

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509389 (CHEMBL4566436) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509387 (CHEMBL4475066) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509386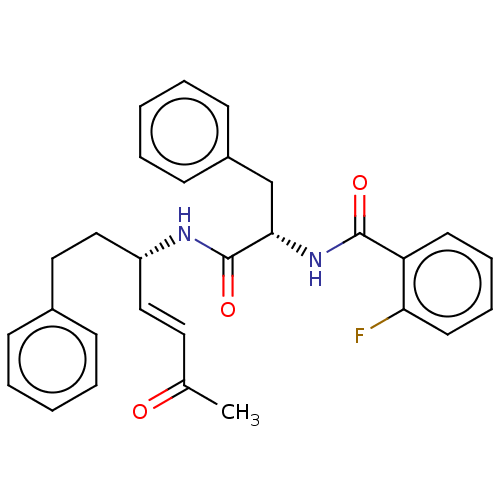 (CHEMBL4515298) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509388 (CHEMBL4587965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509383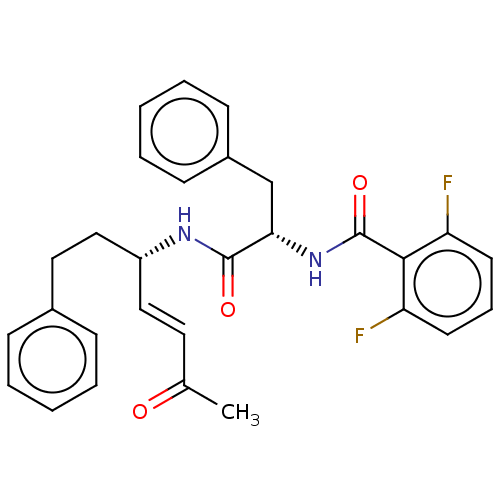 (CHEMBL4441635) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509390 (CHEMBL4436812) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509381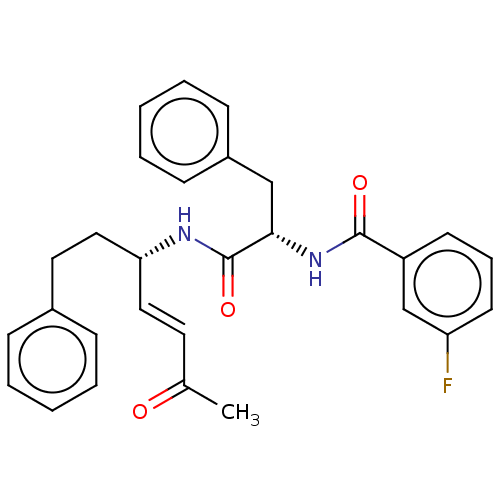 (CHEMBL4453415) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509394 (CHEMBL4584291) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509395 (CHEMBL4437914) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258507 (CHEMBL4078345) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176723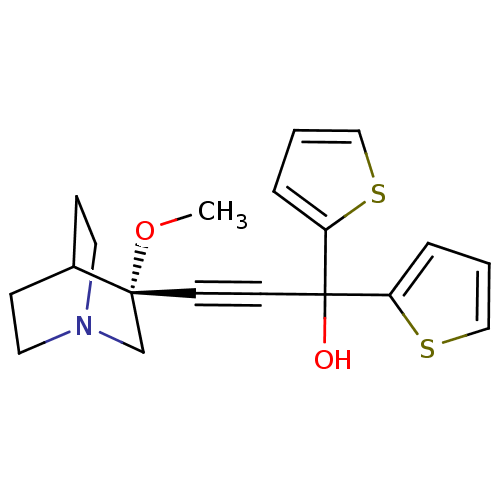 ((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258514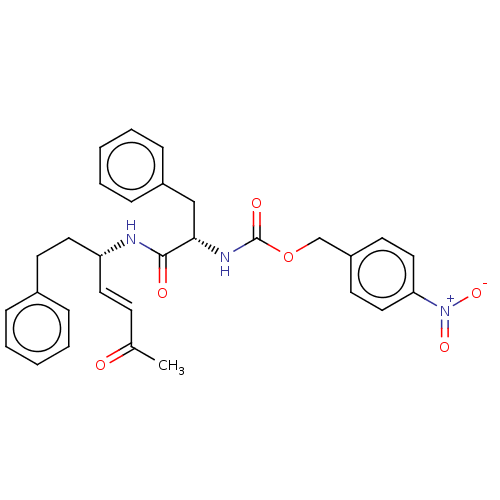 (CHEMBL4062015) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176735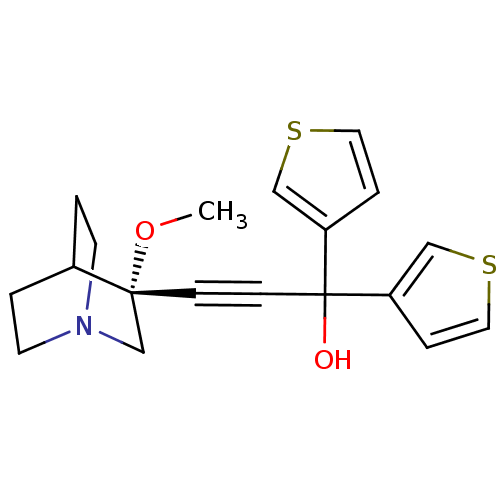 ((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50075000 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074978 (2-[3-(4-Amino-butyl)-ureido]-N-[4-chloro-2-cyano-3...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176732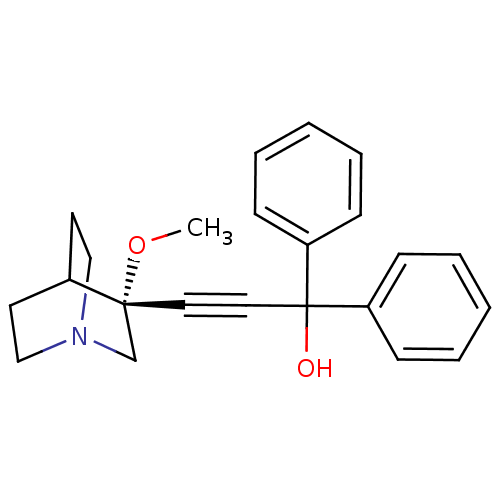 ((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594411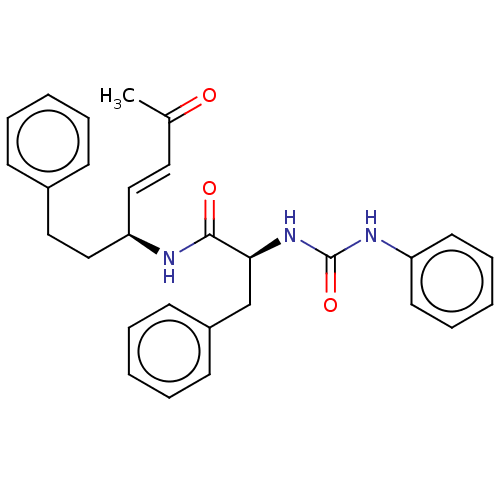 (CHEMBL5173102) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176718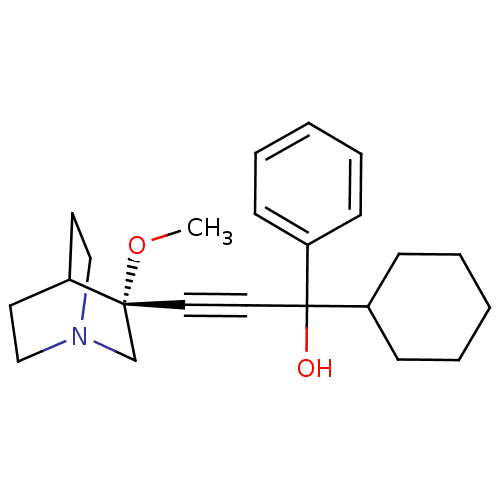 (1-cyclohexyl-3-((R)-3-methoxyquinuclidin-3-yl)-1-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50176723 ((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M2 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176712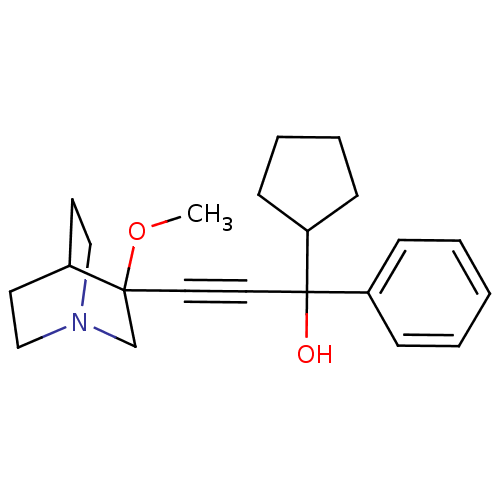 (1-cyclopentyl-3-(3-methoxyquinuclidin-3-yl)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176708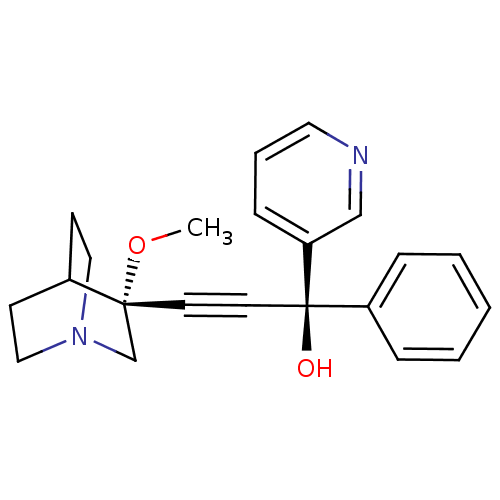 ((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074986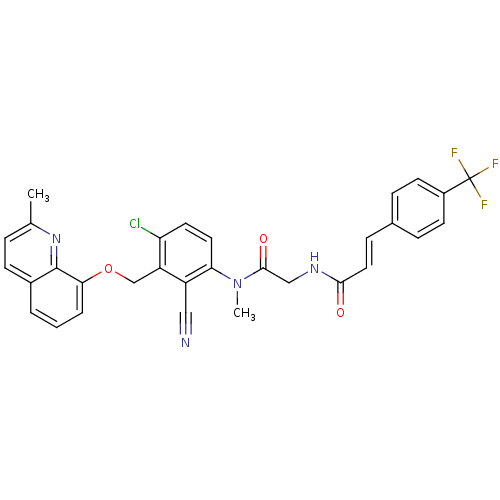 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176706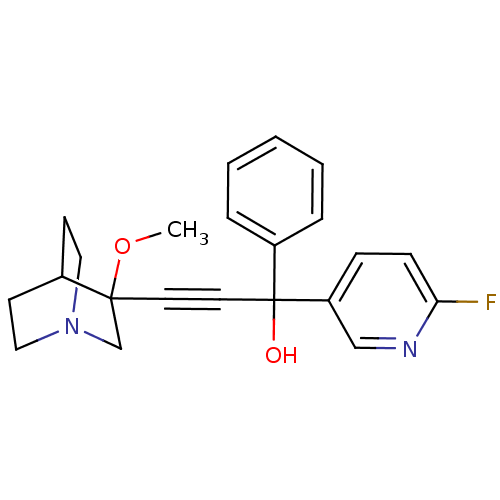 (1-(6-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258507 (CHEMBL4078345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50176735 ((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M2 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176729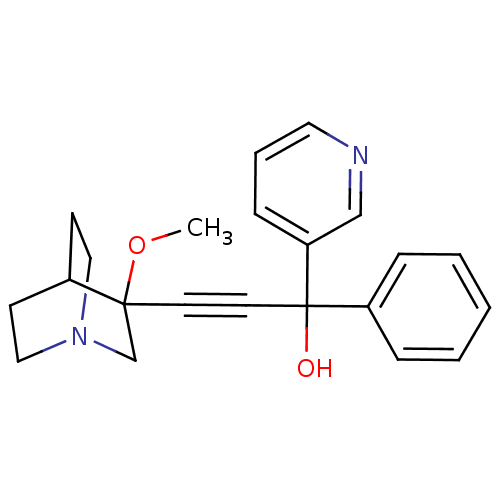 (3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594420 (CHEMBL5200091) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574836 (CHEMBL4871105) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509391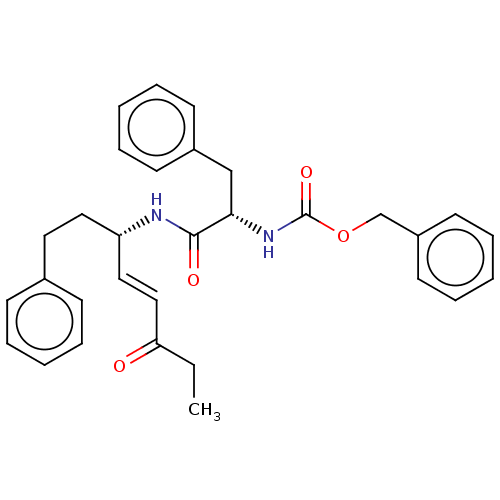 (CHEMBL4459413) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258506 (CHEMBL4072275) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594413 (CHEMBL5209133) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594417 (CHEMBL5170687) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074997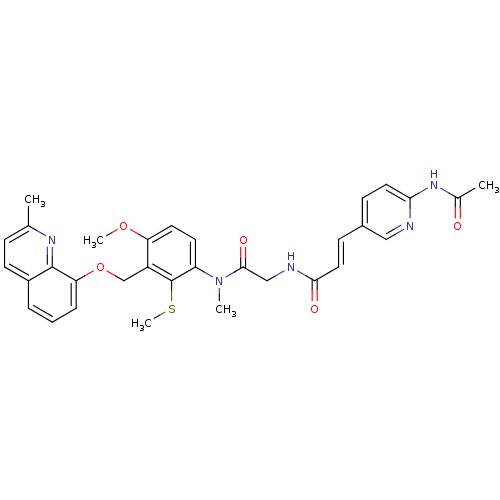 ((E)-3-(6-Acetylamino-pyridin-3-yl)-N-({[4-methoxy-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50509394 (CHEMBL4584291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50258515 (CHEMBL4083754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176717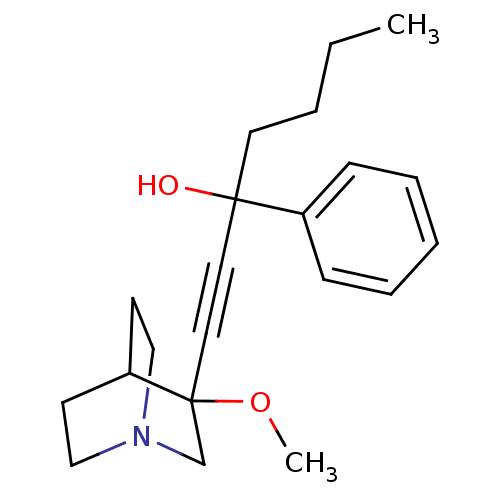 (1-(3-methoxyquinuclidin-3-yl)-3-phenylhept-1-yn-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50509392 (CHEMBL4456010) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... | J Med Chem 62: 10617-10629 (2019) Article DOI: 10.1021/acs.jmedchem.9b00908 BindingDB Entry DOI: 10.7270/Q2Z60SCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594416 (CHEMBL5207517) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50176708 ((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M2 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176726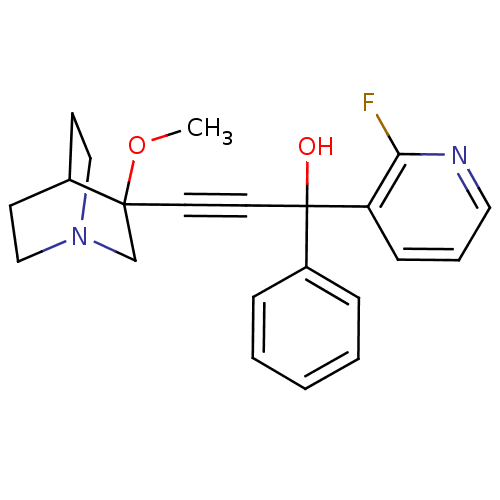 (1-(2-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176711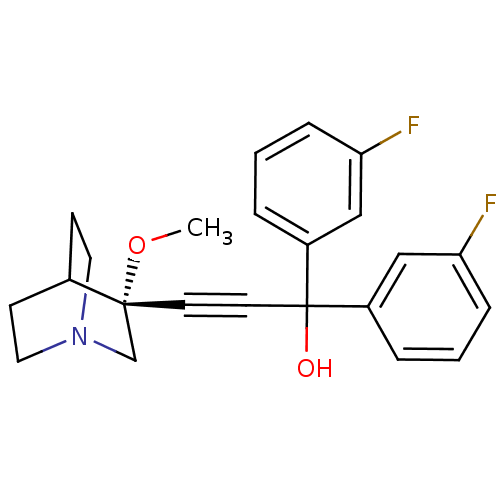 ((R)-1,1-bis(3-fluorophenyl)-3-(3-methoxyquinuclidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50176732 ((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M2 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594414 (CHEMBL5186393) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50258544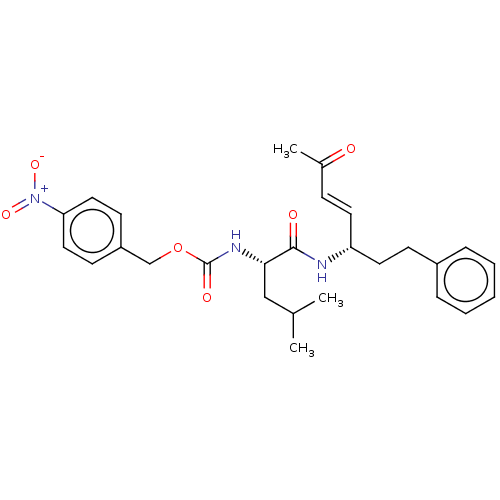 (CHEMBL4096388) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy. Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... | J Med Chem 60: 6911-6923 (2017) Article DOI: 10.1021/acs.jmedchem.7b00405 BindingDB Entry DOI: 10.7270/Q2FJ2K73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594421 (CHEMBL5190766) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176722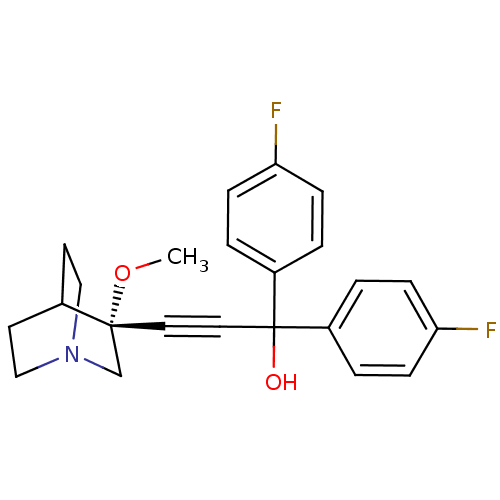 ((R)-1,1-bis(4-fluorophenyl)-3-(3-methoxyquinuclidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594418 (CHEMBL5205818) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176714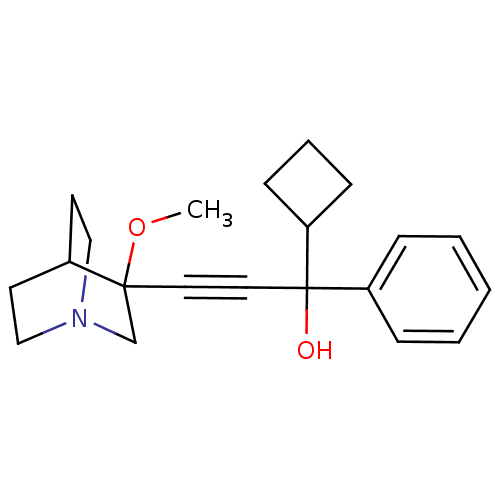 (1-cyclobutyl-3-(3-methoxyquinuclidin-3-yl)-1-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50176704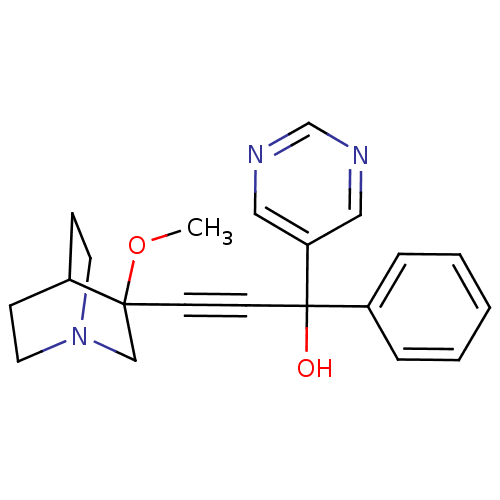 (3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 16: 373-7 (2005) Article DOI: 10.1016/j.bmcl.2005.09.079 BindingDB Entry DOI: 10.7270/Q2T72J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50594415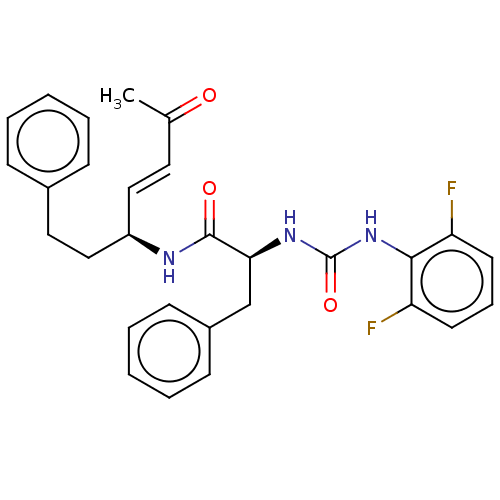 (CHEMBL5176432) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00084 BindingDB Entry DOI: 10.7270/Q2862MF6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 652 total ) | Next | Last >> |