

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417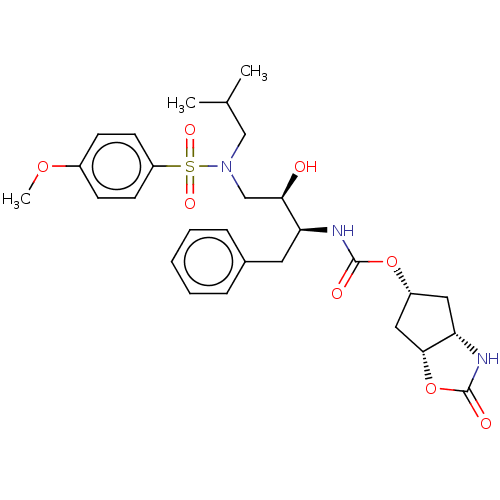 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498523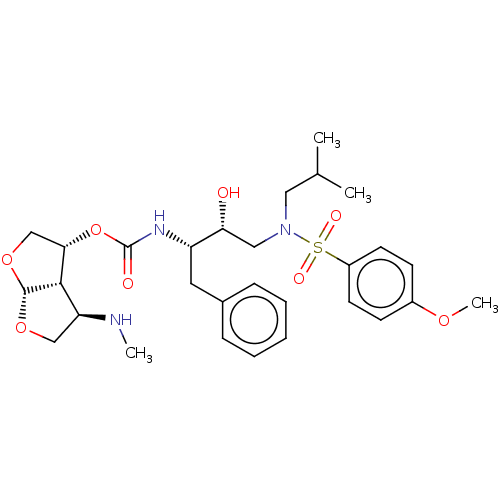 (CHEMBL3605643) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484842 (CHEMBL1958482 | GRL-0249A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110797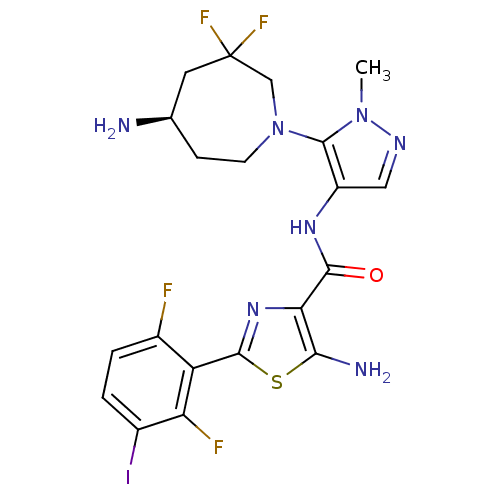 (US8614206, 346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528152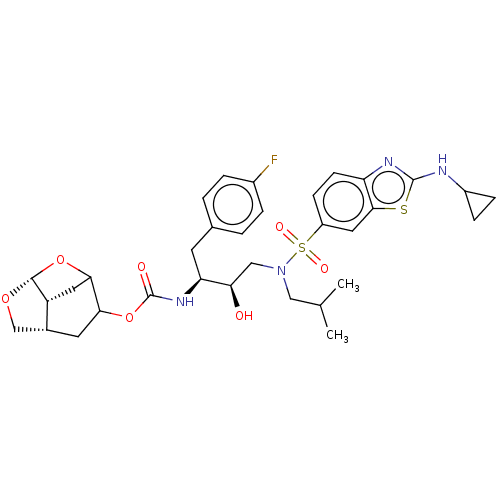 (CHEMBL4532946) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110798 (US8614206, 347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528147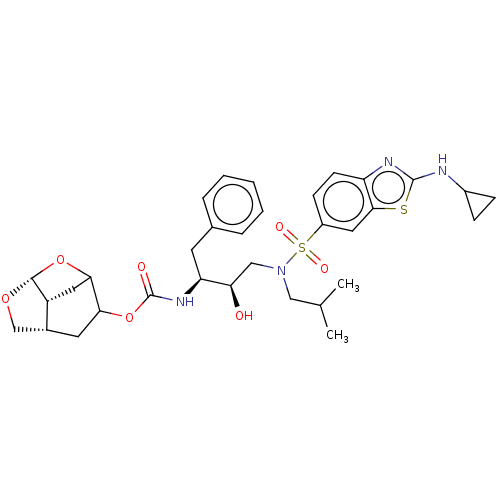 (CHEMBL4514504) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110726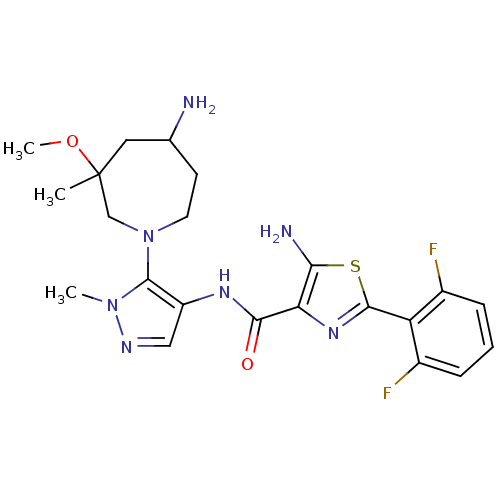 (US8614206, 252 | US8614206, 254 | US8614206, 352 