

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070947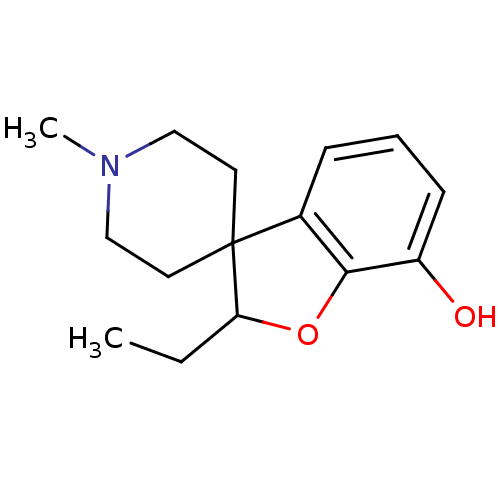 (2-ethyl-1'-methylspiro[2,3-dihydrobenzo[b]furan-3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070948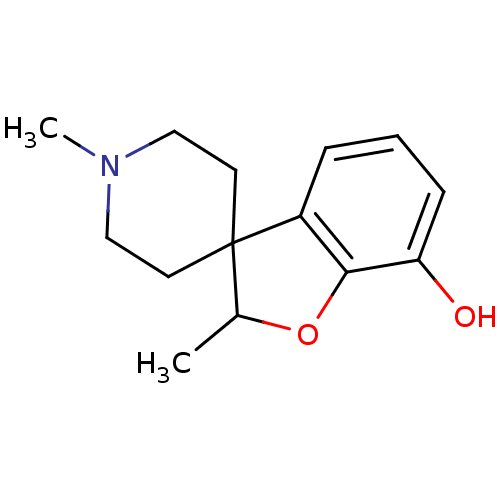 (1',2-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50026752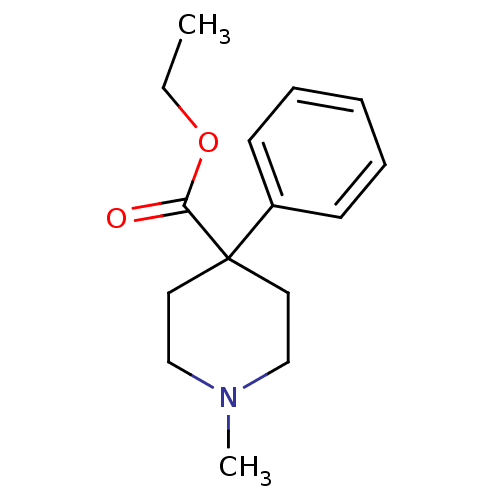 (1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070946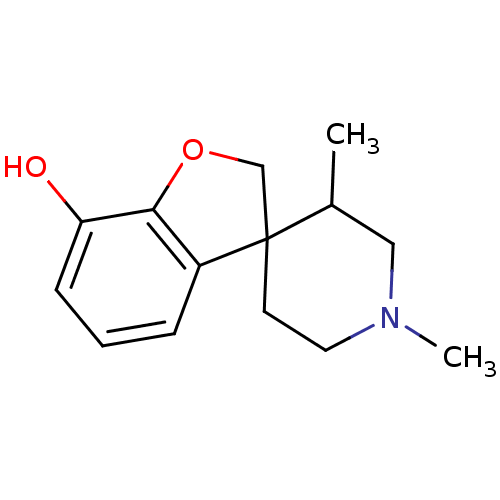 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070946 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286878 (Butyl-carbamic acid 2'-hydroxy-[1,1']binaphthaleny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286878 (Butyl-carbamic acid 2'-hydroxy-[1,1']binaphthaleny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant of the compound (KI) for the Pancreatic cholesterol esterase-catalyzed hydrolysis of 4-nitrophenyl butyrate | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286878 (Butyl-carbamic acid 2'-hydroxy-[1,1']binaphthaleny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50070947 (2-ethyl-1'-methylspiro[2,3-dihydrobenzo[b]furan-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070946 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070946 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070949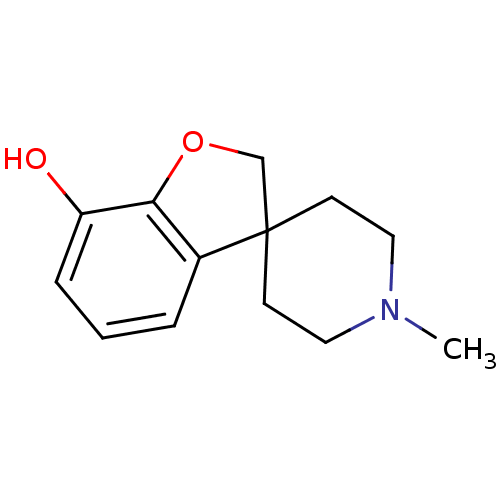 (1'-methylspiro[2,3-dihydrobenzo[b]furan-3,4'-(hexa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50070948 (1',2-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286880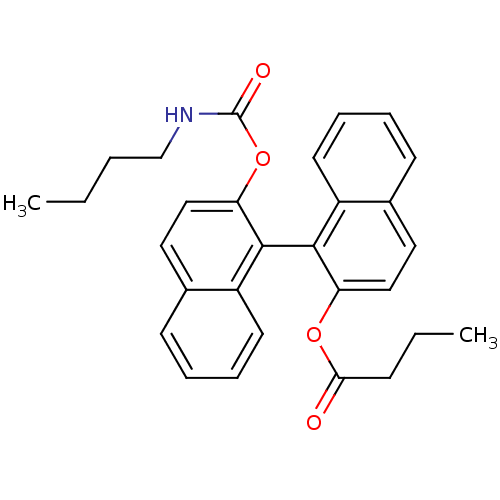 (Butyric acid 2'-butylcarbamoyloxy-[1,1']binaphthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286880 (Butyric acid 2'-butylcarbamoyloxy-[1,1']binaphthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant of the compound (KI) for the Pancreatic cholesterol esterase-catalyzed hydrolysis of 4-nitrophenyl butyrate | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286880 (Butyric acid 2'-butylcarbamoyloxy-[1,1']binaphthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286879 (Butyl-carbamic acid 2'-butylcarbamoyloxy-[1,1']bin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286879 (Butyl-carbamic acid 2'-butylcarbamoyloxy-[1,1']bin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286879 (Butyl-carbamic acid 2'-butylcarbamoyloxy-[1,1']bin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070947 (2-ethyl-1'-methylspiro[2,3-dihydrobenzo[b]furan-3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026752 (1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070948 (1',2-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070949 (1'-methylspiro[2,3-dihydrobenzo[b]furan-3,4'-(hexa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50070949 (1'-methylspiro[2,3-dihydrobenzo[b]furan-3,4'-(hexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428362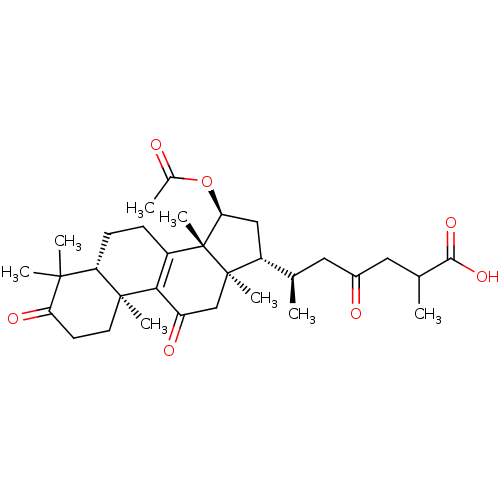 (15-O-Acetylganolucidate A | CHEMBL2331733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428365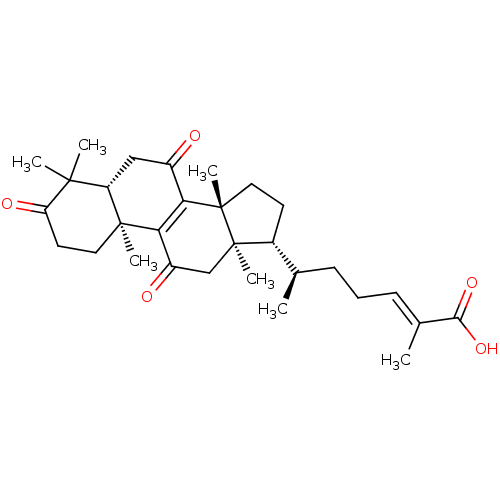 (CHEMBL2331728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428366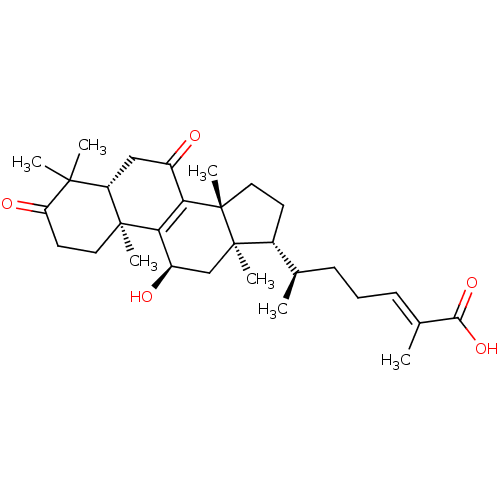 (CHEMBL2203598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428364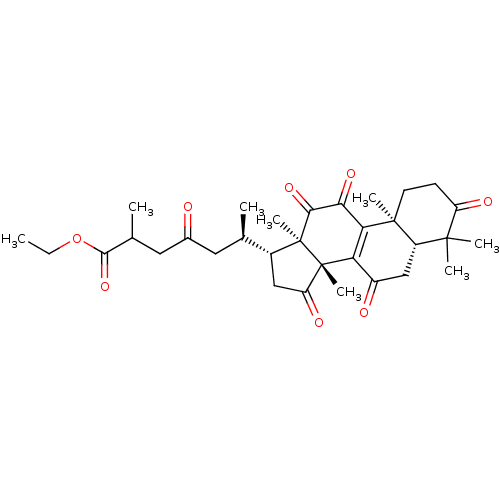 (CHEMBL2331730) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428363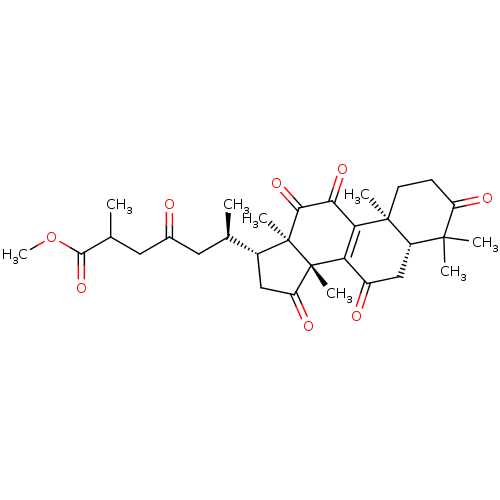 (CHEMBL2331729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428361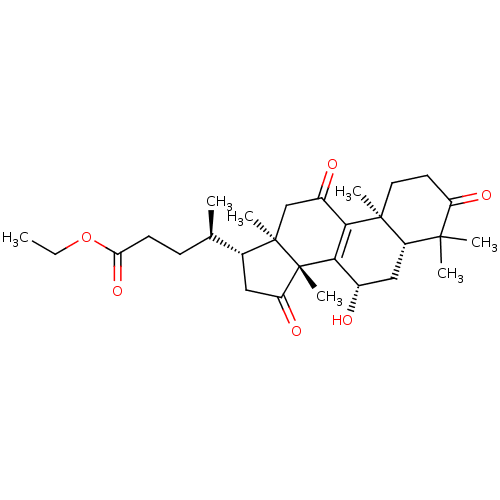 (CHEMBL2331731 | Ethyl Lucidenate A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428360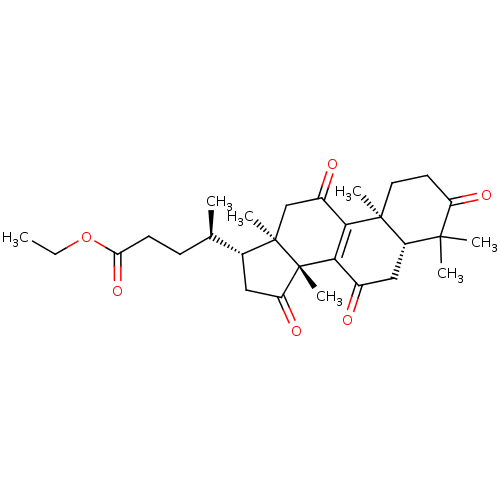 (CHEMBL2331732 | Ethyl Lucidenate F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428359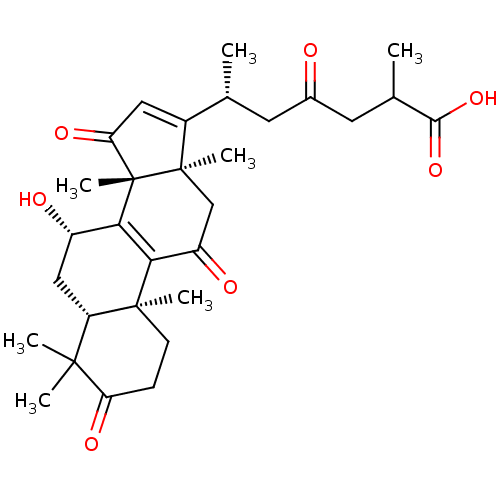 (CHEMBL2331734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428358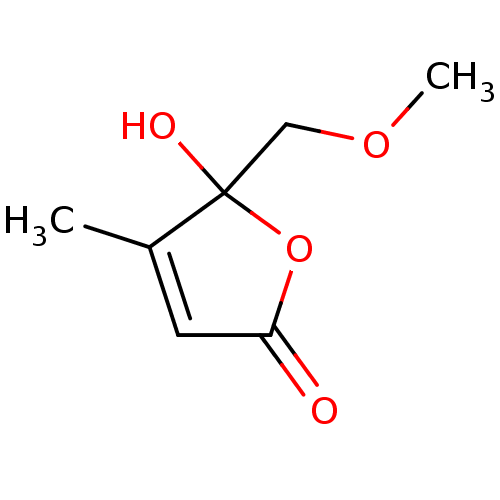 (CHEMBL2331735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50428357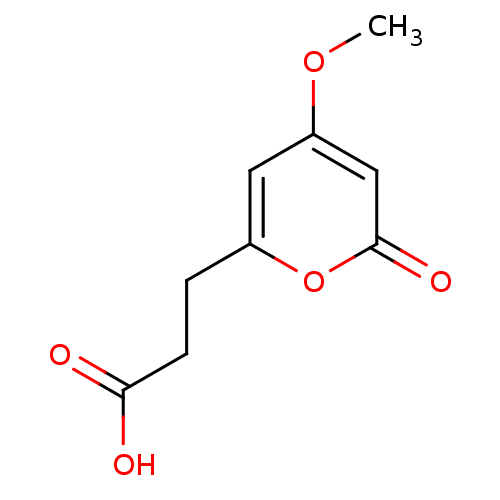 (CHEMBL2331727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reaction analysis | J Nat Prod 76: 489-94 (2013) Article DOI: 10.1021/np300443p BindingDB Entry DOI: 10.7270/Q2PG1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286879 (Butyl-carbamic acid 2'-butylcarbamoyloxy-[1,1']bin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant of the compound (KI) for the Pancreatic cholesterol esterase-catalyzed hydrolysis of 4-nitrophenyl butyrate | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286880 (Butyric acid 2'-butylcarbamoyloxy-[1,1']binaphthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286880 (Butyric acid 2'-butylcarbamoyloxy-[1,1']binaphthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286880 (Butyric acid 2'-butylcarbamoyloxy-[1,1']binaphthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286878 (Butyl-carbamic acid 2'-hydroxy-[1,1']binaphthaleny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 5.00E+5 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286878 (Butyl-carbamic acid 2'-hydroxy-[1,1']binaphthaleny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 5.00E+5 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286879 (Butyl-carbamic acid 2'-butylcarbamoyloxy-[1,1']bin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant of the compound (KI) for the Pancreatic cholesterol esterase-catalyzed hydrolysis of 4-nitrophenyl butyrate | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286879 (Butyl-carbamic acid 2'-butylcarbamoyloxy-[1,1']bin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dissociation constant of the compound (KI) for the Pancreatic cholesterol esterase-catalyzed hydrolysis of 4-nitrophenyl butyrate | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt-activated lipase (Bos taurus) | BDBM50286878 (Butyl-carbamic acid 2'-hydroxy-[1,1']binaphthaleny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 5.00E+5 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 (KDR) | Bioorg Med Chem Lett 6: 43-46 (1996) Article DOI: 10.1016/0960-894X(95)00549-9 BindingDB Entry DOI: 10.7270/Q2RV0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||