

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576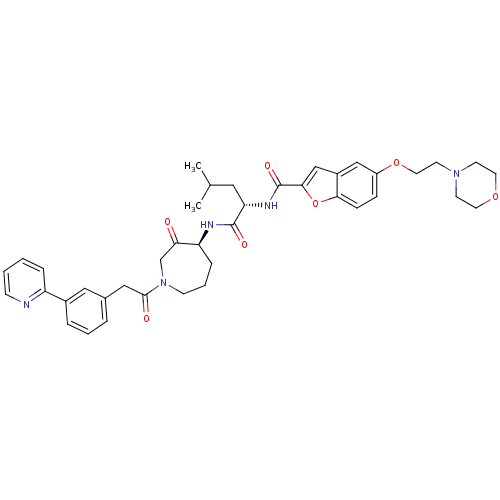 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770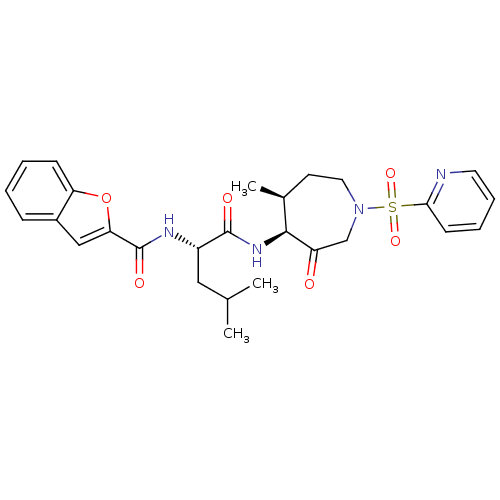 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362044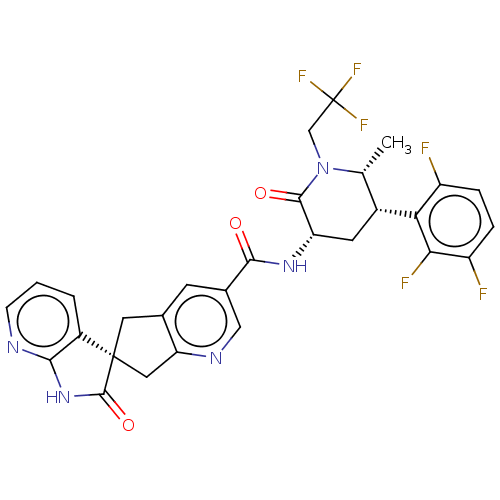 (US10272077, Example 4 | US9833448, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362044 (US10272077, Example 4 | US9833448, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362171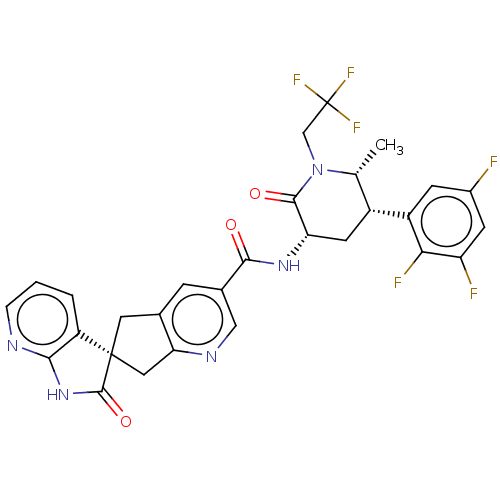 (US10272077, Example 5 | US9833448, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362171 (US10272077, Example 5 | US9833448, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50260646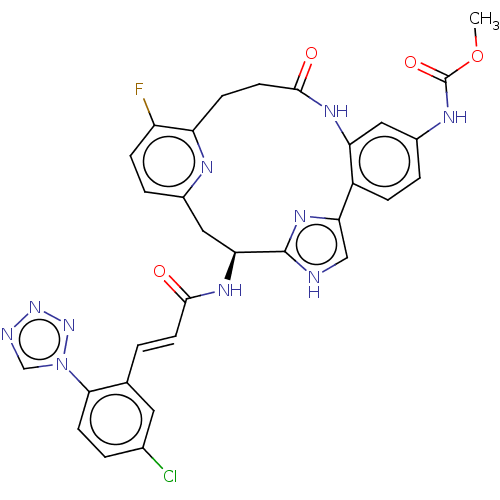 (CHEMBL4096251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company, Research and Development, 350 Carter Road, Hopewell, NJ 08540 United States. Curated by ChEMBL | Assay Description Inhibition of human F11a using peptide substrate by spectrophotometry | Bioorg Med Chem Lett 27: 4056-4060 (2017) Article DOI: 10.1016/j.bmcl.2017.07.048 BindingDB Entry DOI: 10.7270/Q2TB19B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50148931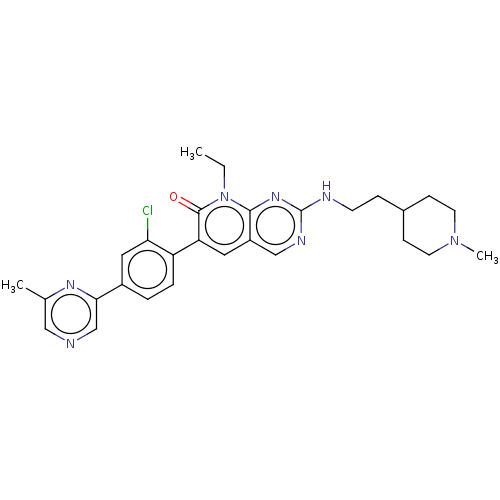 (CHEMBL3770186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112517 BindingDB Entry DOI: 10.7270/Q2Q243W7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50125046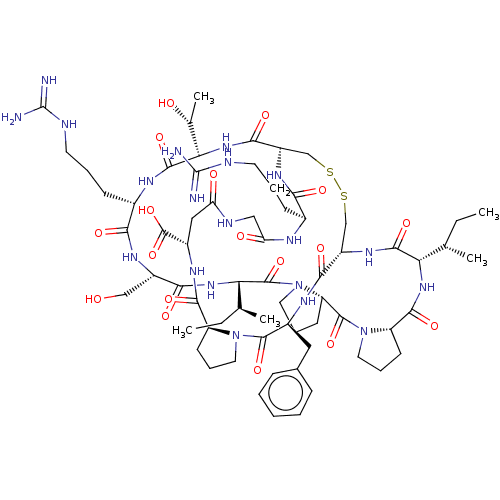 (CHEMBL3623792) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology Curated by ChEMBL | Assay Description Inhibition of beta-trypsin (unknown origin) using Bz-FVRpNA substrate by spectrophotometry method | J Med Chem 58: 8257-68 (2015) Article DOI: 10.1021/acs.jmedchem.5b01148 BindingDB Entry DOI: 10.7270/Q2PR7XSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50185364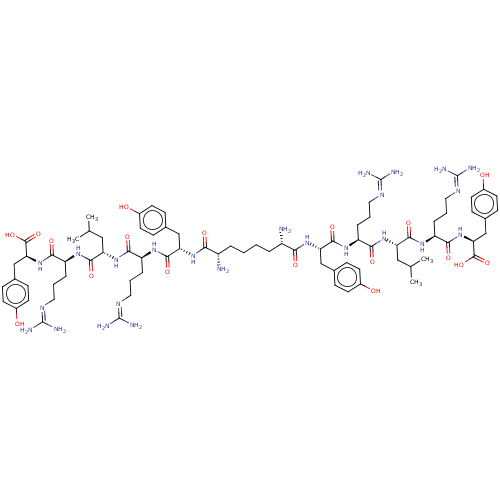 (CHEMBL2371908 | CHEMBL415187 | Sub[-Tyr-Arg-Leu-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Displacement of [125I]hPP from human NPY4 receptor expressed in CHO cells | J Med Chem 49: 2661-5 (2006) Article DOI: 10.1021/jm050907d BindingDB Entry DOI: 10.7270/Q2XS5TZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362174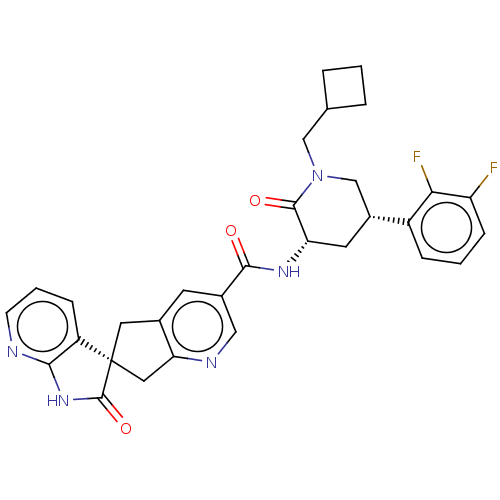 (US9833448, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM381649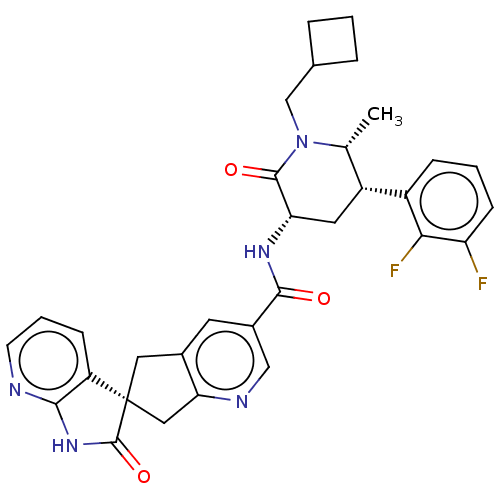 (US10272077, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50266122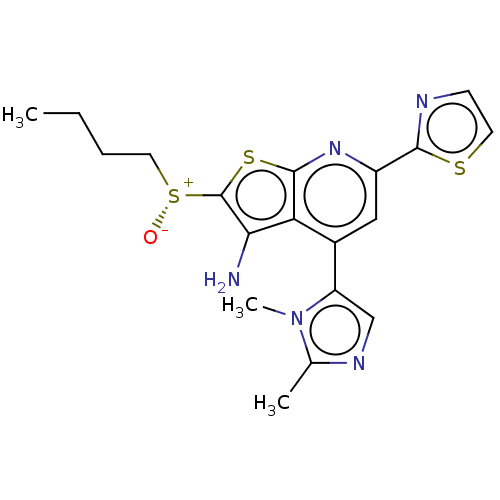 (CHEMBL4070789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UT Southwestern Medical Center Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal 6xHis-tagged 15-PGDH expressed in Escherichia coli using PGE2 as substrate after 15 mins in presence of NA... | J Med Chem 60: 3979-4001 (2017) Article DOI: 10.1021/acs.jmedchem.7b00271 BindingDB Entry DOI: 10.7270/Q2280B21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM361594 (US10272077, Example 1 | US9833448, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM361595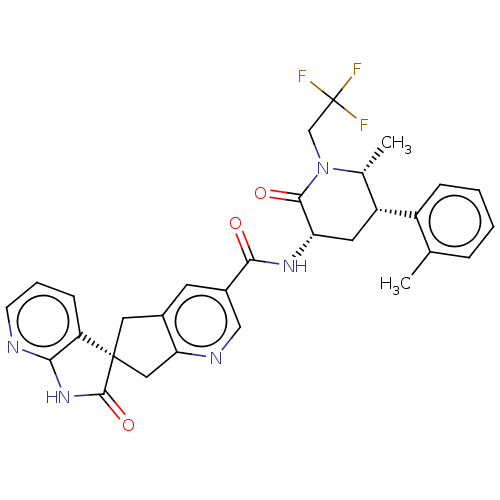 (US10272077, Example 3 | US9833448, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM361594 (US10272077, Example 1 | US9833448, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM361595 (US10272077, Example 3 | US9833448, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50409214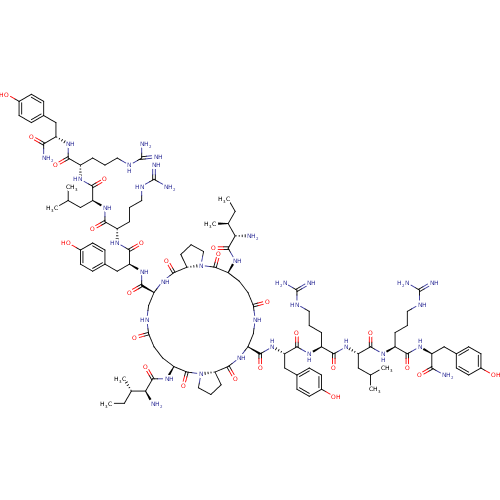 (CHEMBL2110365 | GR-231118) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati and VA Medical Centers Curated by ChEMBL | Assay Description Affinity for cloned Y1 receptor using [125I]-PYY as radioligand | J Med Chem 44: 1479-82 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50099198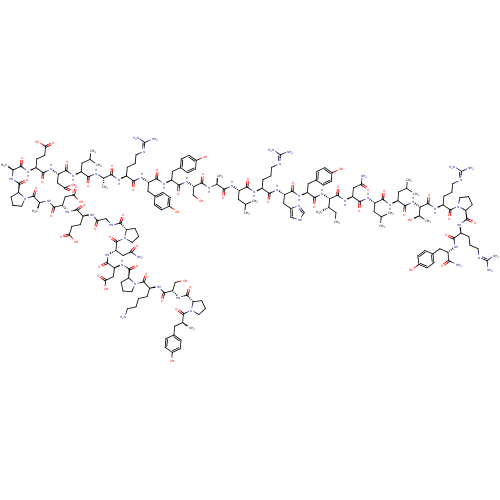 (CHEMBL429531 | Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati and VA Medical Centers Curated by ChEMBL | Assay Description Affinity for cloned Y4 receptor using [125I]-PP as radioligand | J Med Chem 44: 1479-82 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 17: 4419-27 (2007) Article DOI: 10.1016/j.bmcl.2007.06.029 BindingDB Entry DOI: 10.7270/Q2416XVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from mouse recombinant adenosine A3 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2813-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.001 BindingDB Entry DOI: 10.7270/Q2XK8GFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362175 (US10272077, Example 26 | US9833448, Example 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362175 (US10272077, Example 26 | US9833448, Example 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236563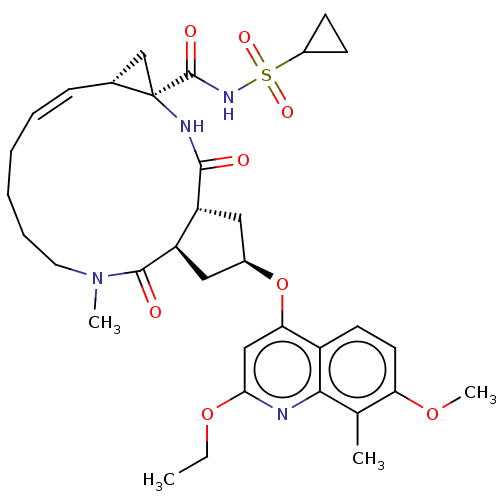 (US9365582, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236565 (US9365582, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50266252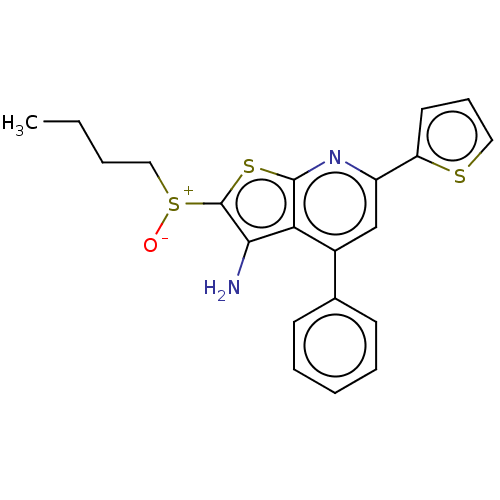 (CHEMBL4061483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UT Southwestern Medical Center Curated by ChEMBL | Assay Description Inhibition of 15-PGDH (unknown origin) using PGE2 as substrate preincubated for 12 hrs followed by dialysis for 12 hrs and subsequent addition of NAD... | J Med Chem 60: 3979-4001 (2017) Article DOI: 10.1021/acs.jmedchem.7b00271 BindingDB Entry DOI: 10.7270/Q2280B21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236571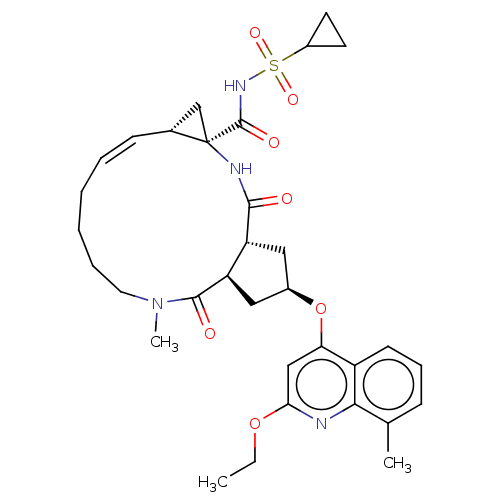 (US9365582, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50124947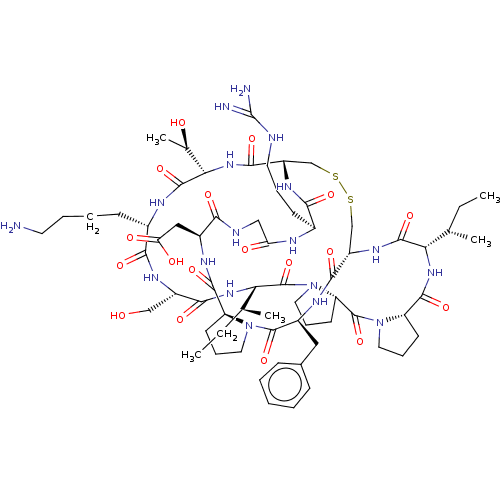 (CHEMBL453539) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) | J Med Chem 58: 8257-68 (2015) Article DOI: 10.1021/acs.jmedchem.5b01148 BindingDB Entry DOI: 10.7270/Q2PR7XSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236566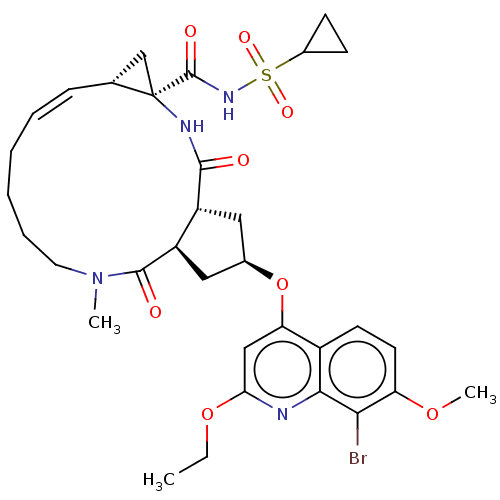 (US9365582, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775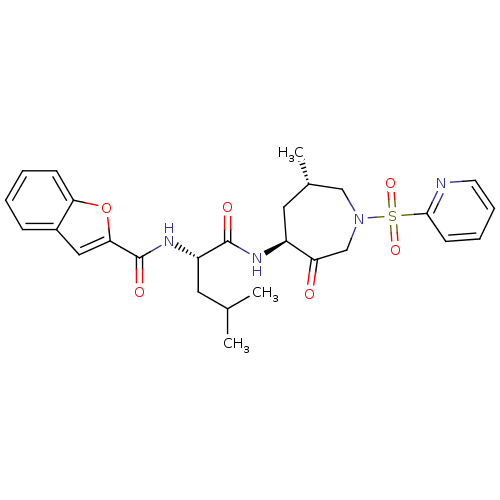 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362176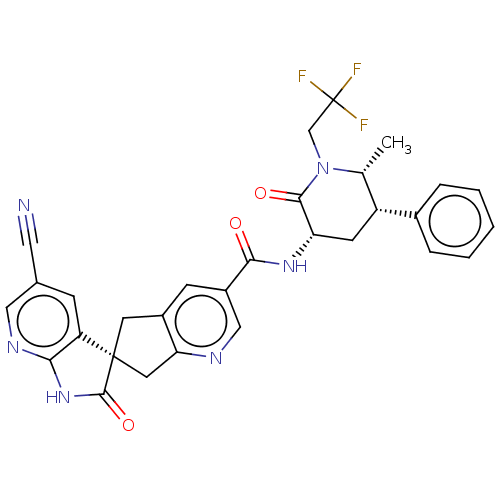 (US10272077, Example 30 | US9833448, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362176 (US10272077, Example 30 | US9833448, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328970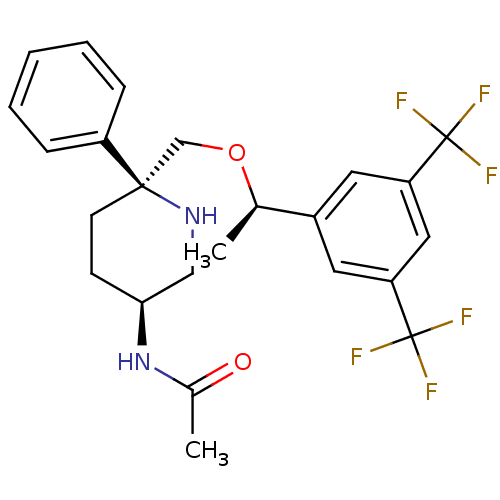 (CHEMBL1270066 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50125045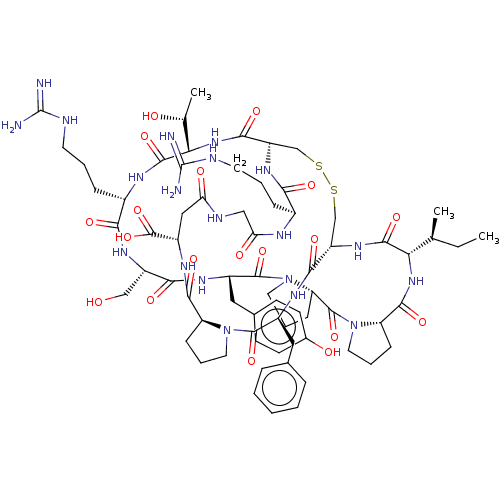 (CHEMBL3623793) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology Curated by ChEMBL | Assay Description Inhibition of beta-trypsin (unknown origin) using Bz-FVRpNA substrate by spectrophotometry method | J Med Chem 58: 8257-68 (2015) Article DOI: 10.1021/acs.jmedchem.5b01148 BindingDB Entry DOI: 10.7270/Q2PR7XSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362177 (US10272077, Example 31 | US9833448, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Cells were resuspended in DMEM/F12 (Hyclone) supplemented with 1 g/L BSA and 300 μM isobutyl-methylxanthine. Cells were then plated in a 384-wel... | US Patent US9833448 (2017) BindingDB Entry DOI: 10.7270/Q2BP052K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM362177 (US10272077, Example 31 | US9833448, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The binding of 125I-CGRP to receptors in SK-N-MC cell membranes was carried out essentially as described (Edvinsson et al. (2001) Eur. J. Pharmacol. ... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q25X2C8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from mouse recombinant adenosine A3 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2813-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.001 BindingDB Entry DOI: 10.7270/Q2XK8GFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328981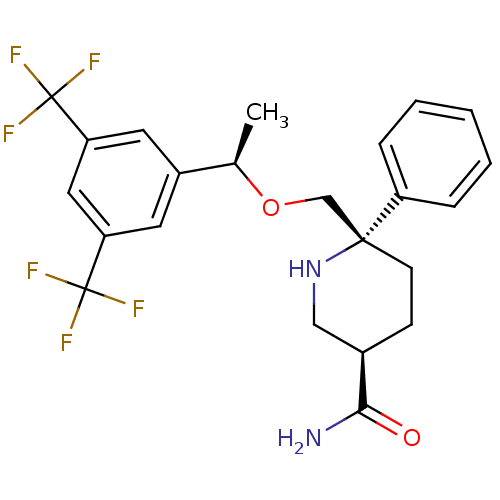 ((3R,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 17: 4419-27 (2007) Article DOI: 10.1016/j.bmcl.2007.06.029 BindingDB Entry DOI: 10.7270/Q2416XVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50448583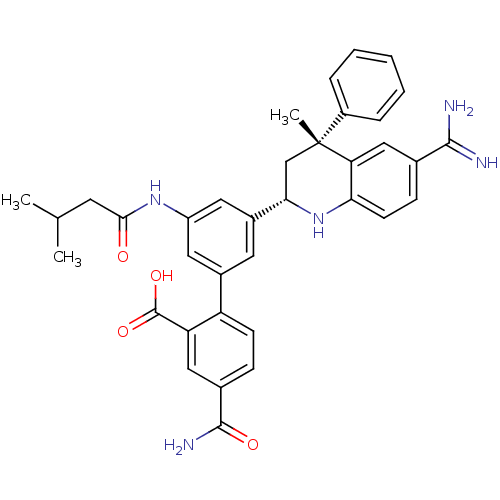 (CHEMBL3127491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236573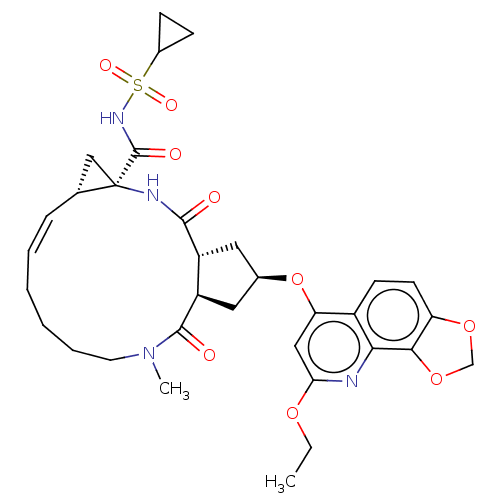 (US9365582, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243428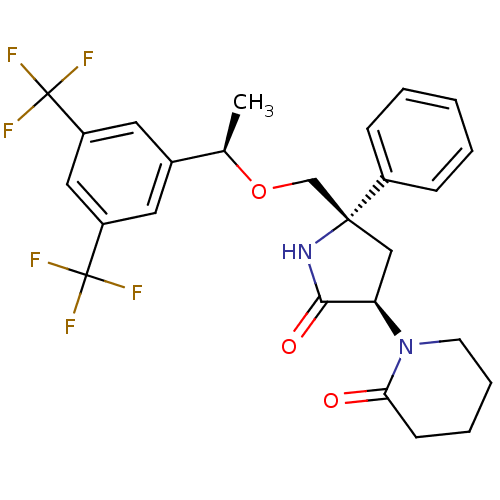 (1-((3R,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243427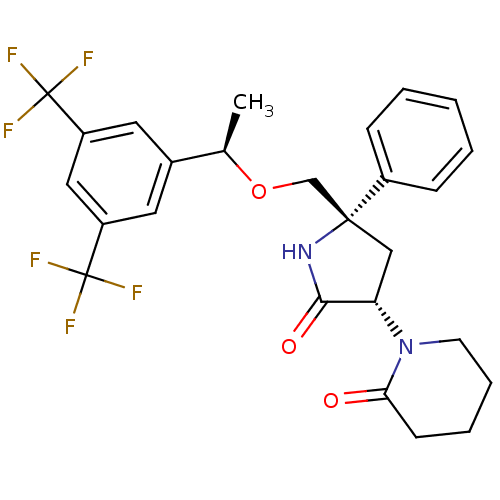 (1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50448581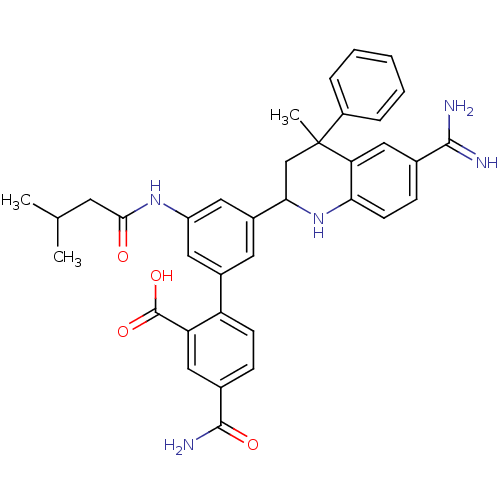 (CHEMBL3127463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human factor 11a assessed as release of p-nitroaniline after 10 to 120 mins by spectrophotometric analysis | J Med Chem 57: 955-69 (2014) Article DOI: 10.1021/jm401670x BindingDB Entry DOI: 10.7270/Q2G44RTQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 21778 total ) | Next | Last >> |