

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50393864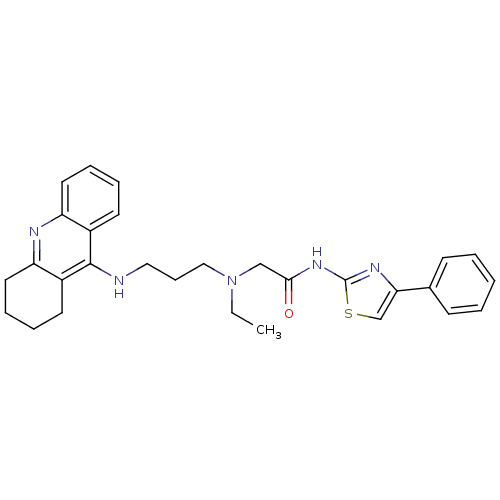 (CHEMBL2158116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0447 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393868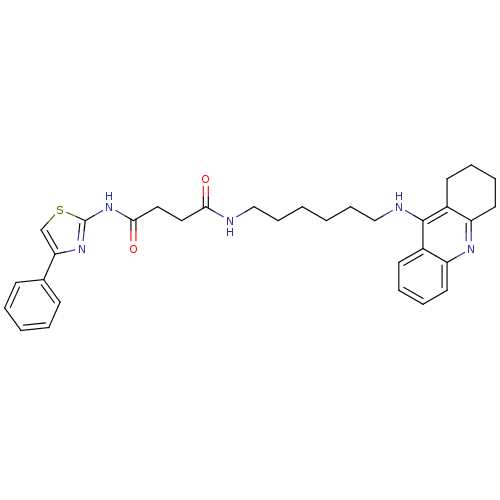 (CHEMBL2158112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393858 (CHEMBL2158122) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393867 (CHEMBL2158113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393870 (CHEMBL2158110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393857 (CHEMBL2158123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393859 (CHEMBL2158121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393855 (CHEMBL2158125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's m... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393862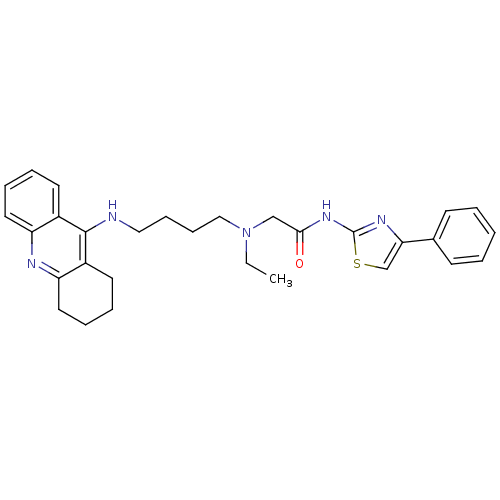 (CHEMBL2158118) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393866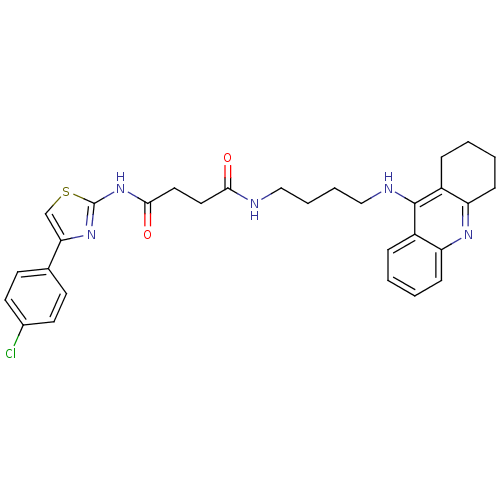 (CHEMBL2158114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393856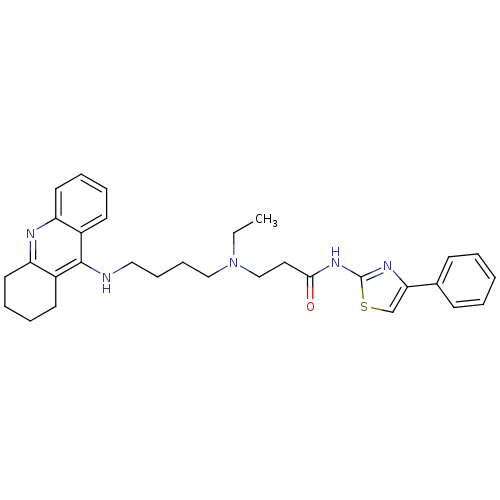 (CHEMBL2158124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393861 (CHEMBL2158119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384894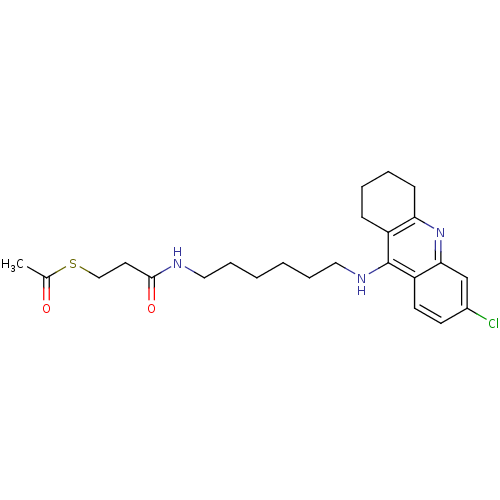 (CHEMBL2036262) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase using acetylcholine chloride as substrate incubated for 5 mins prior to substrate addition measured... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393854 (CHEMBL2158126) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase using acetylcholine chloride as substrate incubated for 5 mins prior to substrate addition measured... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393868 (CHEMBL2158112) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393860 (CHEMBL2158120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50127334 (CHEMBL3628065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393853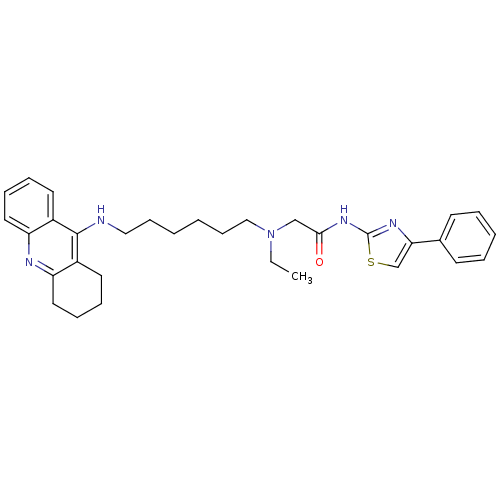 (CHEMBL2158127) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384894 (CHEMBL2036262) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393858 (CHEMBL2158122) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393864 (CHEMBL2158116) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393856 (CHEMBL2158124) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50127381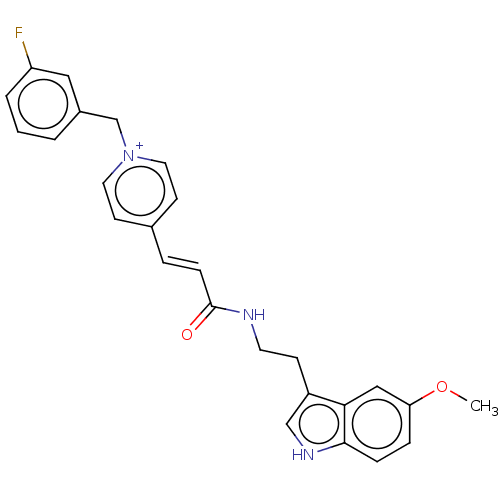 (CHEMBL3628067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's m... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393854 (CHEMBL2158126) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384893 (CHEMBL2036261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384892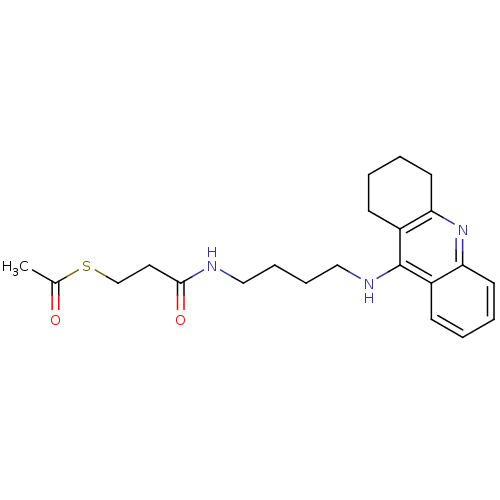 (CHEMBL2036260) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393862 (CHEMBL2158118) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393852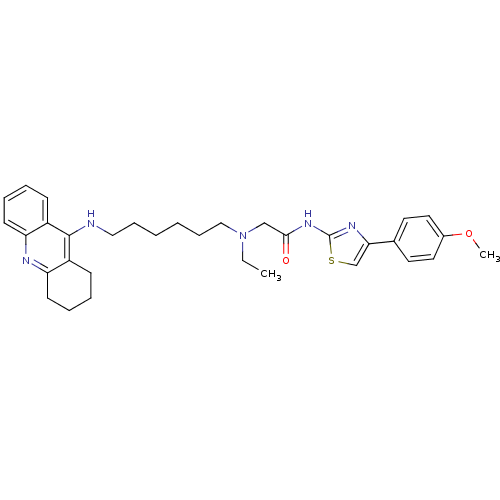 (CHEMBL2158128) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384890 (CHEMBL2036263) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase using acetylcholine chloride as substrate incubated for 5 mins prior to substrate addition measured... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384890 (CHEMBL2036263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384895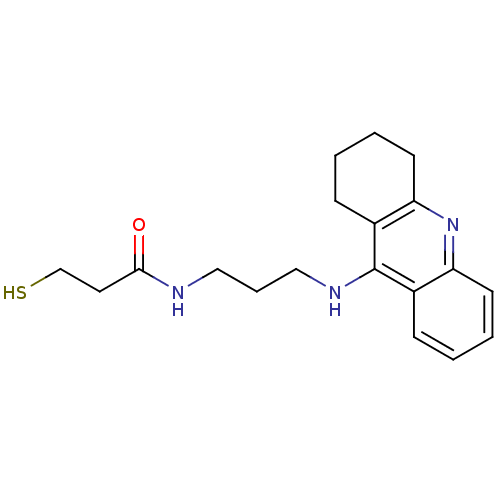 (CHEMBL2036259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50127382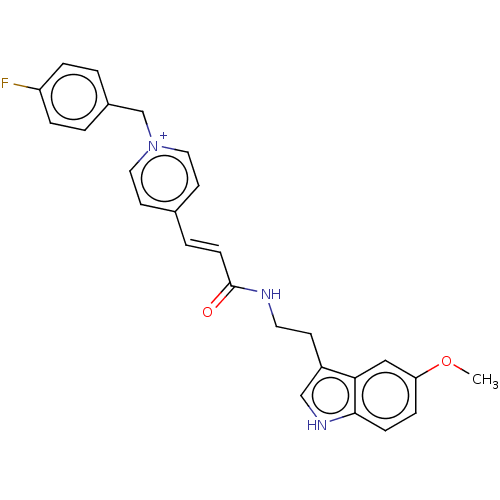 (CHEMBL3628183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's m... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384891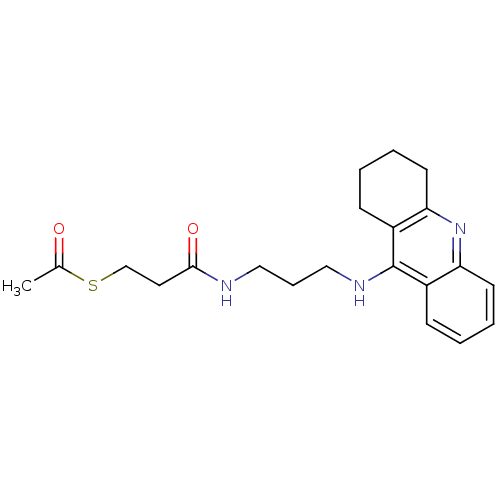 (CHEMBL2036258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50127380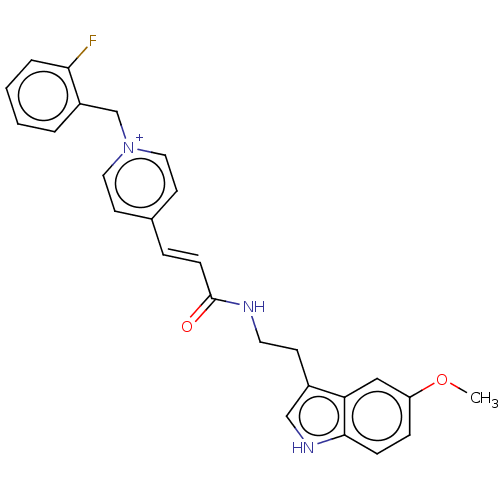 (CHEMBL3628066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's m... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393863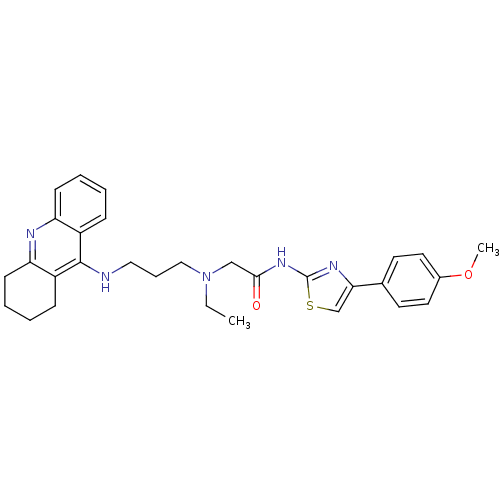 (CHEMBL2158117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50127274 (CHEMBL3628053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393867 (CHEMBL2158113) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393855 (CHEMBL2158125) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393859 (CHEMBL2158121) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50127275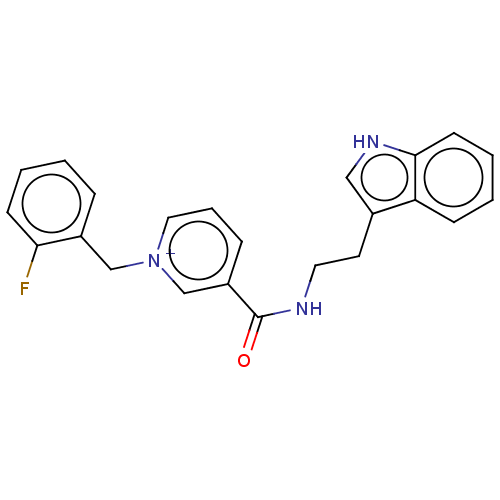 (CHEMBL3628054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393865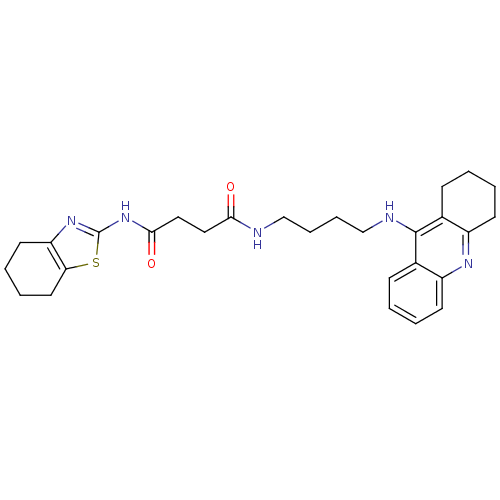 (CHEMBL2158115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393869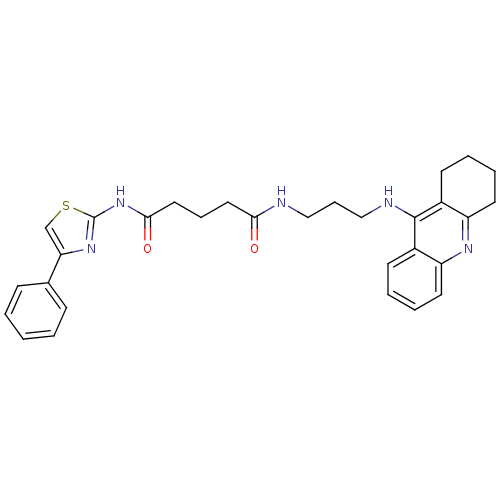 (CHEMBL2158111) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50127393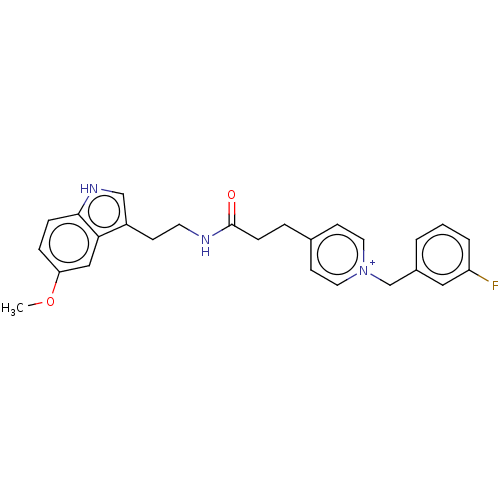 (CHEMBL3628185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
1st Affiliated Hospital of Guangxi Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition by Ellman's m... | Eur J Med Chem 103: 302-11 (2015) Article DOI: 10.1016/j.ejmech.2015.08.052 BindingDB Entry DOI: 10.7270/Q21V5GT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393853 (CHEMBL2158127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393870 (CHEMBL2158110) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |