 Found 412 hits with Last Name = 'wiseman' and Initial = 'j'
Found 412 hits with Last Name = 'wiseman' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415001
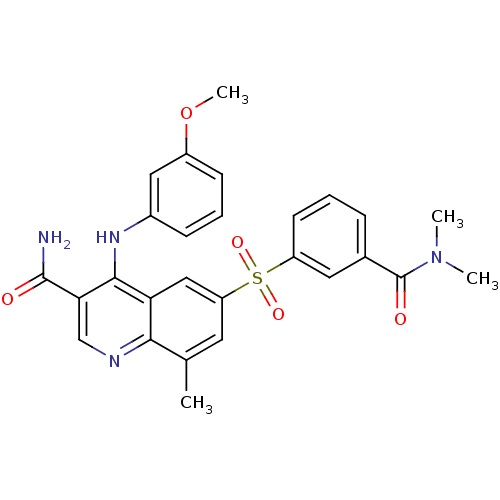
(CHEMBL570015 | GSK-256066 | GSK-256066 (3))Show SMILES COc1cccc(Nc2c(cnc3c(C)cc(cc23)S(=O)(=O)c2cccc(c2)C(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C27H26N4O5S/c1-16-11-21(37(34,35)20-10-5-7-17(12-20)27(33)31(2)3)14-22-24(16)29-15-23(26(28)32)25(22)30-18-8-6-9-19(13-18)36-4/h5-15H,1-4H3,(H2,28,32)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414999

(CHEMBL569791)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2cccc(c2)C(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C26H24N4O5S/c1-30(2)26(32)16-6-4-9-19(12-16)36(33,34)20-10-11-23-21(14-20)24(22(15-28-23)25(27)31)29-17-7-5-8-18(13-17)35-3/h4-15H,1-3H3,(H2,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415009
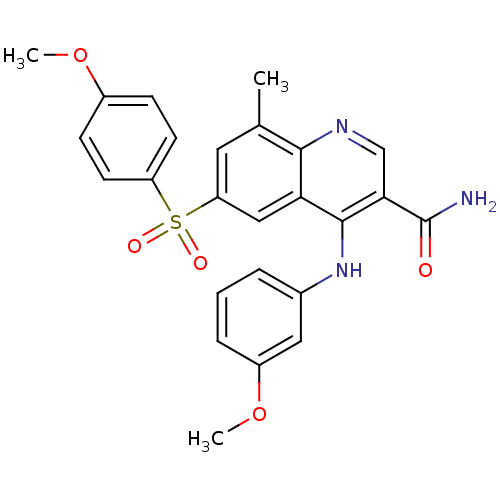
(CHEMBL571381)Show SMILES COc1ccc(cc1)S(=O)(=O)c1cc(C)c2ncc(C(N)=O)c(Nc3cccc(OC)c3)c2c1 Show InChI InChI=1S/C25H23N3O5S/c1-15-11-20(34(30,31)19-9-7-17(32-2)8-10-19)13-21-23(15)27-14-22(25(26)29)24(21)28-16-5-4-6-18(12-16)33-3/h4-14H,1-3H3,(H2,26,29)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415008
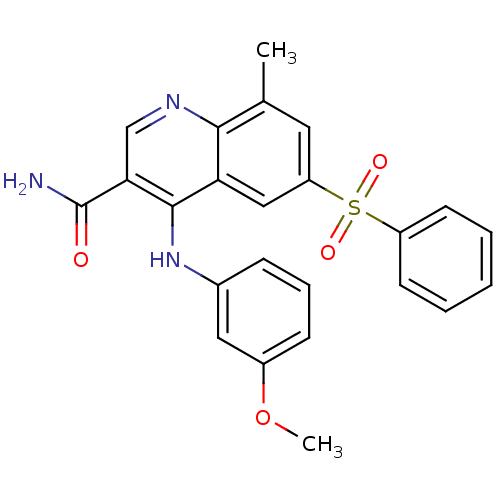
(CHEMBL584327)Show SMILES COc1cccc(Nc2c(cnc3c(C)cc(cc23)S(=O)(=O)c2ccccc2)C(N)=O)c1 Show InChI InChI=1S/C24H21N3O4S/c1-15-11-19(32(29,30)18-9-4-3-5-10-18)13-20-22(15)26-14-21(24(25)28)23(20)27-16-7-6-8-17(12-16)31-2/h3-14H,1-2H3,(H2,25,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414998
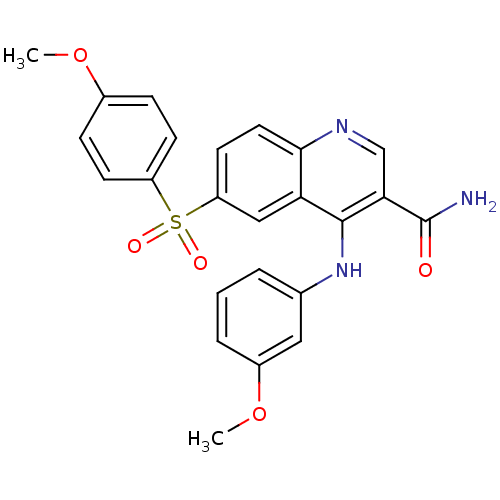
(CHEMBL569556)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc2ncc(C(N)=O)c(Nc3cccc(OC)c3)c2c1 Show InChI InChI=1S/C24H21N3O5S/c1-31-16-6-8-18(9-7-16)33(29,30)19-10-11-22-20(13-19)23(21(14-26-22)24(25)28)27-15-4-3-5-17(12-15)32-2/h3-14H,1-2H3,(H2,25,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297677
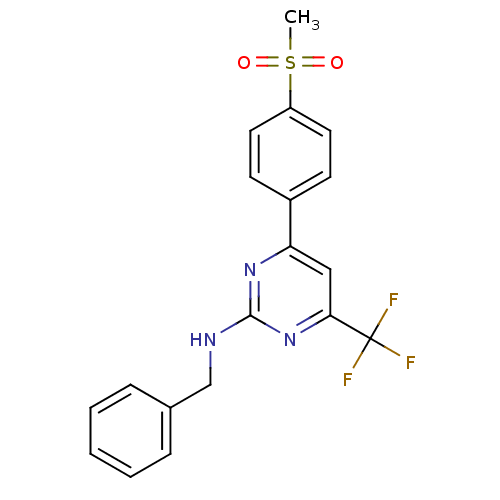
(CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O2S/c1-28(26,27)15-9-7-14(8-10-15)16-11-17(19(20,21)22)25-18(24-16)23-12-13-5-3-2-4-6-13/h2-11H,12H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297675
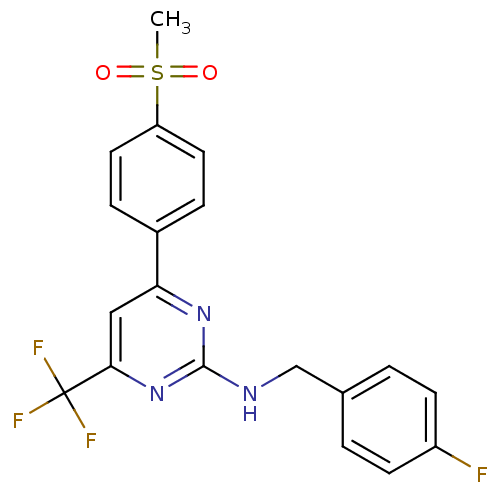
(CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-2-6-14(20)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413296
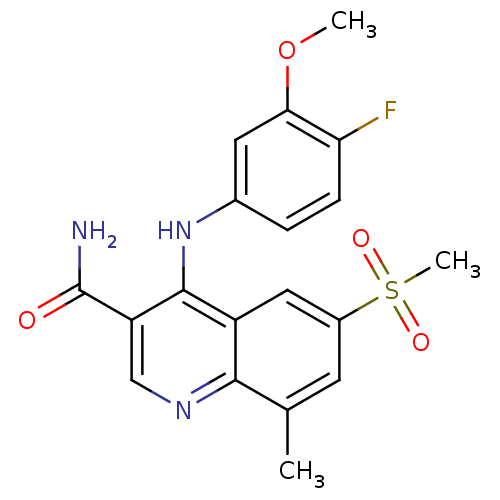
(CHEMBL473764)Show SMILES COc1cc(Nc2c(cnc3c(C)cc(cc23)S(C)(=O)=O)C(N)=O)ccc1F Show InChI InChI=1S/C19H18FN3O4S/c1-10-6-12(28(3,25)26)8-13-17(10)22-9-14(19(21)24)18(13)23-11-4-5-15(20)16(7-11)27-2/h4-9H,1-3H3,(H2,21,24)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413297
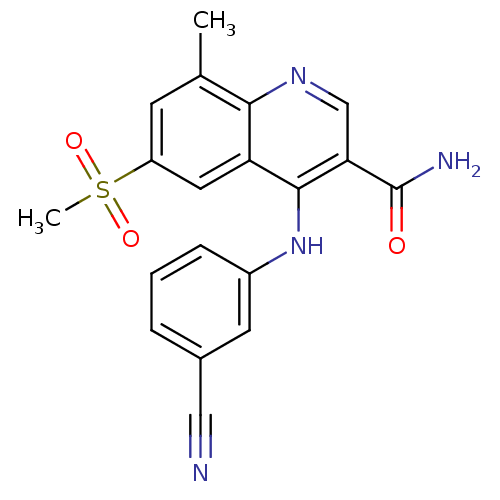
(CHEMBL473560)Show SMILES Cc1cc(cc2c(Nc3cccc(c3)C#N)c(cnc12)C(N)=O)S(C)(=O)=O Show InChI InChI=1S/C19H16N4O3S/c1-11-6-14(27(2,25)26)8-15-17(11)22-10-16(19(21)24)18(15)23-13-5-3-4-12(7-13)9-20/h3-8,10H,1-2H3,(H2,21,24)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50153990
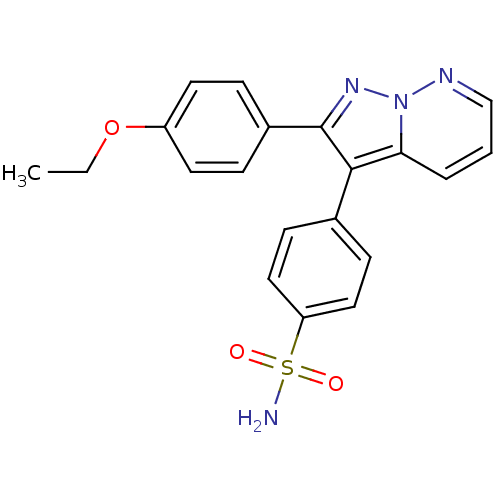
(4-[2-(4-Ethoxy-phenyl)-pyrazolo[1,5-b]pyridazin-3-...)Show SMILES CCOc1ccc(cc1)-c1nn2ncccc2c1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H18N4O3S/c1-2-27-16-9-5-15(6-10-16)20-19(18-4-3-13-22-24(18)23-20)14-7-11-17(12-8-14)28(21,25)26/h3-13H,2H2,1H3,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 expressed in COS cells |
Bioorg Med Chem Lett 14: 5445-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.089
BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297669
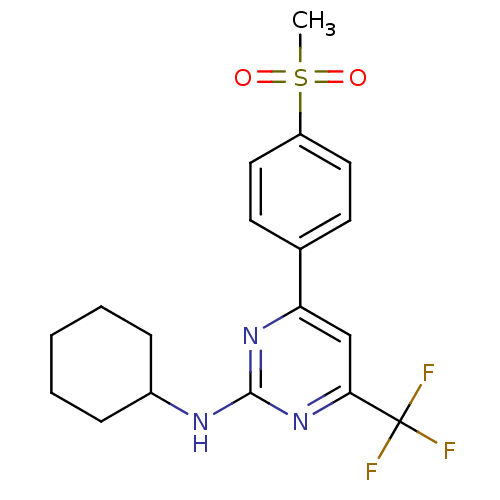
(CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NC2CCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O2S/c1-27(25,26)14-9-7-12(8-10-14)15-11-16(18(19,20)21)24-17(23-15)22-13-5-3-2-4-6-13/h7-11,13H,2-6H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415007
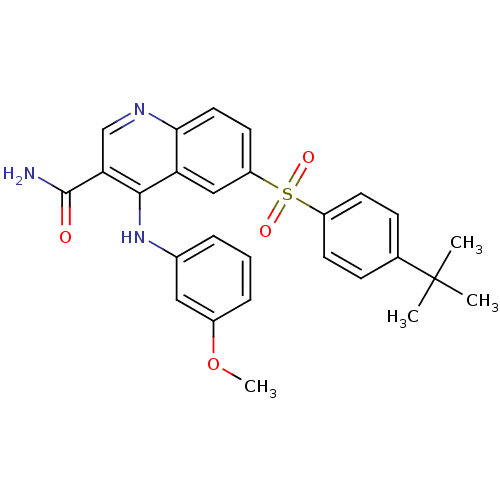
(CHEMBL571171)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccc(cc2)C(C)(C)C)C(N)=O)c1 Show InChI InChI=1S/C27H27N3O4S/c1-27(2,3)17-8-10-20(11-9-17)35(32,33)21-12-13-24-22(15-21)25(23(16-29-24)26(28)31)30-18-6-5-7-19(14-18)34-4/h5-16H,1-4H3,(H2,28,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414992
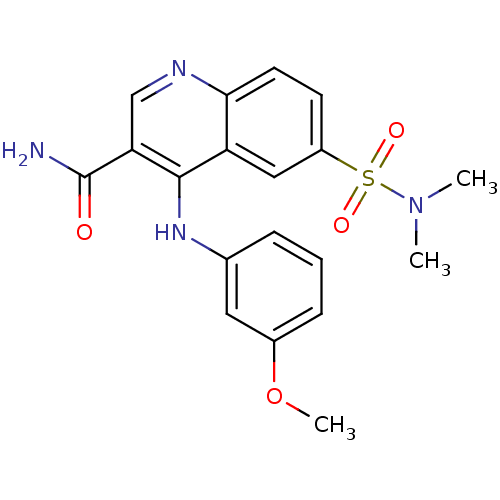
(CHEMBL576479)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C19H20N4O4S/c1-23(2)28(25,26)14-7-8-17-15(10-14)18(16(11-21-17)19(20)24)22-12-5-4-6-13(9-12)27-3/h4-11H,1-3H3,(H2,20,24)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221760

