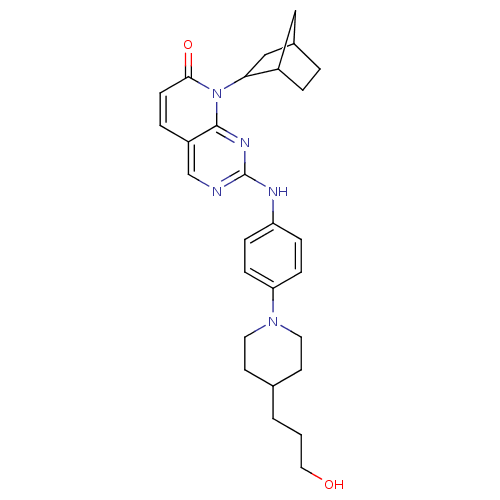 (8-Bicyclo[2.2.1]hept-2-yl-2-{4-[4-(3-hydroxypropyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3635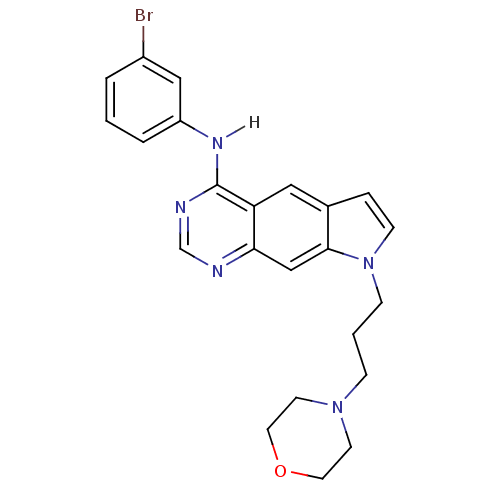 (5-[(3-Bromophenyl)amino]-1-[3-(4-morpholino)propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3095 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 62 | 6-(2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3096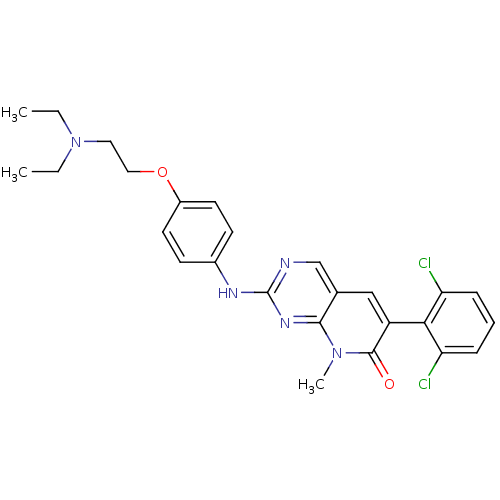 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6276 (8-Cyclopentyl-2-[4-(4-methylpiperazin-1-yl)phenyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3101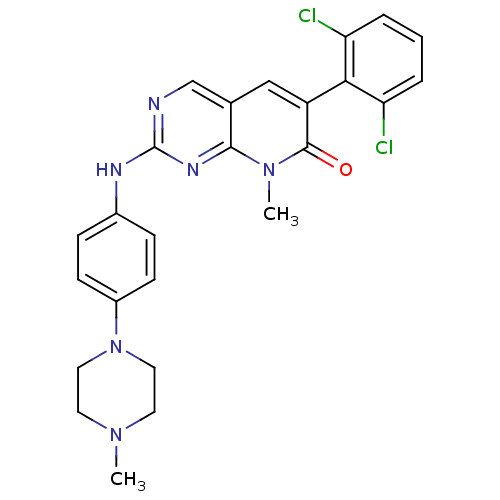 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 68 | 6-(2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3104 (2-(4-{[6-(2,6-dichlorophenyl)-8-methyl-7-oxo-7H,8H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6267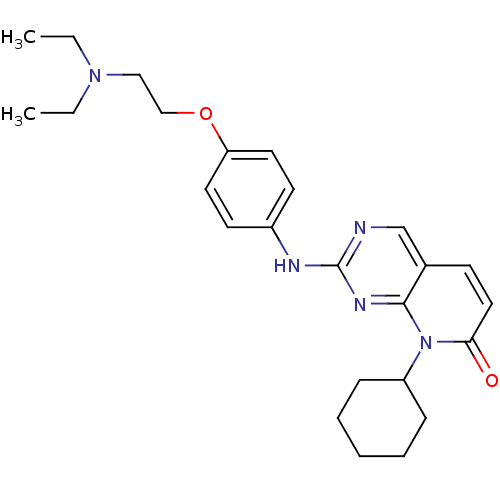 (8-Cyclohexyl-2-[4-(2-diethylaminoethoxy)phenylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3625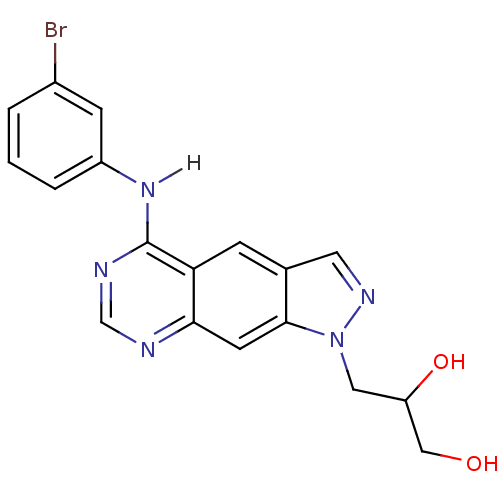 (3-{5-[(3-bromophenyl)amino]-1H-pyrazolo[4,3-g]quin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6275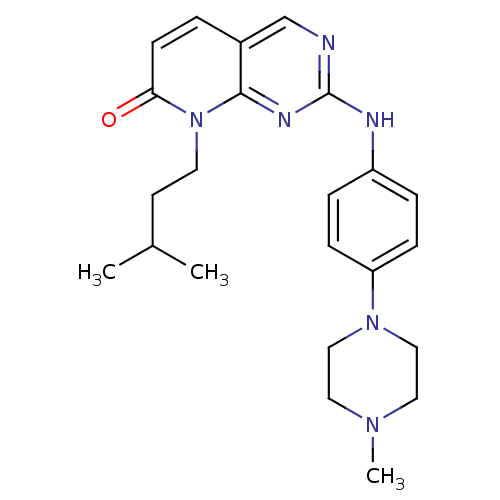 (8-(3-methylbutyl)-2-{[4-(4-methylpiperazin-1-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6278 (8-Cyclohexyl-2-[4-(4-methylpiperazin-1-yl)phenylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6278 (8-Cyclohexyl-2-[4-(4-methylpiperazin-1-yl)phenylam...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3633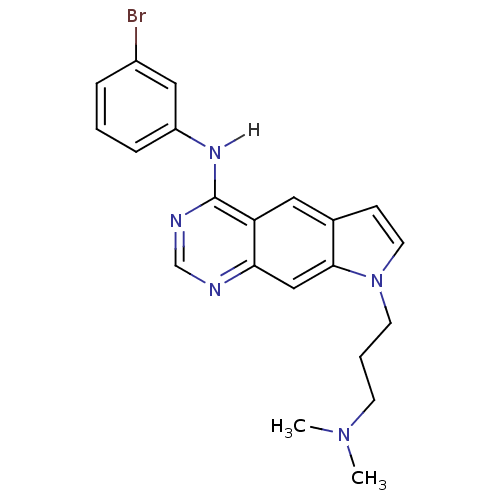 (5-[(3-Bromophenyl)amino]-1-[3-(N,N-dimethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3451 (3-tert-butyl-1-[6-(2,6-dichlorophenyl)-2-{[4-(diet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50064349 (1-tert-Butyl-3-{6-(2,6-dichloro-phenyl)-2-[4-(2-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3071 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 38 | 2-ani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3487 (3-[6-(2,6-dichlorophenyl)-2-{[3-(4-methylpiperazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3473 (1-benzyl-3-[6-(2,6-dichlorophenyl)-2-{[3-(4-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3474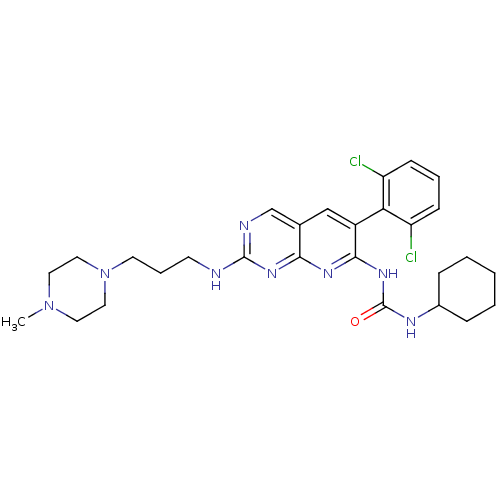 (1-cyclohexyl-3-[6-(2,6-dichlorophenyl)-2-{[3-(4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6274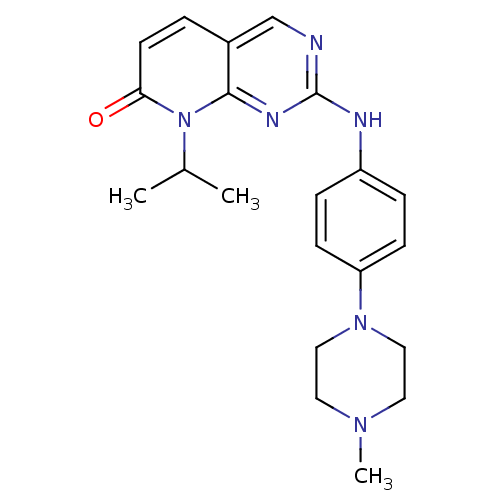 (2-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-8-(pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6279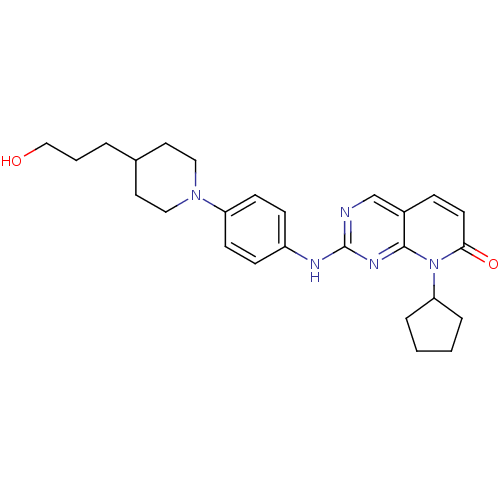 (8-Cyclopentyl-2-{4-[4-(3-hydroxypropyl)piperidin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6250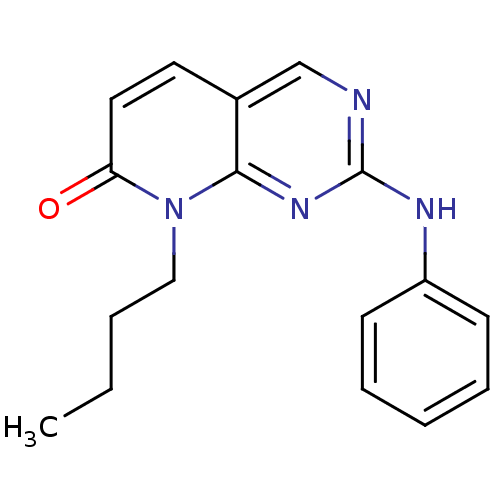 (8-Butyl-2-phenylamino-8H-pyrido[2,3-d]pyrimidin-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6256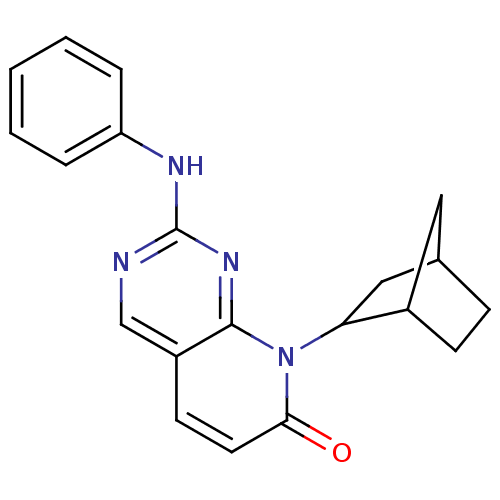 (8-Bicyclo[2.2.1]hept-2-yl-2-phenylamino-8H-pyrido[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3626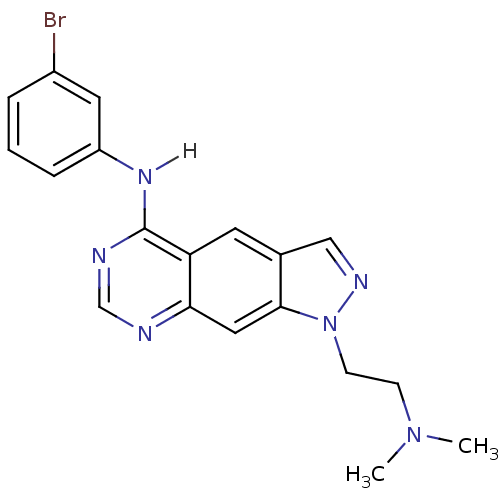 (5-[(3-Bromophenyl)amino]-1-[2-(N,N-dimethylamino)e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3632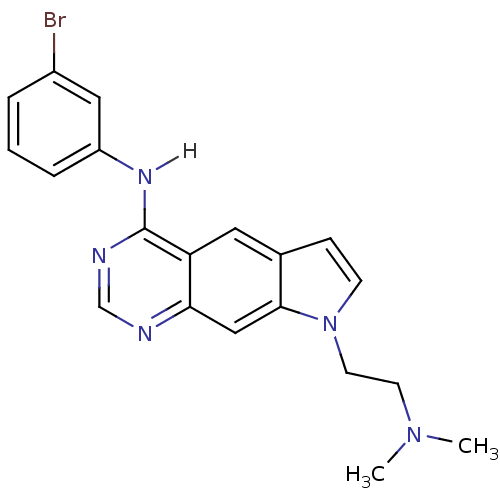 (5-[(3-Bromophenyl)amino]-1-[2-(N,N-dimethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6265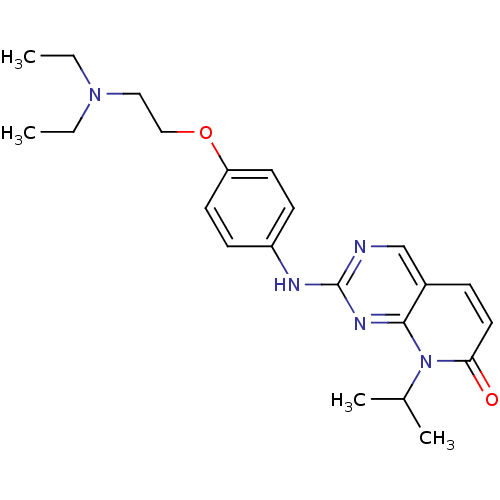 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-(pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6254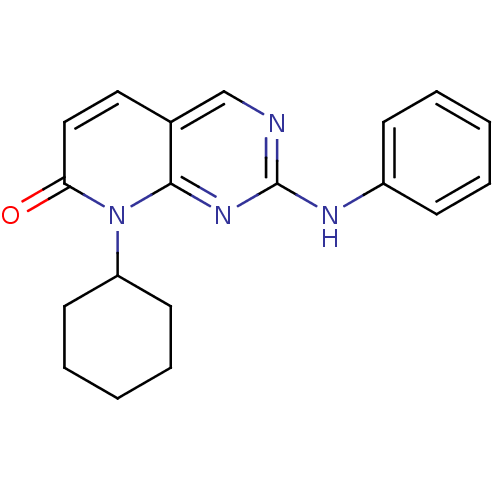 (8-Cyclohexyl-2-phenylamino-8H-pyrido[2,3-d]pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6248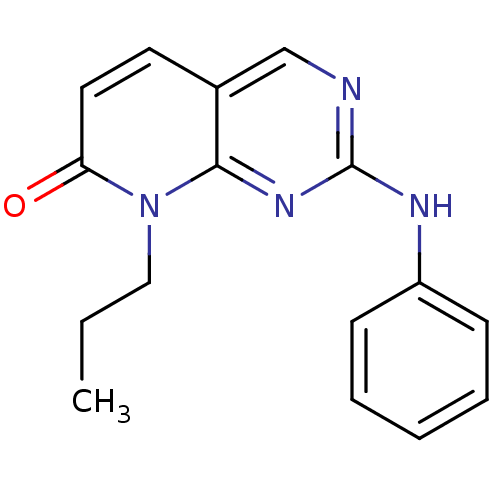 (2-(phenylamino)-8-propyl-7H,8H-pyrido[2,3-d]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6278 (8-Cyclohexyl-2-[4-(4-methylpiperazin-1-yl)phenylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3628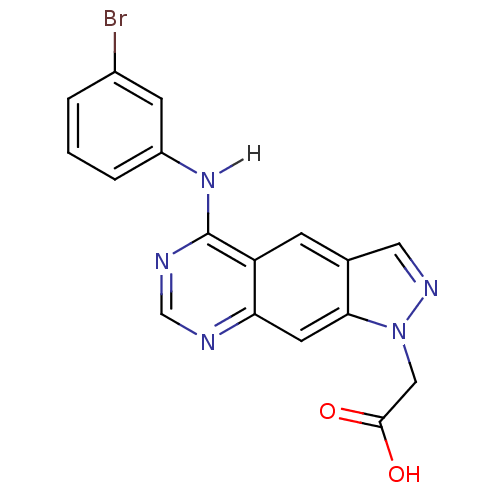 (2-{5-[(3-bromophenyl)amino]-1H-pyrazolo[4,3-g]quin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6274 (2-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-8-(pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6250 (8-Butyl-2-phenylamino-8H-pyrido[2,3-d]pyrimidin-7-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | J Med Chem 43: 4606-16 (2000) Article DOI: 10.1021/jm000271k BindingDB Entry DOI: 10.7270/Q25B00N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 291 total ) | Next | Last >> |