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457611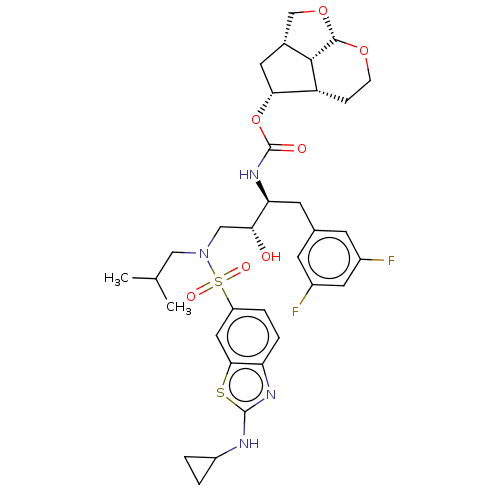 (CHEMBL4214453) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930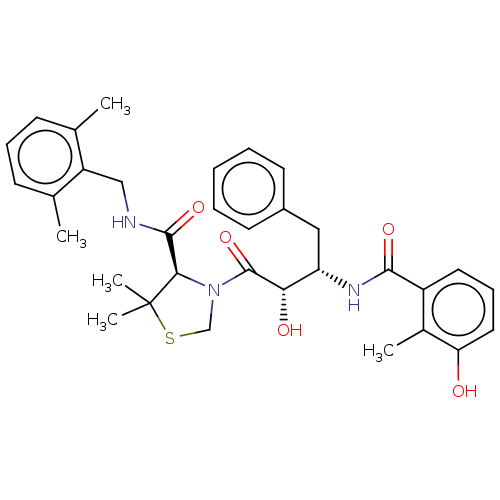 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336 (CHEMBL1651153 | GRL-0476) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110938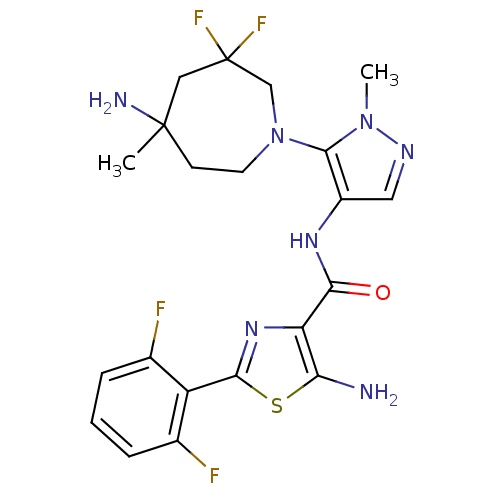 (US8614206, 495) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110712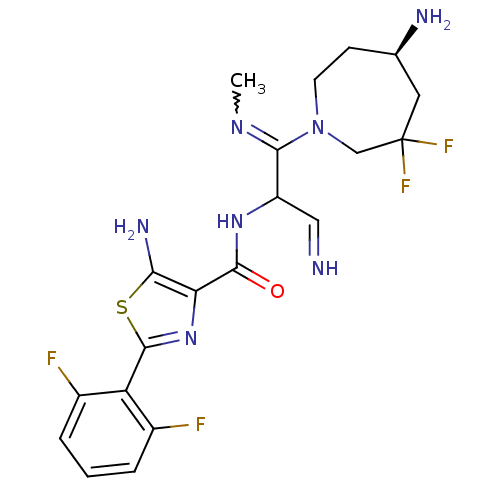 (US8614206, 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925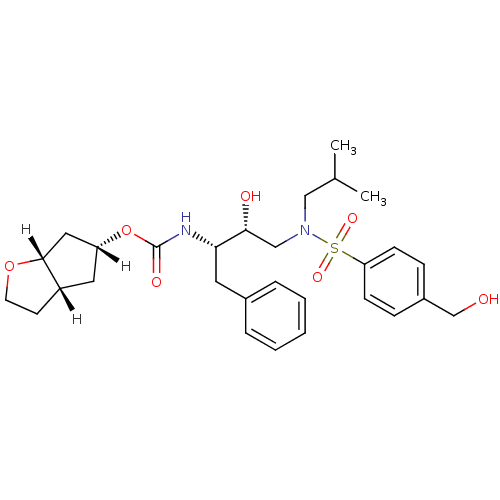 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498524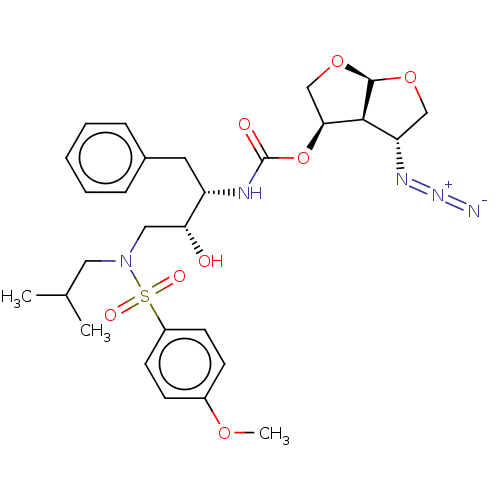 (CHEMBL3605638) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110749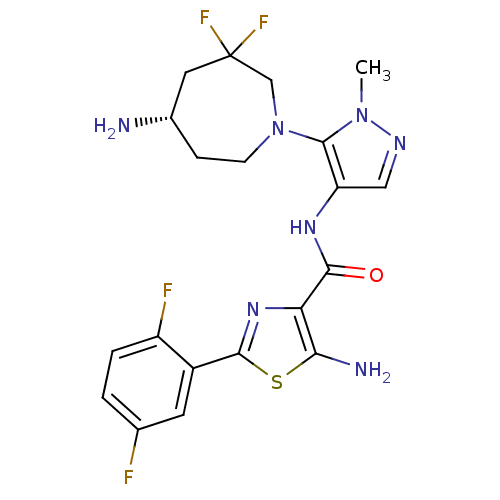 (US8614206, 282) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110701 (US8614206, 121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206677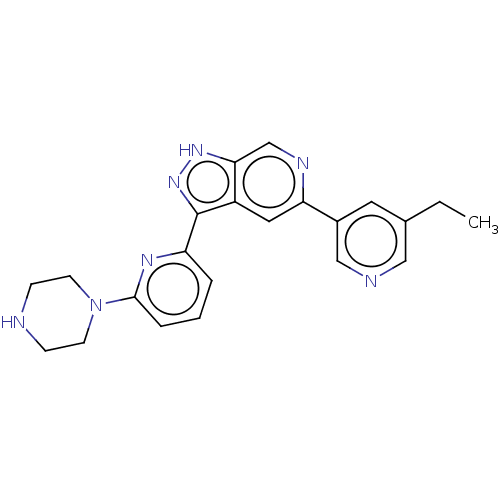 (US9260425, 438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484847 (CHEMBL1958483 | GRL-0289A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110963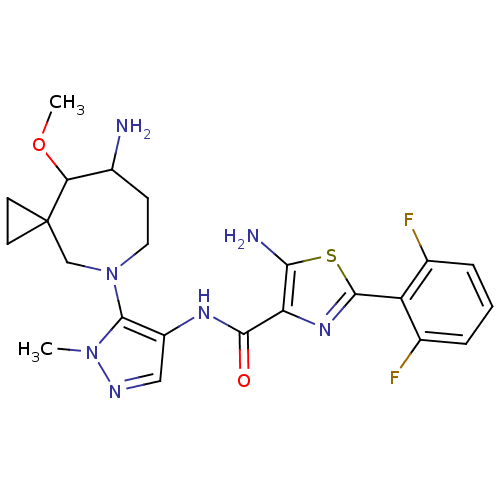 (US8614206, 520 | US8614206, 521) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13925 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457604 (CHEMBL4213229) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338 (CHEMBL1651155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 15: 7576-80 (2007) Article DOI: 10.1016/j.bmc.2007.09.010 BindingDB Entry DOI: 10.7270/Q2VD726P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484190 (CHEMBL1817686) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481584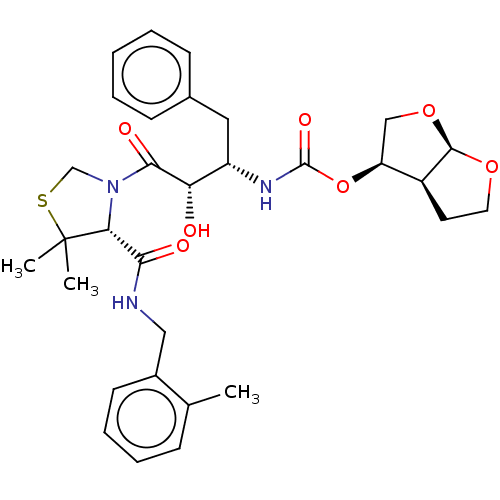 (CHEMBL589988 | GRL-0355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484193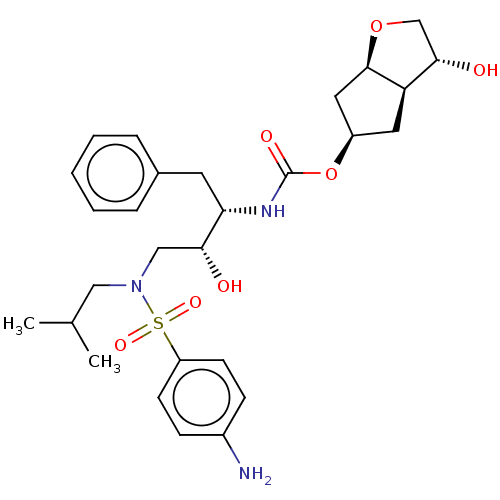 (CHEMBL1819294) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484191 (CHEMBL1819295) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50489369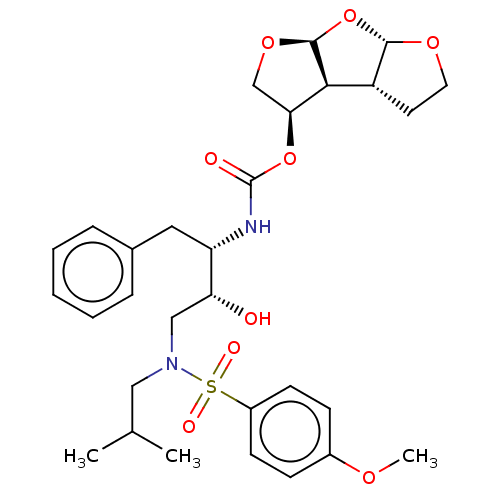 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 56: 6792-802 (2013) Article DOI: 10.1021/jm400768f BindingDB Entry DOI: 10.7270/Q2K07764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528144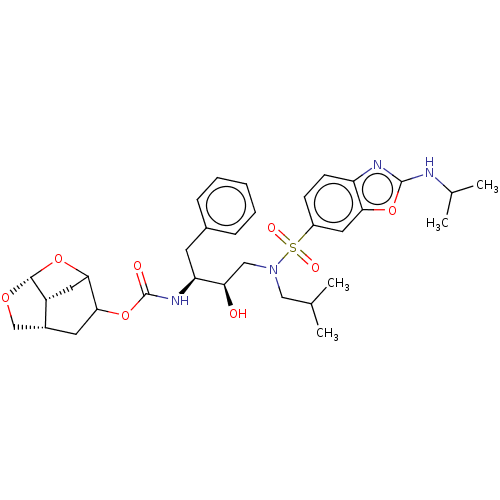 (CHEMBL4435411) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110711 (US8614206, 166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110820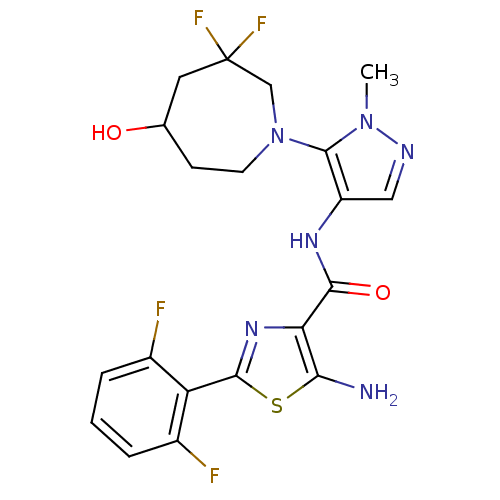 (US8614206, 371 | US8614206, 372) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110906 (US8614206, 459) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498534 (CHEMBL3605635) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528145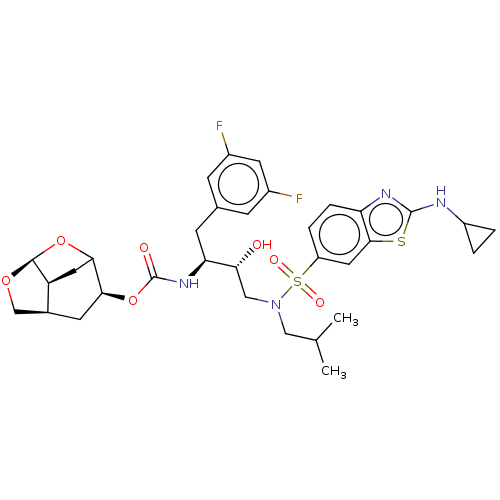 (CHEMBL4586218) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110767 (US8614206, 316 | US8614206, 317 | US8614206, 320 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110857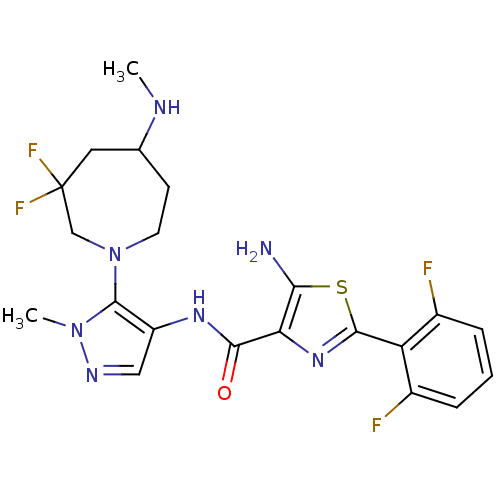 (US8614206, 408 | US8614206, 409) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110796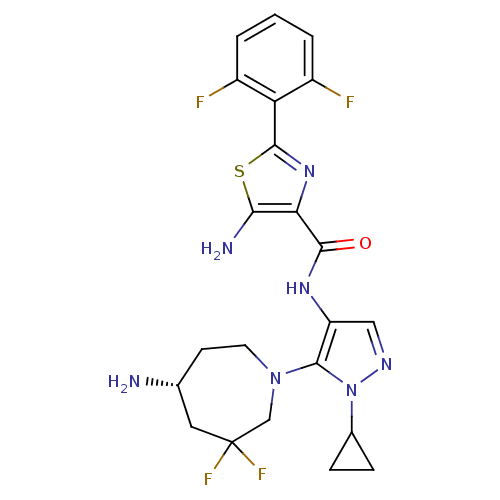 (US8614206, 345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110722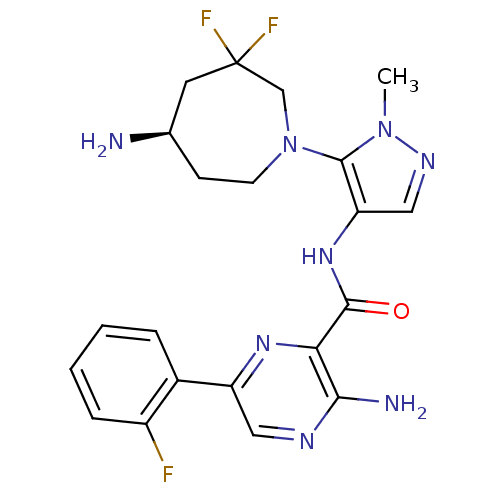 (US8614206, 248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484845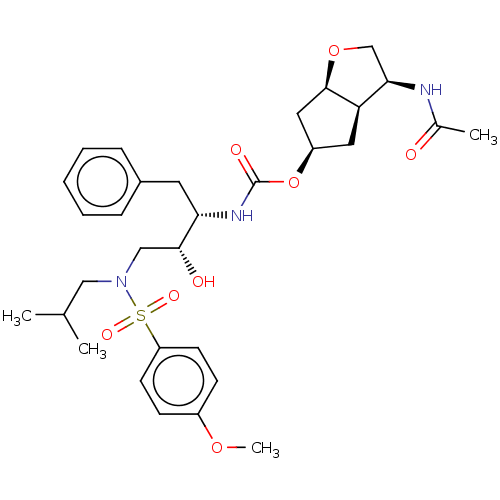 (CHEMBL1958480) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484841 (CHEMBL1958481) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206495 (US9260425, 245 | US9260425, 340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110726 (US8614206, 252 | US8614206, 254 | US8614206, 352 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528150 (CHEMBL4449179) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110887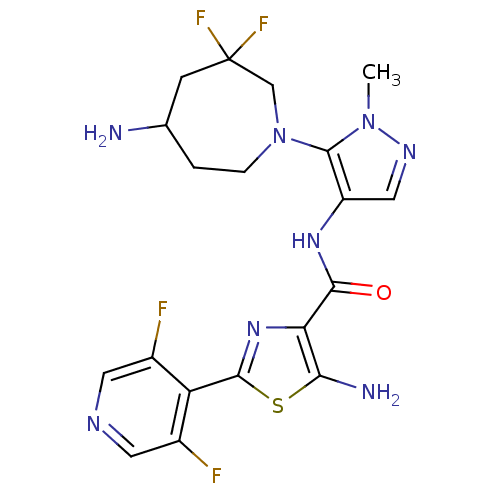 (US8614206, 438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457612 (CHEMBL4218164) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110750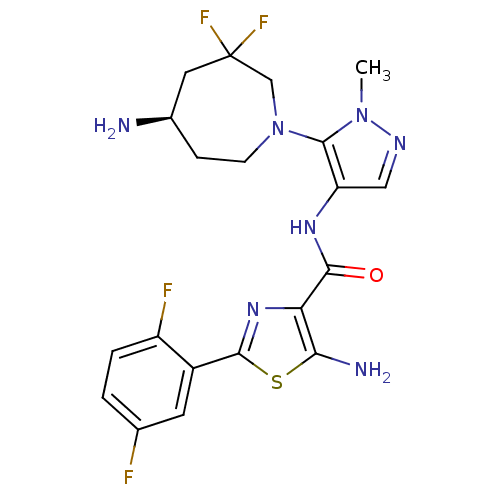 (US8614206, 283) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520752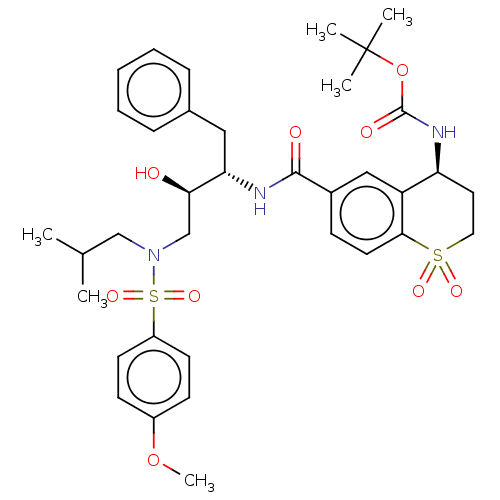 (CHEMBL4474261) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528154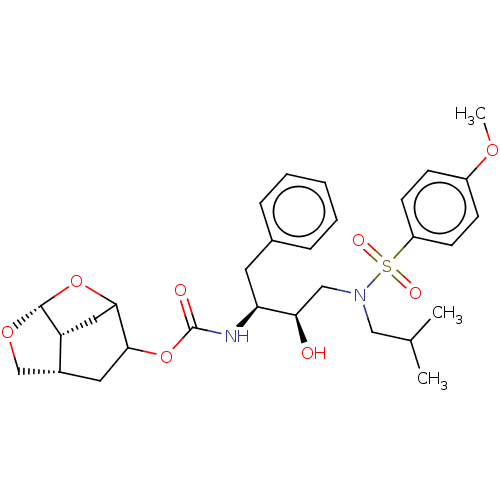 (CHEMBL4545005) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 15418 total ) | Next | Last >> |