(US9314468, Table 7, Compound 47)Show SMILES CC(C)(C)OC(=O)Cn1c2ccccc2c2ccnc(CN(CCCCN)[C@H]3CCCc4cccnc34)c12 |r| Show InChI InChI=1S/C31H39N5O2/c1-31(2,3)38-28(37)21-36-26-13-5-4-12-23(26)24-15-18-33-25(30(24)36)20-35(19-7-6-16-32)27-14-8-10-22-11-9-17-34-29(22)27/h4-5,9,11-13,15,17-18,27H,6-8,10,14,16,19-21,32H2,1-3H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221860
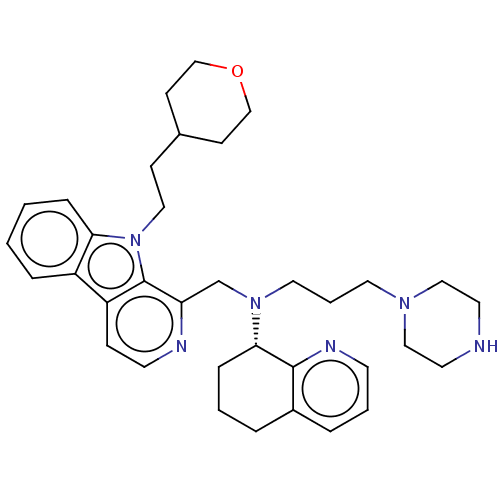
(US9314468, Table 7, Compound 147)Show SMILES C(CN(Cc1nccc2c3ccccc3n(CCC3CCOCC3)c12)[C@H]1CCCc2cccnc12)CN1CCNCC1 |r| Show InChI InChI=1S/C35H46N6O/c1-2-9-32-29(8-1)30-11-16-37-31(35(30)41(32)21-12-27-13-24-42-25-14-27)26-40(20-5-19-39-22-17-36-18-23-39)33-10-3-6-28-7-4-15-38-34(28)33/h1-2,4,7-9,11,15-16,27,33,36H,3,5-6,10,12-14,17-26H2/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297672
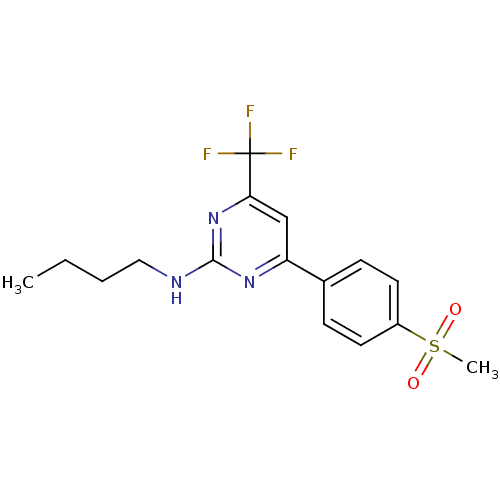
(CHEMBL549393 | N-butyl-4-(4-(methylsulfonyl)phenyl...)Show SMILES CCCCNc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C16H18F3N3O2S/c1-3-4-9-20-15-21-13(10-14(22-15)16(17,18)19)11-5-7-12(8-6-11)25(2,23)24/h5-8,10H,3-4,9H2,1-2H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297671
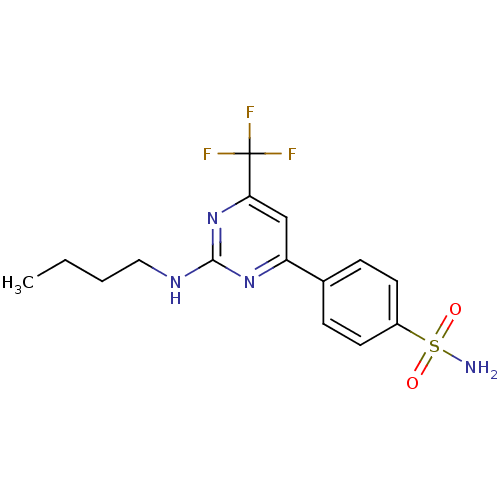
(4-(2-(butylamino)-6-(trifluoromethyl)pyrimidin-4-y...)Show SMILES CCCCNc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H17F3N4O2S/c1-2-3-8-20-14-21-12(9-13(22-14)15(16,17)18)10-4-6-11(7-5-10)25(19,23)24/h4-7,9H,2-3,8H2,1H3,(H2,19,23,24)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413291
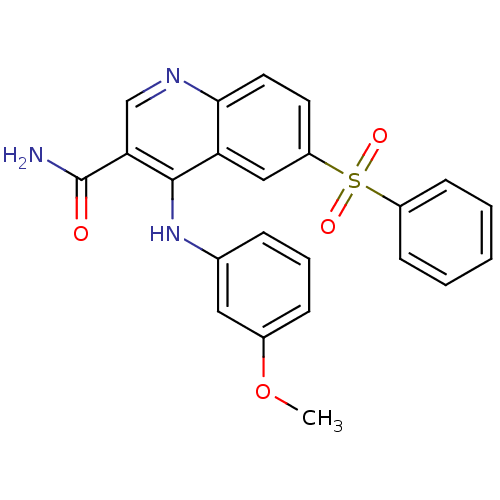
(CHEMBL515240)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccccc2)C(N)=O)c1 Show InChI InChI=1S/C23H19N3O4S/c1-30-16-7-5-6-15(12-16)26-22-19-13-18(31(28,29)17-8-3-2-4-9-17)10-11-21(19)25-14-20(22)23(24)27/h2-14H,1H3,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413291

(CHEMBL515240)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccccc2)C(N)=O)c1 Show InChI InChI=1S/C23H19N3O4S/c1-30-16-7-5-6-15(12-16)26-22-19-13-18(31(28,29)17-8-3-2-4-9-17)10-11-21(19)25-14-20(22)23(24)27/h2-14H,1H3,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415000
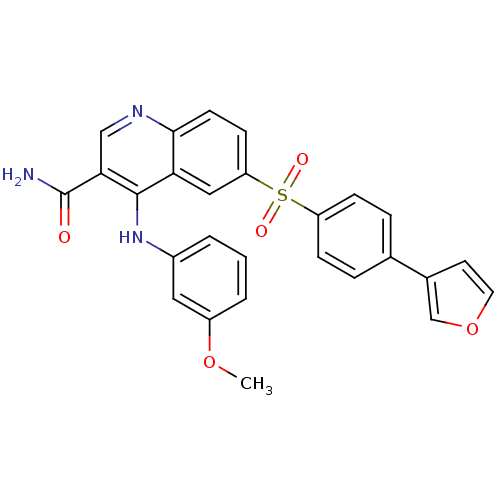
(CHEMBL570029)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccc(cc2)-c2ccoc2)C(N)=O)c1 Show InChI InChI=1S/C27H21N3O5S/c1-34-20-4-2-3-19(13-20)30-26-23-14-22(9-10-25(23)29-15-24(26)27(28)31)36(32,33)21-7-5-17(6-8-21)18-11-12-35-16-18/h2-16H,1H3,(H2,28,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50153994
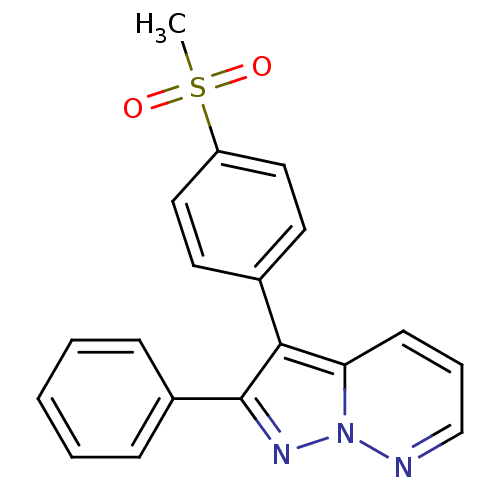
(3-(4-Methanesulfonyl-phenyl)-2-phenyl-pyrazolo[1,5...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1c(nn2ncccc12)-c1ccccc1 Show InChI InChI=1S/C19H15N3O2S/c1-25(23,24)16-11-9-14(10-12-16)18-17-8-5-13-20-22(17)21-19(18)15-6-3-2-4-7-15/h2-13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 expressed in COS cells |
Bioorg Med Chem Lett 14: 5445-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.089
BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415004
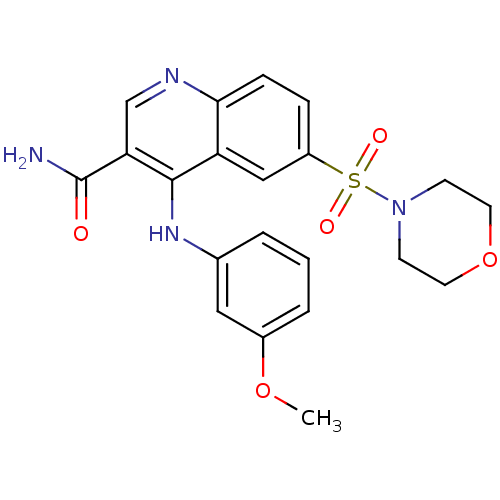
(CHEMBL571593)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)N2CCOCC2)C(N)=O)c1 Show InChI InChI=1S/C21H22N4O5S/c1-29-15-4-2-3-14(11-15)24-20-17-12-16(31(27,28)25-7-9-30-10-8-25)5-6-19(17)23-13-18(20)21(22)26/h2-6,11-13H,7-10H2,1H3,(H2,22,26)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029600
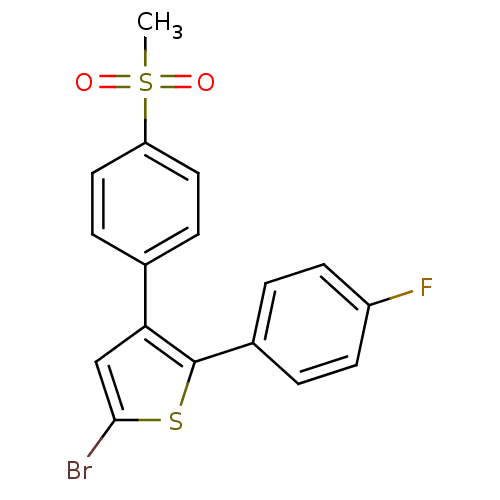
(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... |
Bioorg Med Chem Lett 19: 4509-14 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.089
BindingDB Entry DOI: 10.7270/Q2W66KSC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297670
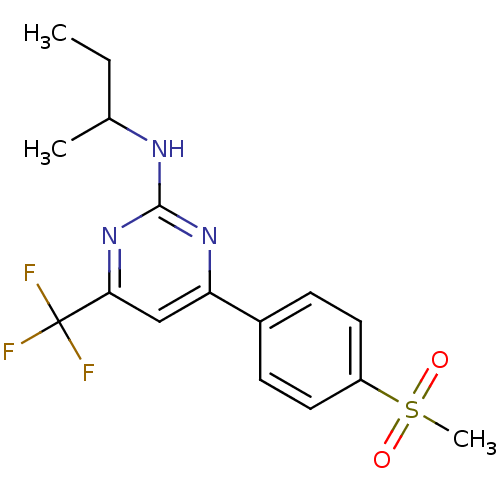
(CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...)Show SMILES CCC(C)Nc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C16H18F3N3O2S/c1-4-10(2)20-15-21-13(9-14(22-15)16(17,18)19)11-5-7-12(8-6-11)25(3,23)24/h5-10H,4H2,1-3H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297668
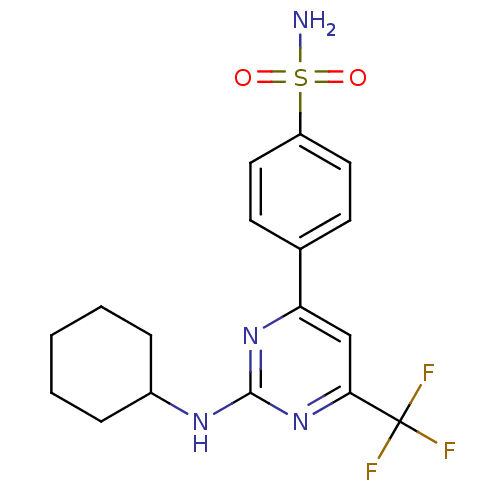
(4-(2-(cyclohexylamino)-6-(trifluoromethyl)pyrimidi...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(nc(NC2CCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O2S/c18-17(19,20)15-10-14(11-6-8-13(9-7-11)27(21,25)26)23-16(24-15)22-12-4-2-1-3-5-12/h6-10,12H,1-5H2,(H2,21,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415006
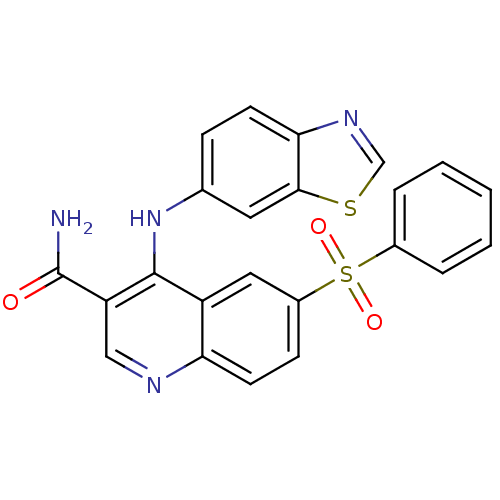
(CHEMBL585528)Show SMILES NC(=O)c1cnc2ccc(cc2c1Nc1ccc2ncsc2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C23H16N4O3S2/c24-23(28)18-12-25-19-9-7-16(32(29,30)15-4-2-1-3-5-15)11-17(19)22(18)27-14-6-8-20-21(10-14)31-13-26-20/h1-13H,(H2,24,28)(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413286
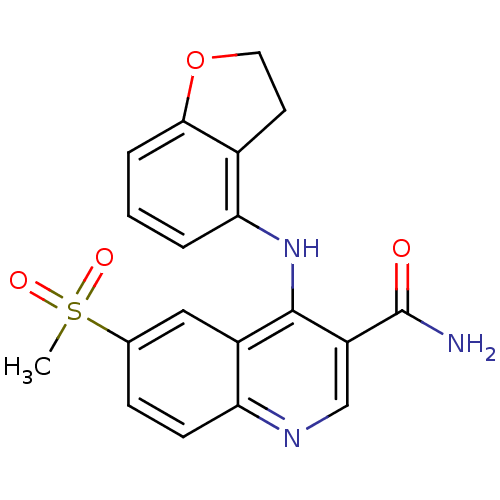
(CHEMBL517766)Show SMILES CS(=O)(=O)c1ccc2ncc(C(N)=O)c(Nc3cccc4OCCc34)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-27(24,25)11-5-6-15-13(9-11)18(14(10-21-15)19(20)23)22-16-3-2-4-17-12(16)7-8-26-17/h2-6,9-10H,7-8H2,1H3,(H2,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297664
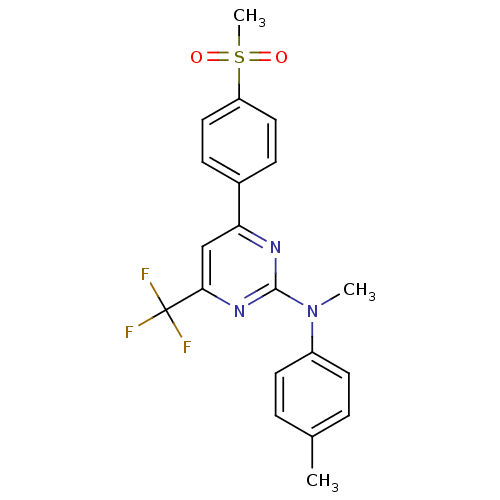
(CHEMBL559613 | N-methyl-4-(4-(methylsulfonyl)pheny...)Show SMILES CN(c1ccc(C)cc1)c1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H18F3N3O2S/c1-13-4-8-15(9-5-13)26(2)19-24-17(12-18(25-19)20(21,22)23)14-6-10-16(11-7-14)29(3,27)28/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50153982
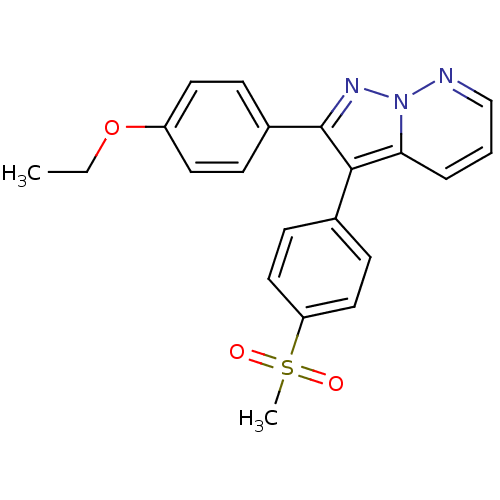
(2-(4-Ethoxy-phenyl)-3-(4-methanesulfonyl-phenyl)-p...)Show SMILES CCOc1ccc(cc1)-c1nn2ncccc2c1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H19N3O3S/c1-3-27-17-10-6-16(7-11-17)21-20(19-5-4-14-22-24(19)23-21)15-8-12-18(13-9-15)28(2,25)26/h4-14H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 expressed in COS cells |
Bioorg Med Chem Lett 14: 5445-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.089
BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50412414

(CHEMBL521203)Show SMILES CCn1ncc2c(NC3CCOCC3)c(cnc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C21H25N5O2/c1-2-26-20-17(14-24-26)19(25-16-8-10-28-11-9-16)18(13-22-20)21(27)23-12-15-6-4-3-5-7-15/h3-7,13-14,16H,2,8-12H2,1H3,(H,22,25)(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B in Sf9 cells |
Bioorg Med Chem Lett 18: 4237-41 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.052
BindingDB Entry DOI: 10.7270/Q2ZP47B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414989
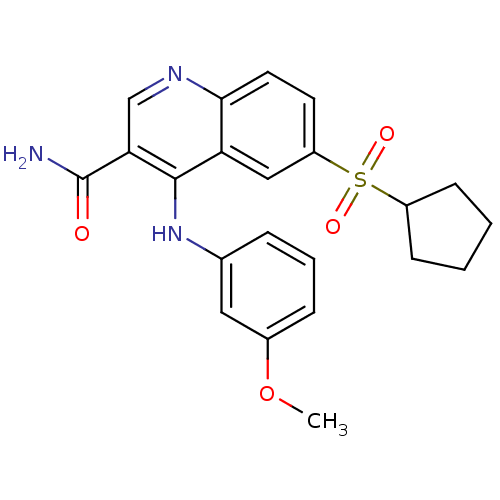
(CHEMBL585937)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)C2CCCC2)C(N)=O)c1 Show InChI InChI=1S/C22H23N3O4S/c1-29-15-6-4-5-14(11-15)25-21-18-12-17(30(27,28)16-7-2-3-8-16)9-10-20(18)24-13-19(21)22(23)26/h4-6,9-13,16H,2-3,7-8H2,1H3,(H2,23,26)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414994
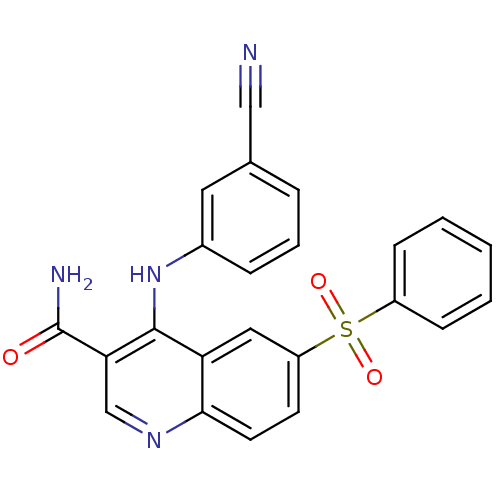
(CHEMBL571386)Show SMILES NC(=O)c1cnc2ccc(cc2c1Nc1cccc(c1)C#N)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C23H16N4O3S/c24-13-15-5-4-6-16(11-15)27-22-19-12-18(31(29,30)17-7-2-1-3-8-17)9-10-21(19)26-14-20(22)23(25)28/h1-12,14H,(H2,25,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413298
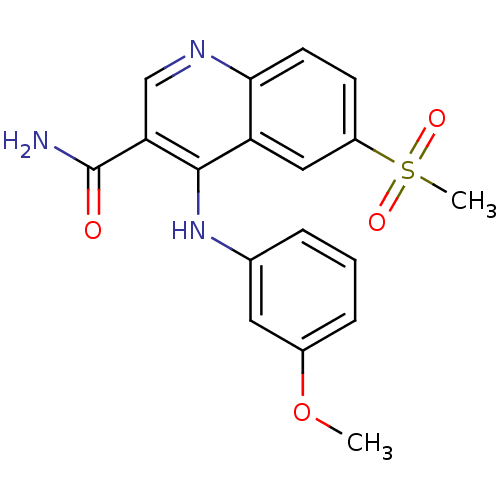
(CHEMBL462150)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(C)(=O)=O)C(N)=O)c1 Show InChI InChI=1S/C18H17N3O4S/c1-25-12-5-3-4-11(8-12)21-17-14-9-13(26(2,23)24)6-7-16(14)20-10-15(17)18(19)22/h3-10H,1-2H3,(H2,19,22)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413298

(CHEMBL462150)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(C)(=O)=O)C(N)=O)c1 Show InChI InChI=1S/C18H17N3O4S/c1-25-12-5-3-4-11(8-12)21-17-14-9-13(26(2,23)24)6-7-16(14)20-10-15(17)18(19)22/h3-10H,1-2H3,(H2,19,22)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221796

(US9314468, Table 7, Compound 83)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CCN3CCOCC3)c12)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C31H40N6O/c32-13-3-4-16-36(29-11-5-7-24-8-6-14-34-30(24)29)23-27-31-26(12-15-33-27)25-9-1-2-10-28(25)37(31)18-17-35-19-21-38-22-20-35/h1-2,6,8-10,12,14-15,29H,3-5,7,11,13,16-23,32H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221857
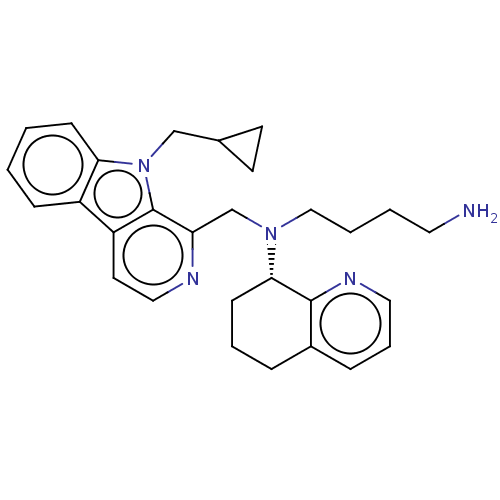
(US9314468, Table 7, Compound 144)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CC3CC3)c12)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C29H35N5/c30-15-3-4-18-33(27-11-5-7-22-8-6-16-32-28(22)27)20-25-29-24(14-17-31-25)23-9-1-2-10-26(23)34(29)19-21-12-13-21/h1-2,6,8-10,14,16-17,21,27H,3-5,7,11-13,15,18-20,30H2/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221834
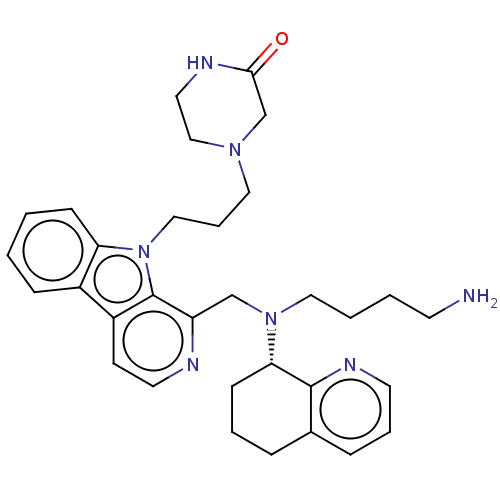
(US9314468, Table 7, Compound 121)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CCCN3CCNC(=O)C3)c12)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C32H41N7O/c33-14-3-4-19-38(29-12-5-8-24-9-6-15-36-31(24)29)22-27-32-26(13-16-34-27)25-10-1-2-11-28(25)39(32)20-7-18-37-21-17-35-30(40)23-37/h1-2,6,9-11,13,15-16,29H,3-5,7-8,12,14,17-23,33H2,(H,35,40)/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221854

(US9314468, Table 7, Compound 141)Show SMILES NCCCn1c2ccccc2c2ccnc(CN(CCCN3CCNCC3)[C@H]3CCCc4cccnc34)c12 |r| Show InChI InChI=1S/C31H41N7/c32-13-5-20-38-28-10-2-1-9-25(28)26-12-15-34-27(31(26)38)23-37(19-6-18-36-21-16-33-17-22-36)29-11-3-7-24-8-4-14-35-30(24)29/h1-2,4,8-10,12,14-15,29,33H,3,5-7,11,13,16-23,32H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297673
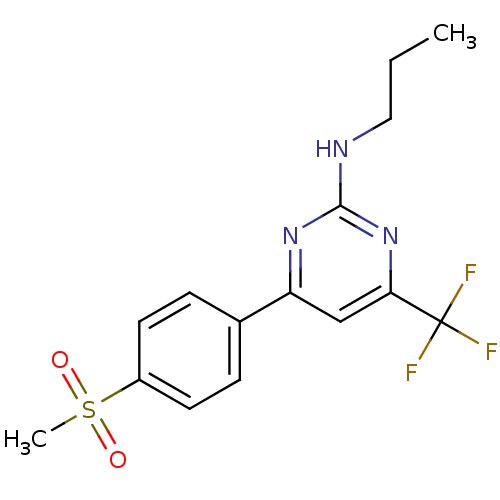
(4-(4-(methylsulfonyl)phenyl)-N-propyl-6-(trifluoro...)Show SMILES CCCNc1nc(cc(n1)C(F)(F)F)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C15H16F3N3O2S/c1-3-8-19-14-20-12(9-13(21-14)15(16,17)18)10-4-6-11(7-5-10)24(2,22)23/h4-7,9H,3,8H2,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297665
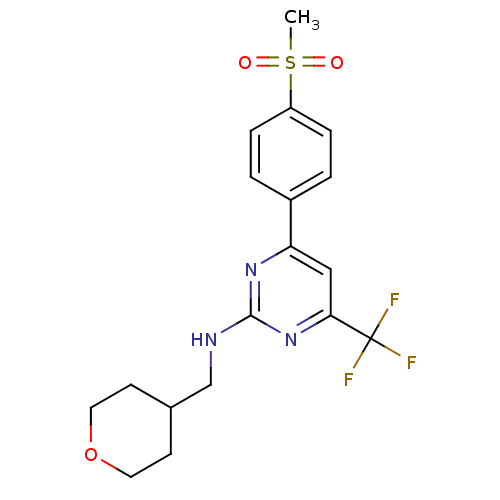
(4-(4-(methylsulfonyl)phenyl)-N-((tetrahydro-2H-pyr...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCC2CCOCC2)n1)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O3S/c1-28(25,26)14-4-2-13(3-5-14)15-10-16(18(19,20)21)24-17(23-15)22-11-12-6-8-27-9-7-12/h2-5,10,12H,6-9,11H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413306
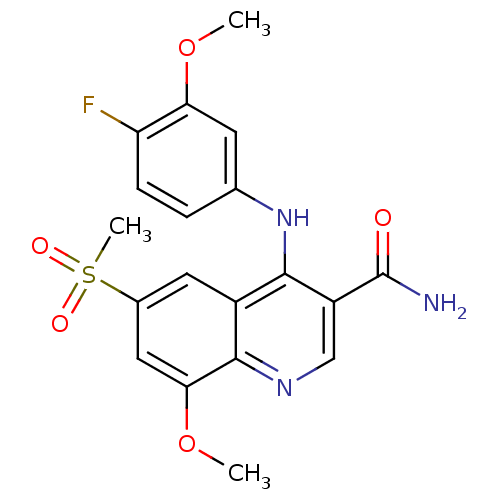
(CHEMBL515098)Show SMILES COc1cc(Nc2c(cnc3c(OC)cc(cc23)S(C)(=O)=O)C(N)=O)ccc1F Show InChI InChI=1S/C19H18FN3O5S/c1-27-15-6-10(4-5-14(15)20)23-17-12-7-11(29(3,25)26)8-16(28-2)18(12)22-9-13(17)19(21)24/h4-9H,1-3H3,(H2,21,24)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221864

(US9314468, Table 8, Compound 2)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CC#C)c12)C1CCCc2cccnc12 Show InChI InChI=1S/C28H31N5/c1-2-18-33-25-12-4-3-11-22(25)23-14-17-30-24(28(23)33)20-32(19-6-5-15-29)26-13-7-9-21-10-8-16-31-27(21)26/h1,3-4,8,10-12,14,16-17,26H,5-7,9,13,15,18-20,29H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
This assay measures the change in impedance that occurs when cells are stimulated with SDF-1a. Changes in shape and cytoskeleton result in a change o... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297680
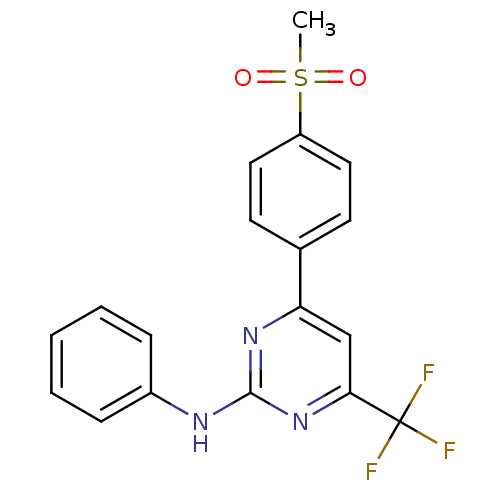
(4-(4-(methylsulfonyl)phenyl)-N-phenyl-6-(trifluoro...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(Nc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C18H14F3N3O2S/c1-27(25,26)14-9-7-12(8-10-14)15-11-16(18(19,20)21)24-17(23-15)22-13-5-3-2-4-6-13/h2-11H,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221865
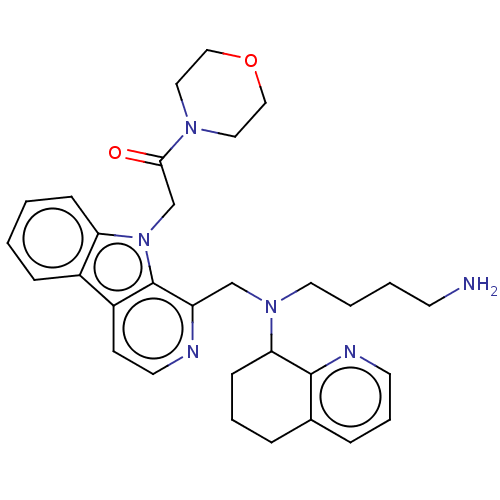
(US9314468, Table 8, Compound 3)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CC(=O)N3CCOCC3)c12)C1CCCc2cccnc12 Show InChI InChI=1S/C31H38N6O2/c32-13-3-4-16-36(28-11-5-7-23-8-6-14-34-30(23)28)21-26-31-25(12-15-33-26)24-9-1-2-10-27(24)37(31)22-29(38)35-17-19-39-20-18-35/h1-2,6,8-10,12,14-15,28H,3-5,7,11,13,16-22,32H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
This assay measures the change in impedance that occurs when cells are stimulated with SDF-1a. Changes in shape and cytoskeleton result in a change o... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221863
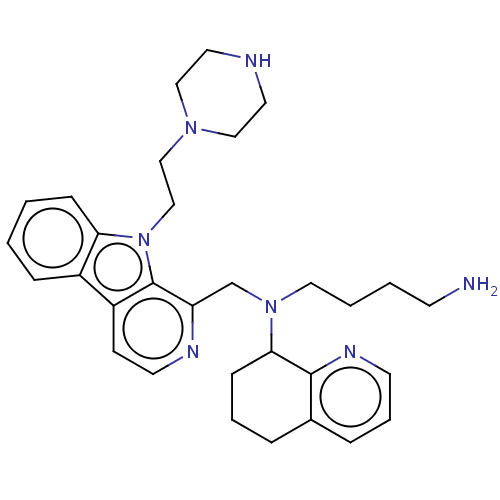
(US9314468, Table 8, Compound 1)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CCN3CCNCC3)c12)C1CCCc2cccnc12 Show InChI InChI=1S/C31H41N7/c32-13-3-4-18-37(29-11-5-7-24-8-6-14-35-30(24)29)23-27-31-26(12-15-34-27)25-9-1-2-10-28(25)38(31)22-21-36-19-16-33-17-20-36/h1-2,6,8-10,12,14-15,29,33H,3-5,7,11,13,16-23,32H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
This assay measures the change in impedance that occurs when cells are stimulated with SDF-1a. Changes in shape and cytoskeleton result in a change o... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM221866

(US9314468, Table 8, Compound 4)Show SMILES NCCCCN(Cc1nccc2c3ccccc3n(CC(N)=O)c12)C1CCCc2cccnc12 Show InChI InChI=1S/C27H32N6O/c28-13-3-4-16-32(24-11-5-7-19-8-6-14-31-26(19)24)17-22-27-21(12-15-30-22)20-9-1-2-10-23(20)33(27)18-25(29)34/h1-2,6,8-10,12,14-15,24H,3-5,7,11,13,16-18,28H2,(H2,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Altiris Therapeutics, Inc.
US Patent
| Assay Description
This assay measures the change in impedance that occurs when cells are stimulated with SDF-1a. Changes in shape and cytoskeleton result in a change o... |
US Patent US9314468 (2016)
BindingDB Entry DOI: 10.7270/Q2DF6Q2P |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413288
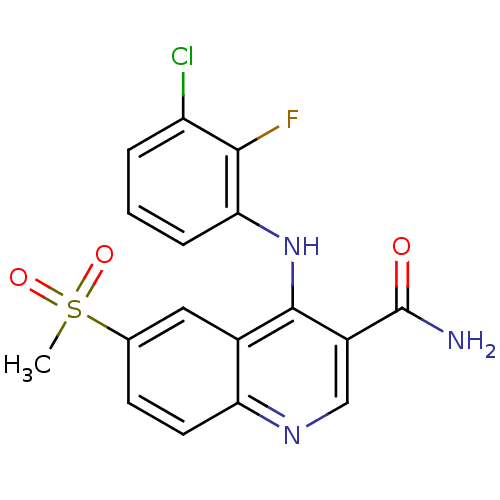
(CHEMBL517590)Show SMILES CS(=O)(=O)c1ccc2ncc(C(N)=O)c(Nc3cccc(Cl)c3F)c2c1 Show InChI InChI=1S/C17H13ClFN3O3S/c1-26(24,25)9-5-6-13-10(7-9)16(11(8-21-13)17(20)23)22-14-4-2-3-12(18)15(14)19/h2-8H,1H3,(H2,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1380-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.045
BindingDB Entry DOI: 10.7270/Q2FJ2J0J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415005
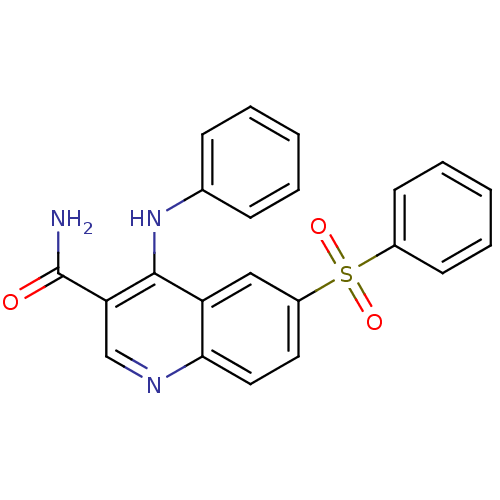
(CHEMBL569352)Show SMILES NC(=O)c1cnc2ccc(cc2c1Nc1ccccc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C22H17N3O3S/c23-22(26)19-14-24-20-12-11-17(29(27,28)16-9-5-2-6-10-16)13-18(20)21(19)25-15-7-3-1-4-8-15/h1-14H,(H2,23,26)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data