

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50462575 (CHEMBL4238121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) pre-incubated for 2 hrs before fluorescence substrate addition and measured after 5 mins by fluorescence as... | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50746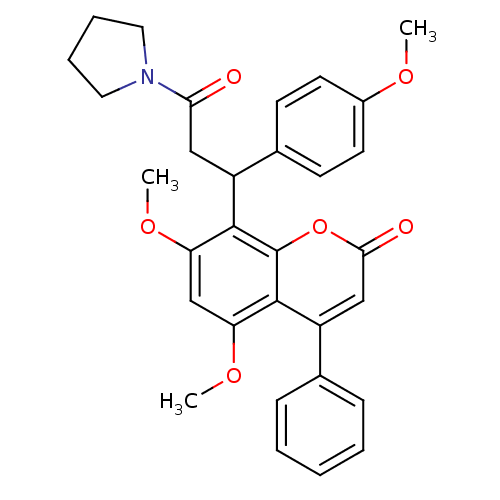 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxidanylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240685 (CHEMBL1522581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description In vitro inhibitory activity against [3H]-DTG in guinea pig brain membrane homogenates | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50745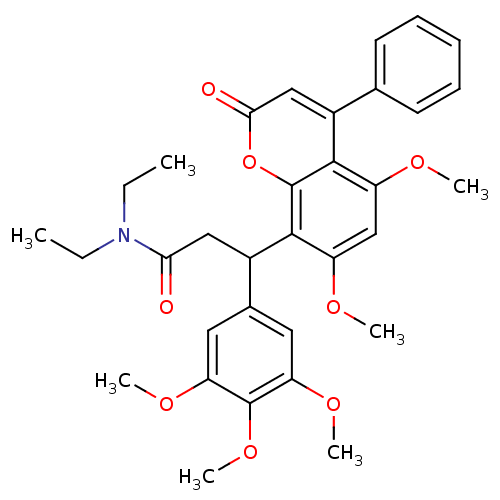 (3-(5,7-dimethoxy-2-oxidanylidene-4-phenyl-chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161470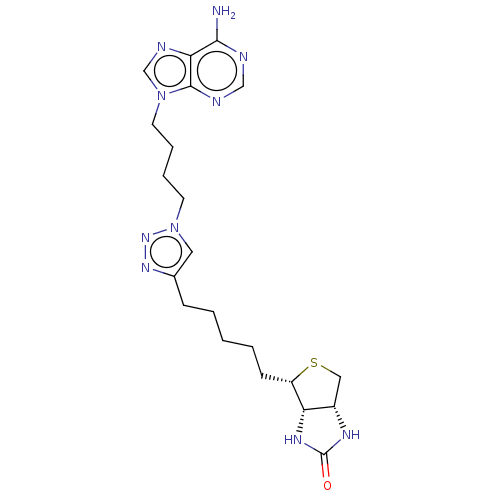 (US9108978, 4.01) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus biotin protein ligase by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM51309 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240684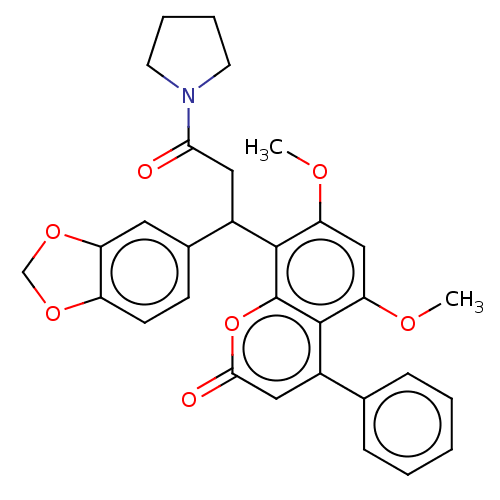 (CHEMBL1309685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM61111 (5,7-dimethoxy-8-[3-oxidanylidene-3-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50746 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxidanylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240685 (CHEMBL1522581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50745 (3-(5,7-dimethoxy-2-oxidanylidene-4-phenyl-chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM51309 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240684 (CHEMBL1309685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM61111 (5,7-dimethoxy-8-[3-oxidanylidene-3-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM84460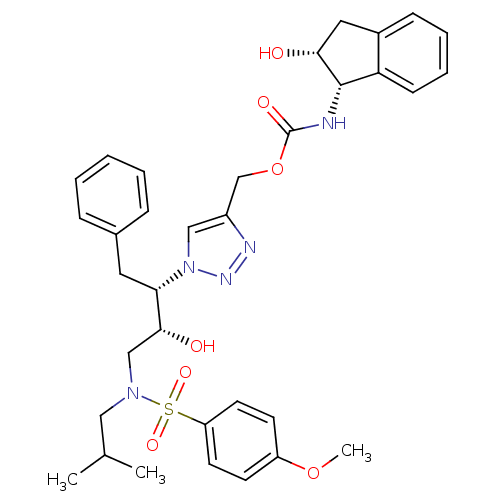 (HIV-1 PR Inhibitor, compound 6) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of HIV1 protease after 24 hrs by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50462584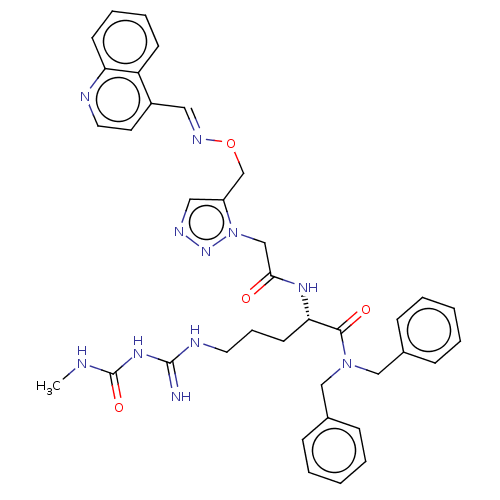 (CHEMBL4245260) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens 6x-His-tagged ChiB after 20 hrs by LCMS-SIR analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50462574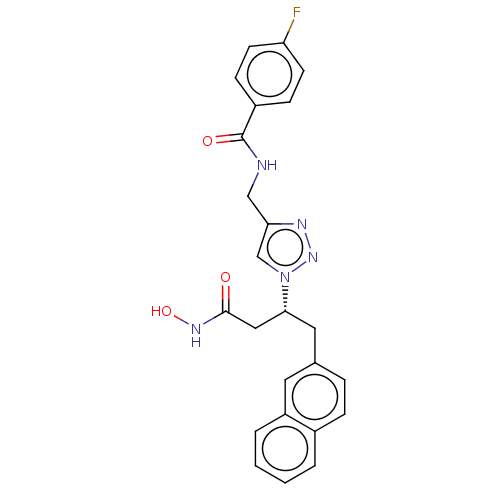 (CHEMBL4241044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of human IDE using insulin as substrate preincubated for 10 mis followed by substrate addition and measured after 30 mins | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50462579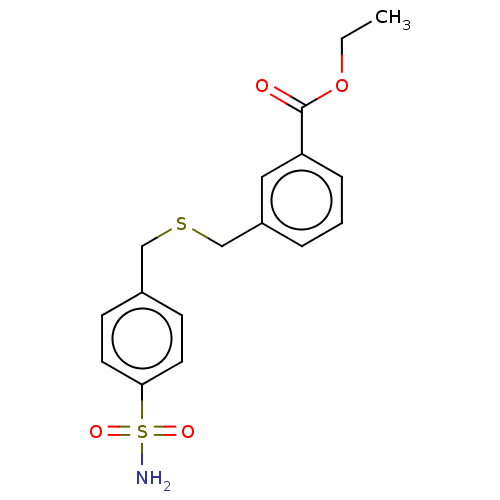 (CHEMBL4250387) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of CA2 (unknown origin) | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50423877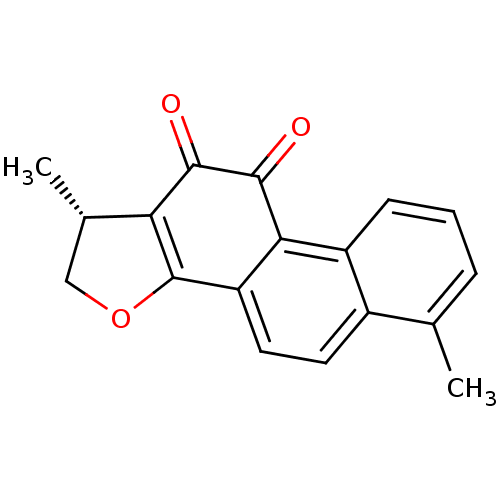 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE TNFalpha complex formation after 90 mins by AlphaScreen assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50423877 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE TNFalpha complex formation after 3 hrs by REMSA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 3 (Homo sapiens) | BDBM50240690 (CHEMBL4088244) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV3 (unknown origin)-artificial ARE complex formation after 30 mins in the presence of biotin-labeled RNA probe by chemiluminescence ... | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 3 (Homo sapiens) | BDBM50153015 ((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV3 (unknown origin)-artificial ARE complex formation after 30 mins in the