

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551316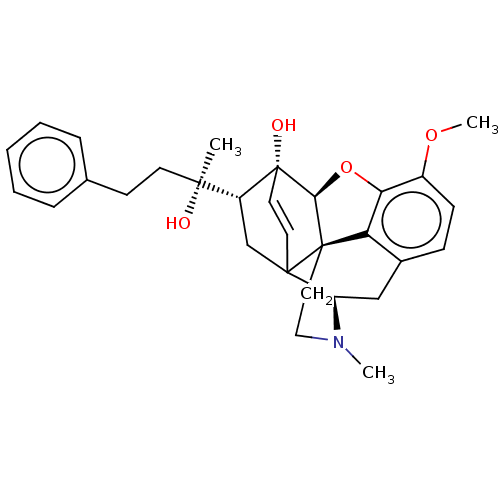 (CHEMBL4776706) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551315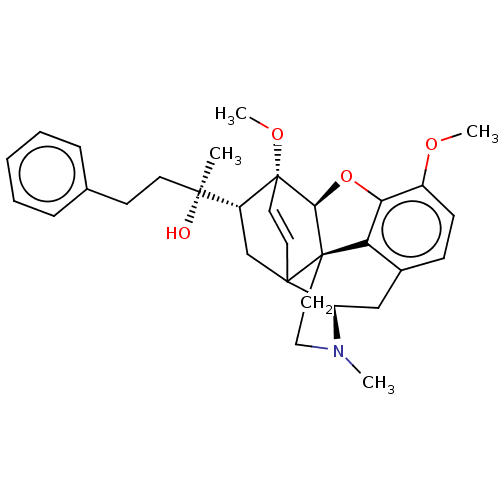 (CHEMBL4762529) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551313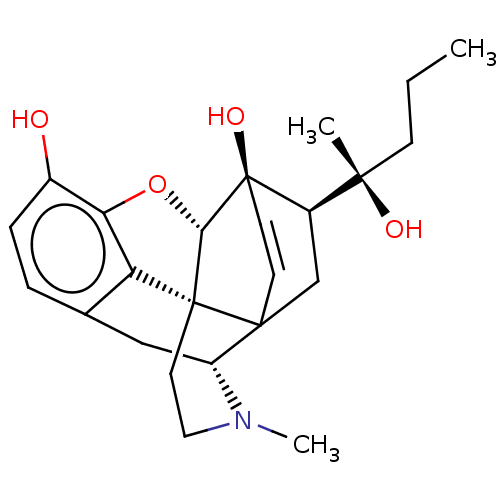 (CHEMBL4745764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551314 (CHEMBL4751305) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551311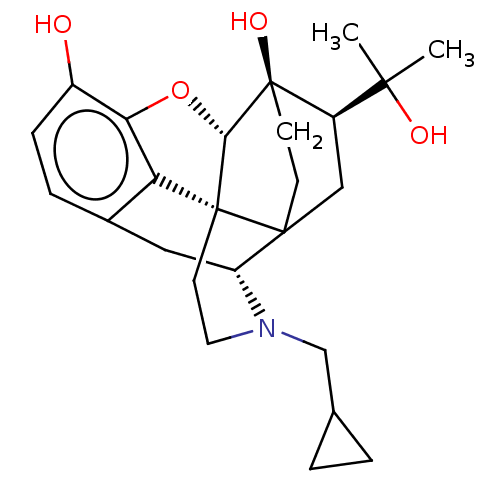 (CHEMBL4746512) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551309 (CHEMBL4758369) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551311 (CHEMBL4746512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551314 (CHEMBL4751305) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551319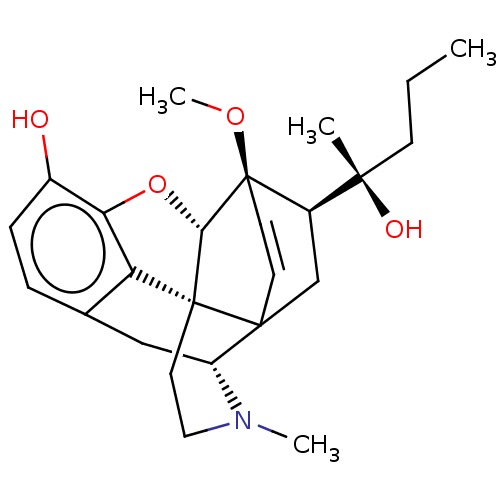 (CHEMBL4753839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551307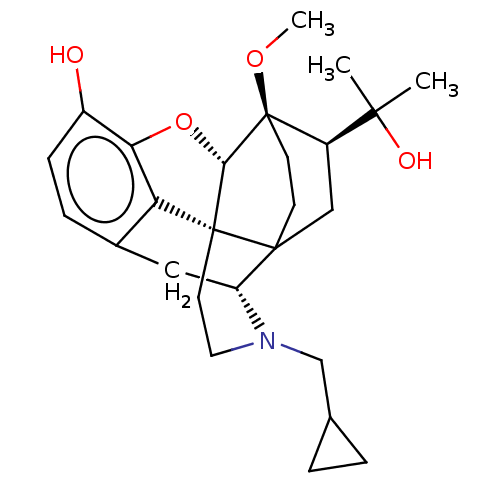 (CHEMBL4754079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551312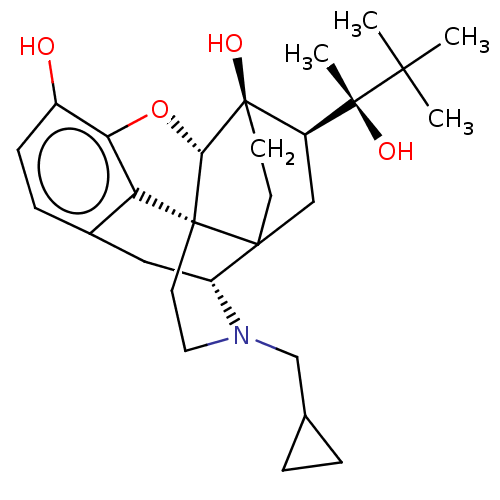 (CHEMBL4761619) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551317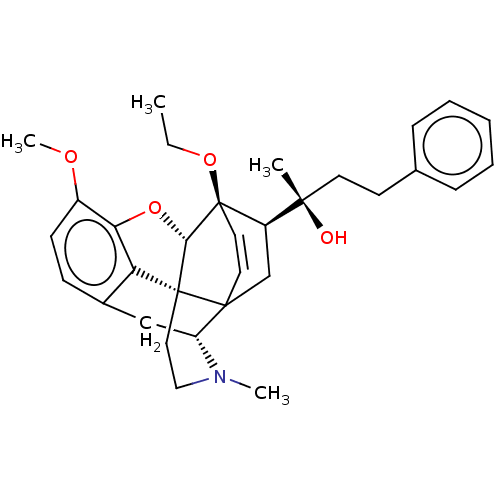 (CHEMBL4764372) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551312 (CHEMBL4761619) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551308 (CHEMBL4758814) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551316 (CHEMBL4776706) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.321 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551318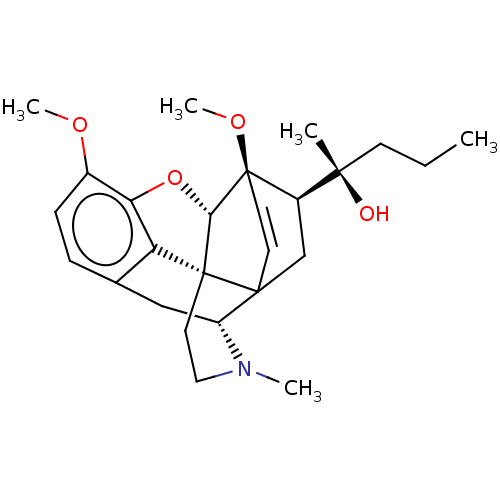 (CHEMBL4787652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.326 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551310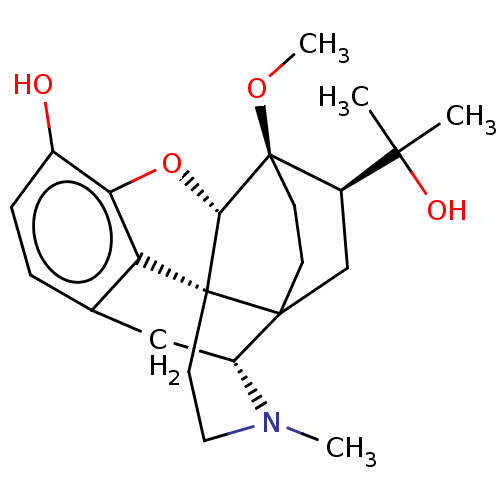 (CHEMBL4752367) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551308 (CHEMBL4758814) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.531 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551317 (CHEMBL4764372) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551313 (CHEMBL4745764) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.796 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551319 (CHEMBL4753839) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551311 (CHEMBL4746512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551307 (CHEMBL4754079) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551316 (CHEMBL4776706) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551307 (CHEMBL4754079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551314 (CHEMBL4751305) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551315 (CHEMBL4762529) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551319 (CHEMBL4753839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551313 (CHEMBL4745764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551309 (CHEMBL4758369) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551310 (CHEMBL4752367) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551318 (CHEMBL4787652) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551315 (CHEMBL4762529) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551312 (CHEMBL4761619) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551308 (CHEMBL4758814) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551317 (CHEMBL4764372) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551310 (CHEMBL4752367) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551309 (CHEMBL4758369) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50551318 (CHEMBL4787652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Ile5'6-deltorphin II from DOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50013509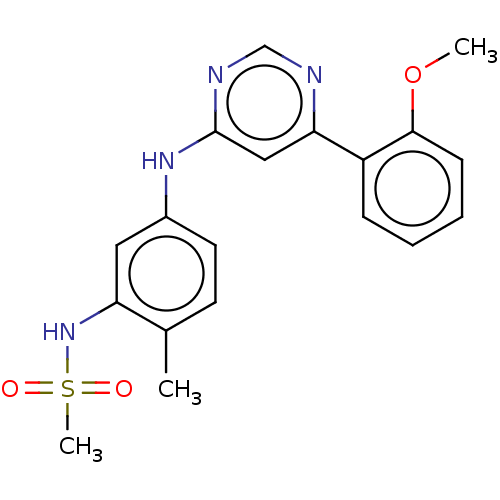 (CHEMBL3263770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) expressed in HEK293 cells using GST-CTDII as substrate | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50013510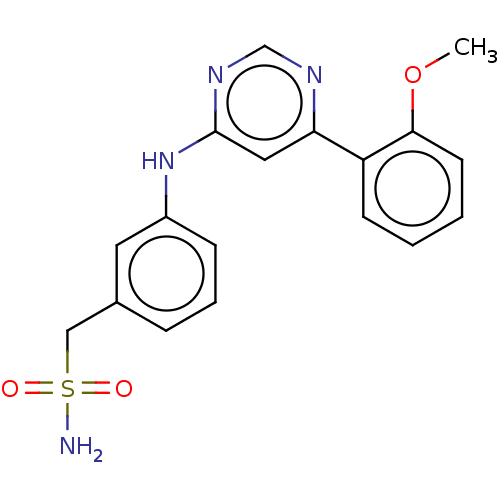 (CHEMBL3263773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) using MBP peptide substrate after 1 hr by FRET-based LANCE assay | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50013602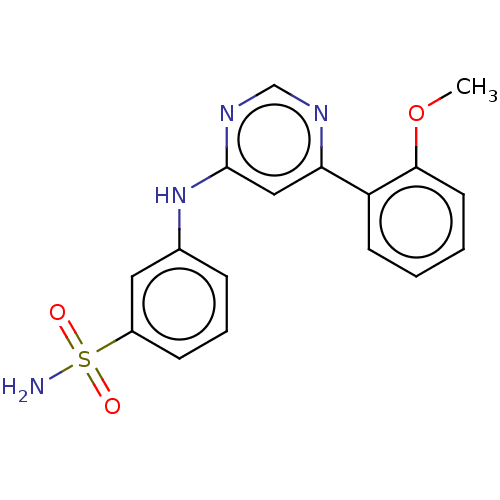 (CHEMBL3263772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) expressed in HEK293 cells using GST-CTDII as substrate | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316211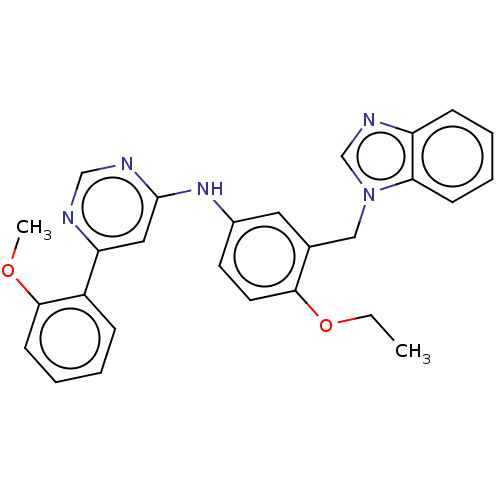 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-ethoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316212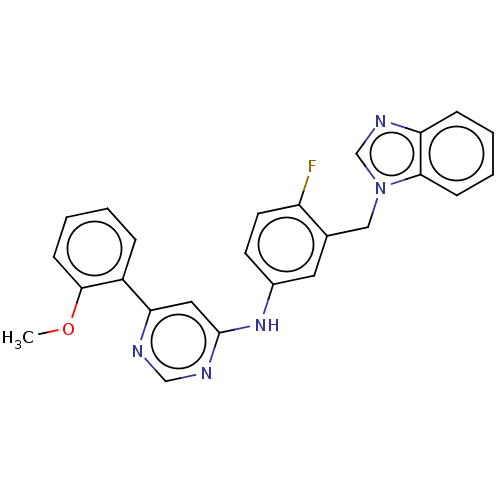 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-fluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316227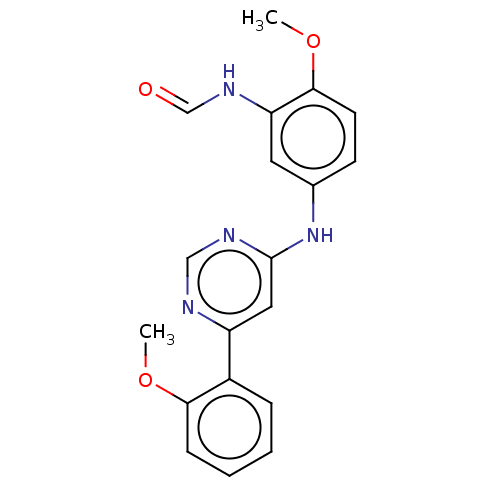 (N-{2-Methoxy-5-[6-(2-methoxy-phenyl)-pyrimidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316285 (2-Dimethylamino-5-[6-(4-fluoro-2-methoxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316288 (US10294218, Example 116 | US9617225, Example 116 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316293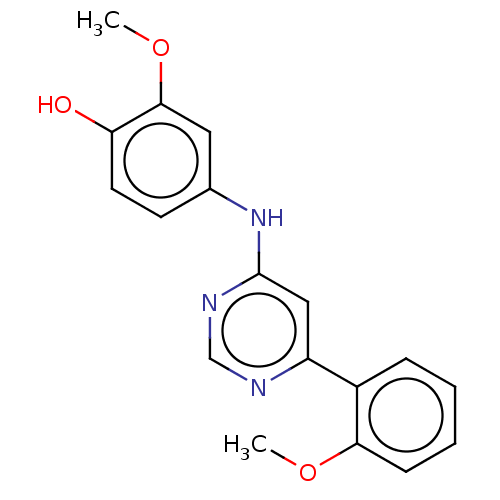 (2-Methoxy-4-[6-(2-methoxy-phenyl)-pyrimidin-4-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316301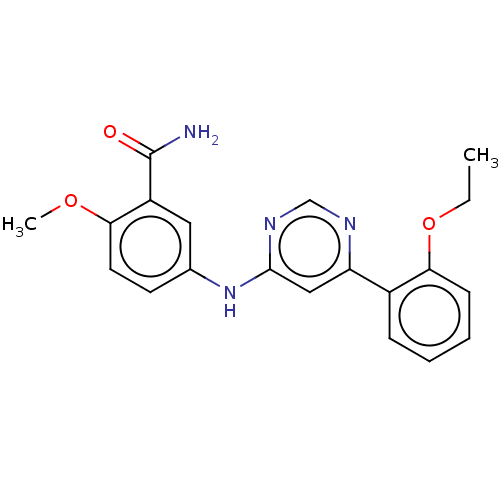 (5-[6-(2-Ethoxy-phenyl)-pyrimidin-4- ylamino]-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 497 total ) | Next | Last >> |