presence of biotin-labeled RNA probe by chemiluminescence ... | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240691 (CHEMBL4061748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE TNFalpha complex formation after 20 mins by liquid scintillation counting method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator EthR (Mycobacterium tuberculosis) | BDBM50462583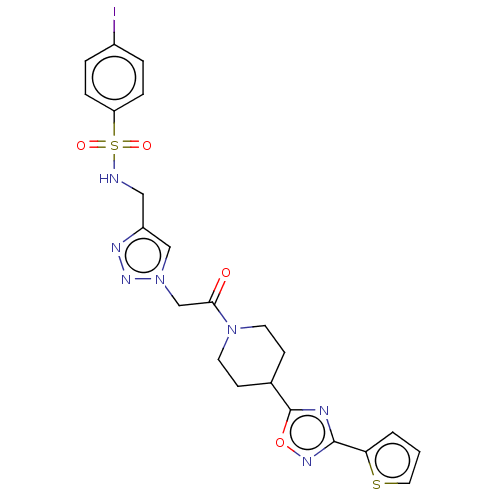 (CHEMBL1234901) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis EthR | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ELAV-like protein 3 (Homo sapiens) | BDBM4078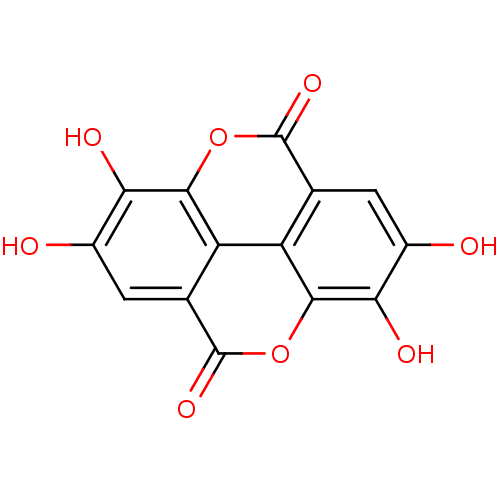 (6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV3 (unknown origin)-artificial ARE complex formation after 30 mins in the presence of biotin-labeled RNA probe by chemiluminescence ... | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 3 (Homo sapiens) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV3 (unknown origin)-artificial ARE complex formation after 30 mins in the presence of biotin-labeled RNA probe by chemiluminescence ... | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 3 (Homo sapiens) | BDBM23410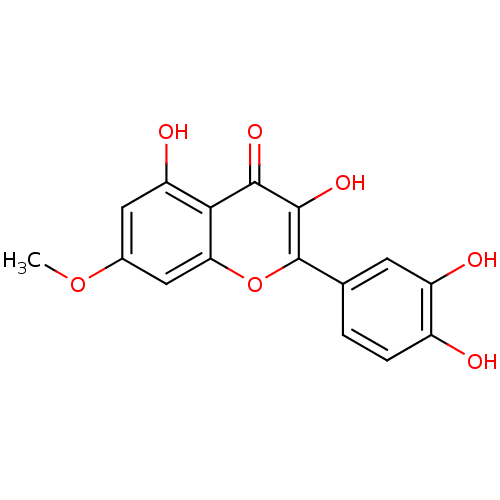 (2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-methoxy-4H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV3 (unknown origin)-artificial ARE complex formation after 30 mins in the presence of biotin-labeled RNA probe by chemiluminescence ... | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE TNFalpha complex formation after 20 mins by liquid scintillation counting method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 3 (Homo sapiens) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description In vitro inhibitory activity against [3H]- (+)-3-PPP binding to Sigma opioid receptor in guinea pig brain membrane homogenates | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50746 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxidanylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240685 (CHEMBL1522581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50745 (3-(5,7-dimethoxy-2-oxidanylidene-4-phenyl-chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM51309 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description In vitro inhibitory activity against [3H]DTG binding to Sigma opioid receptor in guinea pig brain membrane homogenates | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240686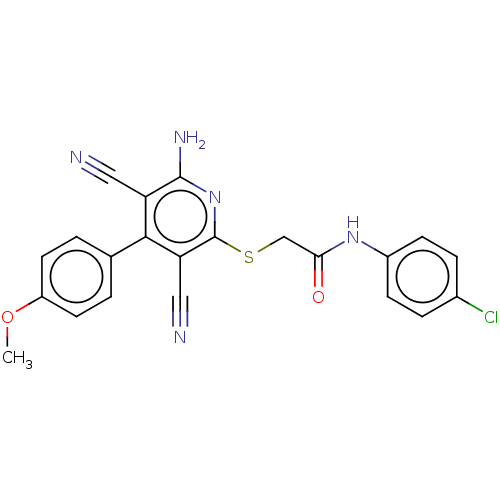 (CHEMBL4095140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description In vitro inhibitory activity against [3H]DTG binding to Sigma opioid receptor in guinea pig brain membrane homogenates | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240684 (CHEMBL1309685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM61111 (5,7-dimethoxy-8-[3-oxidanylidene-3-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of Mushashi1 transcript complex formation after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50746 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxidanylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240685 (CHEMBL1522581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50745 (3-(5,7-dimethoxy-2-oxidanylidene-4-phenyl-chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM51309 (5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240684 (CHEMBL1309685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM61111 (5,7-dimethoxy-8-[3-oxidanylidene-3-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 RRM1/RRM2 domains (unknown origin) interaction with ARE sequence of Mushashi1 transcript after 2 hrs by AlphaLISA method | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240687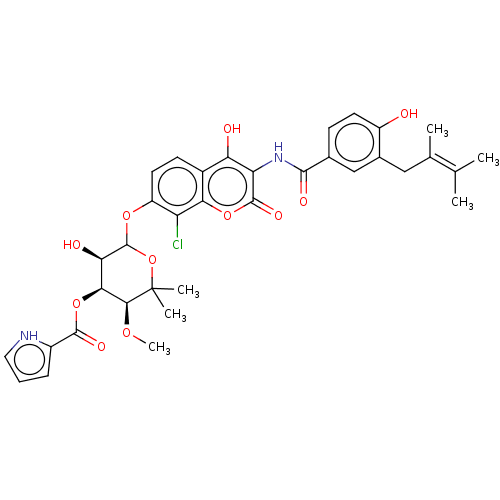 (CHEMBL4066821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of c-fos complex formation by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM94953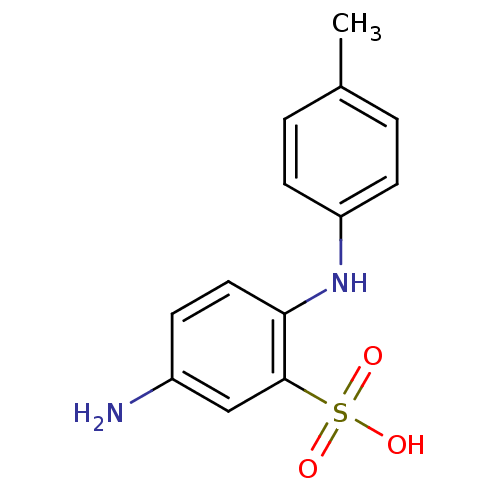 (5-Amino-2-p-toluidinobenzenesulfonic acid | 5-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of c-fos complex formation by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM50240688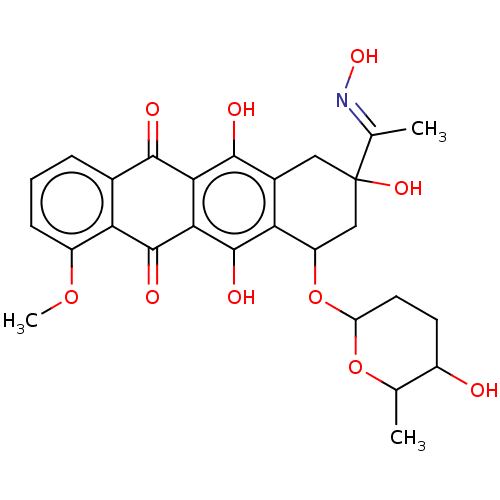 (CHEMBL4077803) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description In vitro inhibitory activity against [3H]- (+)-3-PPP binding to Sigma opioid receptor in guinea pig brain membrane homogenates | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ELAV-like protein 1 (Homo sapiens (Human)) | BDBM82867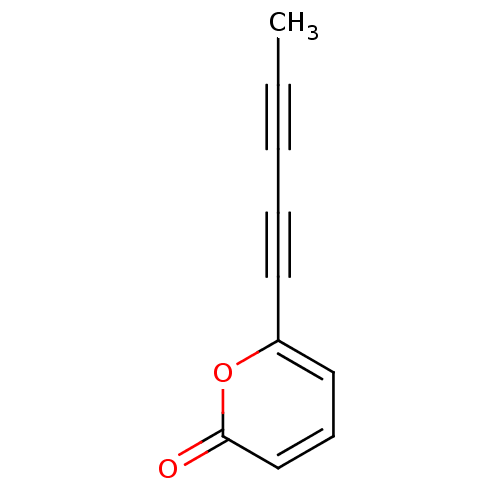 (6-penta-1,3-diynyl-2-pyranone | 6-penta-1,3-diynyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Inhibition of ELAV1 (unknown origin)-ARE sequence of c-fos complex formation by fluorescence polarization assay | J Med Chem 60: 8257-8267 (2017) Article DOI: 10.1021/acs.jmedchem.6b01871 BindingDB Entry DOI: 10.7270/Q2377BWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50044549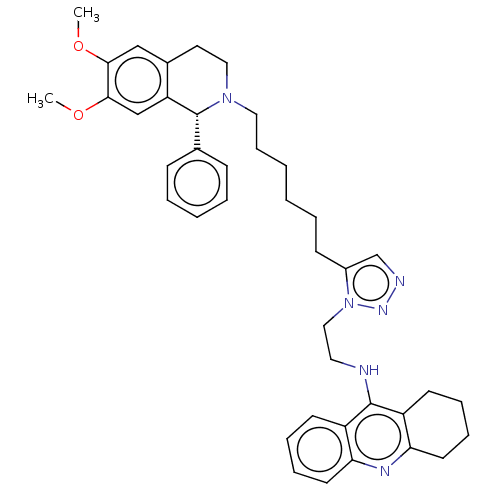 (CHEMBL3314093) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50462577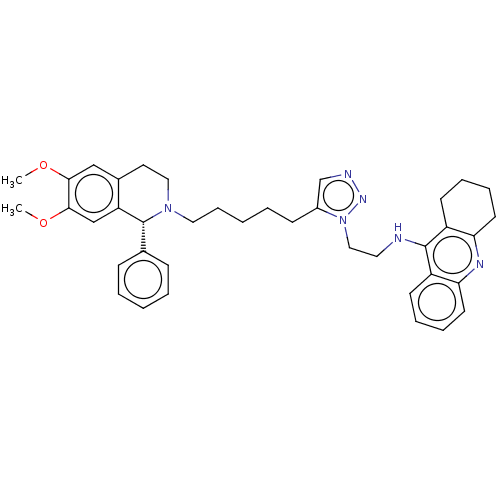 (CHEMBL4243833) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50462581 (CHEMBL4246013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50462578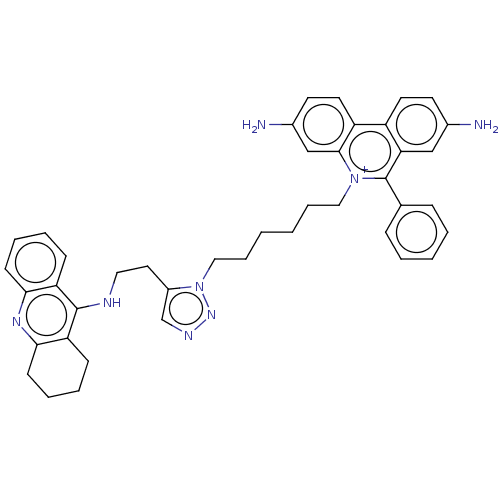 (CHEMBL4239697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |