

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051007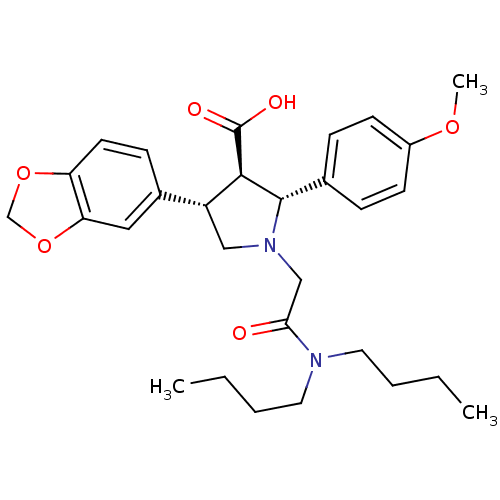 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50050976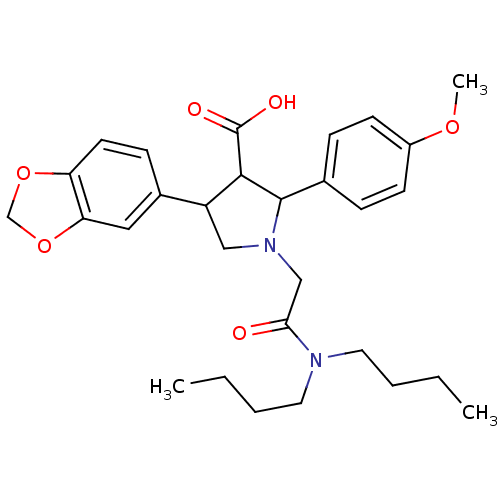 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50050964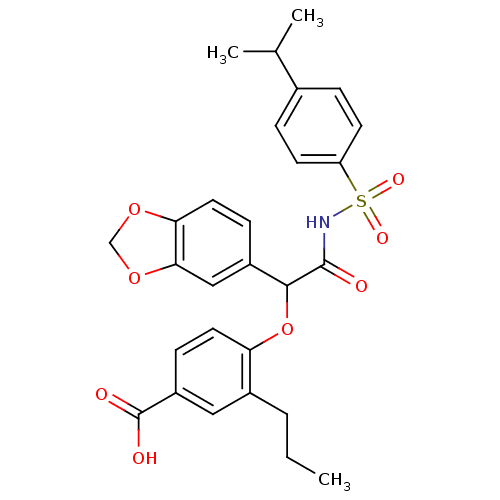 (4-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50066370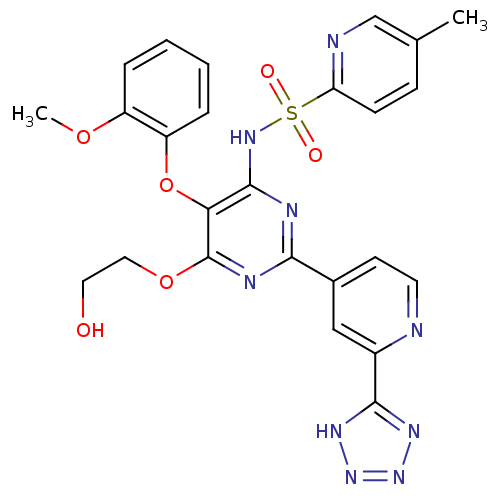 (5-Methyl-pyridine-2-sulfonic acid {6-(2-hydroxy-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) | J Med Chem 41: 3261-75 (1998) Article DOI: 10.1021/jm980217s BindingDB Entry DOI: 10.7270/Q20Z72D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267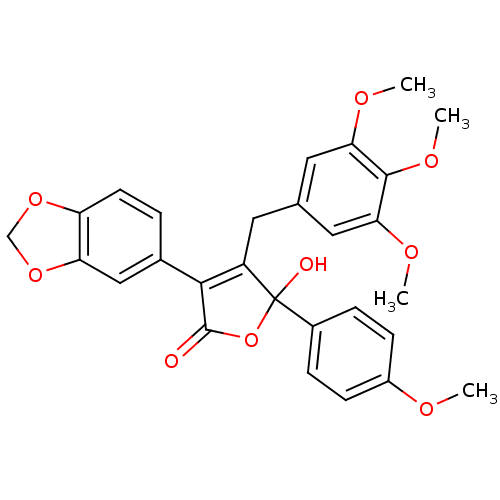 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31592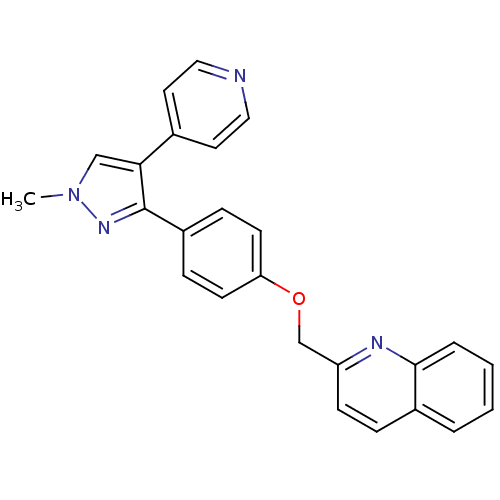 (PF-2545920 | US9138494, MP-10 | substituted pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034263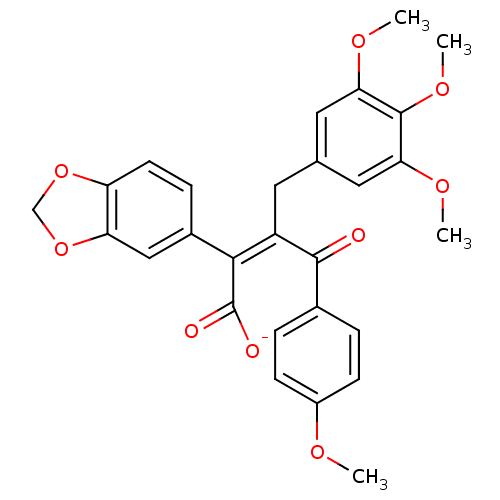 (CHEMBL25438 | PD-156707 | Sodium; (Z)-2-benzo[1,3]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) | J Med Chem 41: 3261-75 (1998) Article DOI: 10.1021/jm980217s BindingDB Entry DOI: 10.7270/Q20Z72D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50041617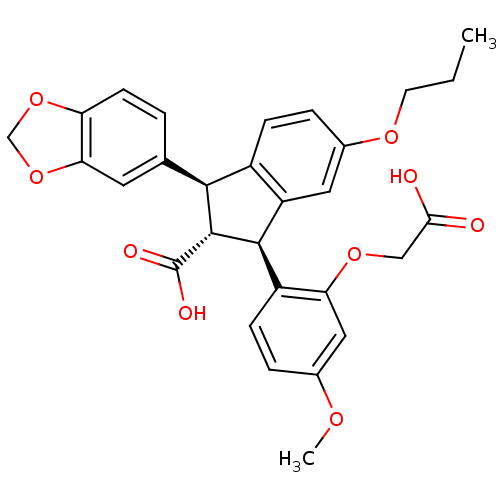 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50066398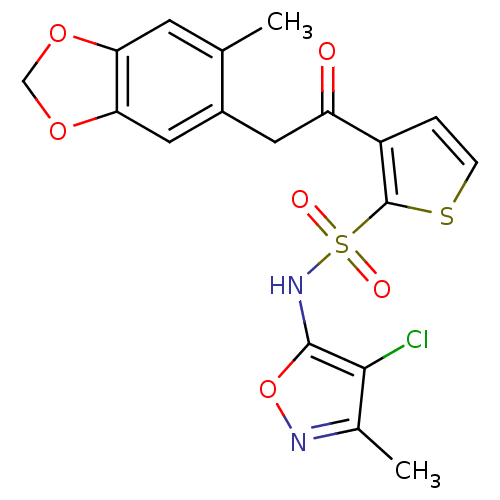 (3-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) | J Med Chem 41: 3261-75 (1998) Article DOI: 10.1021/jm980217s BindingDB Entry DOI: 10.7270/Q20Z72D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50066391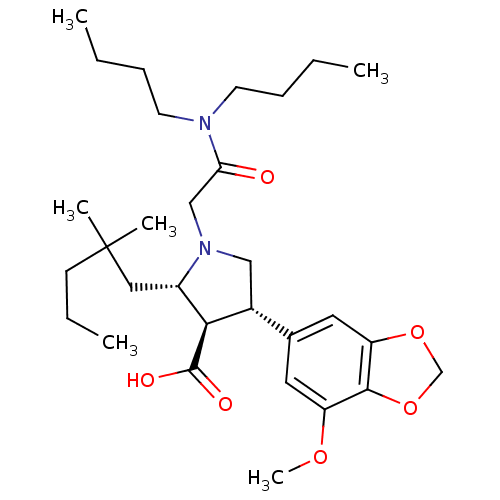 ((2S,3R,4S)-1-Dibutylcarbamoylmethyl-2-(2,2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) | J Med Chem 41: 3261-75 (1998) Article DOI: 10.1021/jm980217s BindingDB Entry DOI: 10.7270/Q20Z72D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065428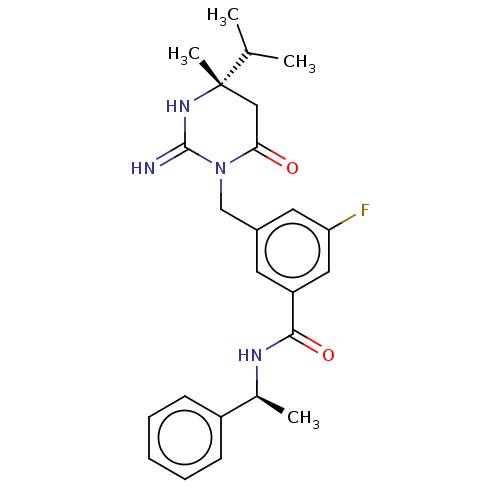 (CHEMBL3401350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50061077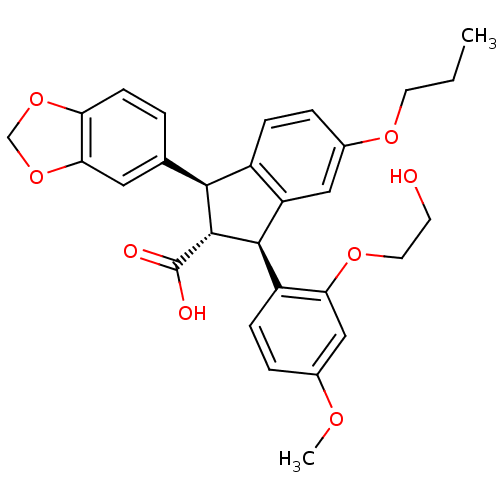 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) | J Med Chem 41: 3261-75 (1998) Article DOI: 10.1021/jm980217s BindingDB Entry DOI: 10.7270/Q20Z72D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM50365964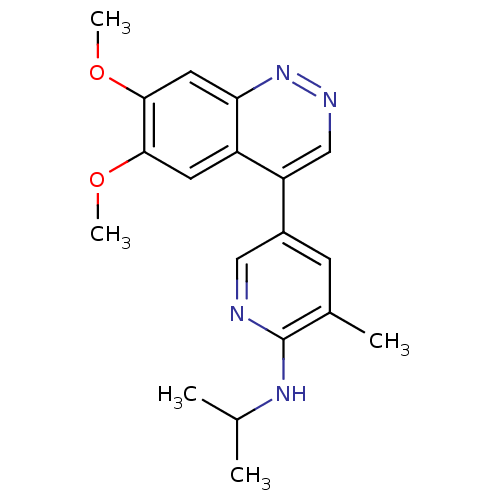 (CHEMBL1956235 | CHEMBL2070530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156461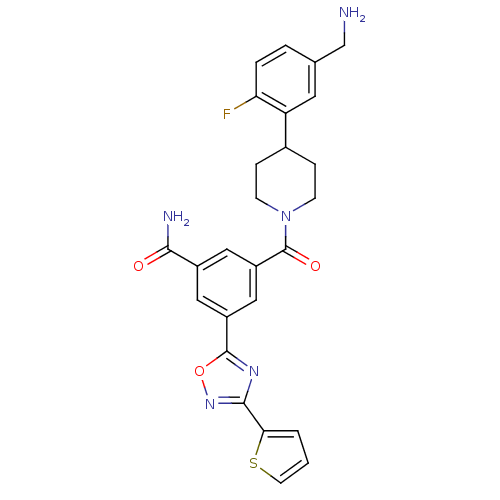 (3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156460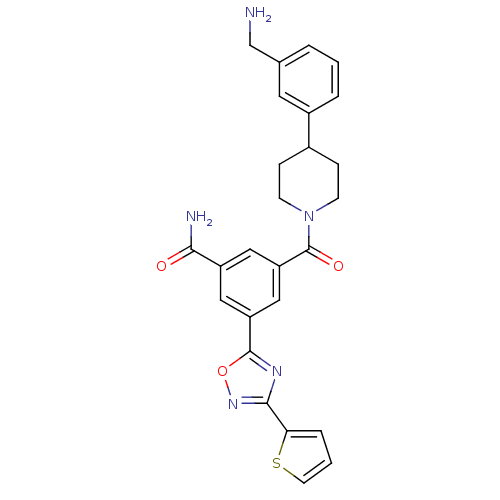 (3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065395 (CHEMBL3401345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065426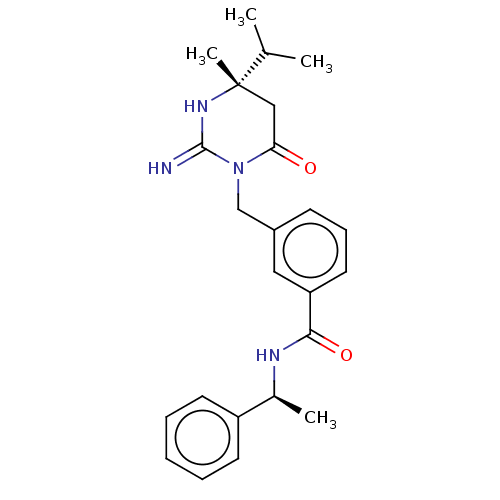 (CHEMBL3401348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM22868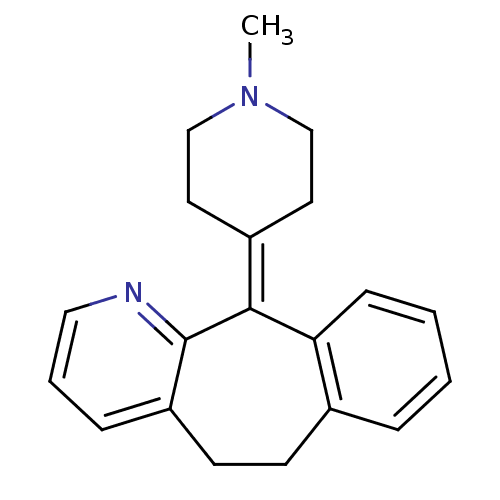 (11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5H-b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor in rat brain membranes was evaluated using [3H]-pyrilamine as radioligand | J Med Chem 34: 457-61 (1991) BindingDB Entry DOI: 10.7270/Q2BV7FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50156457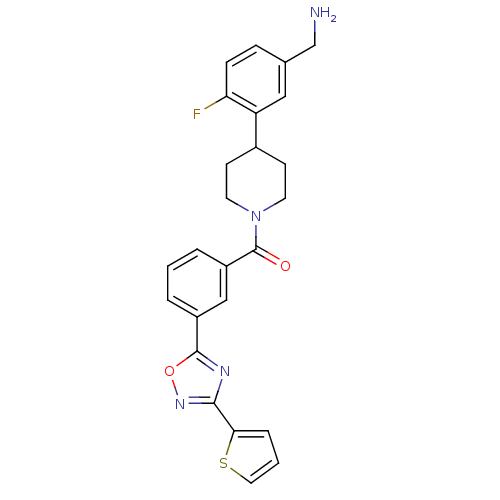 (CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human mast cell tryptase beta | Bioorg Med Chem Lett 14: 6053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.065 BindingDB Entry DOI: 10.7270/Q2KH0MST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50050964 (4-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin B receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM35938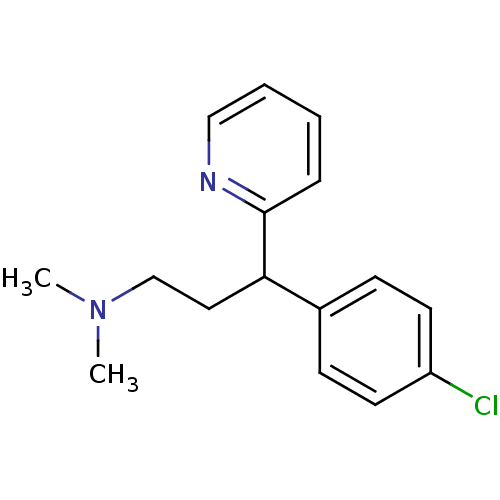 (1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM35938 (1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor in rat brain membranes was evaluated using [3H]-pyrilamine as radioligand | J Med Chem 34: 457-61 (1991) BindingDB Entry DOI: 10.7270/Q2BV7FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065424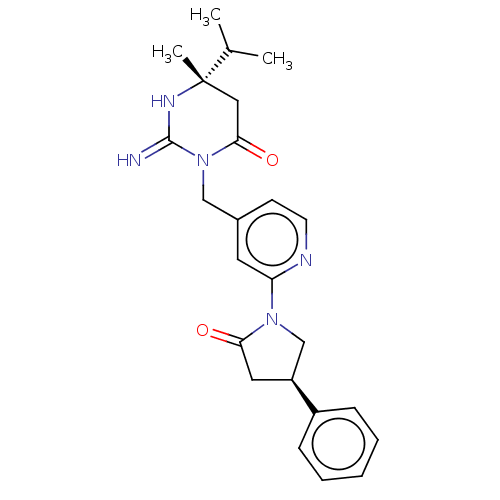 (CHEMBL3401346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50063449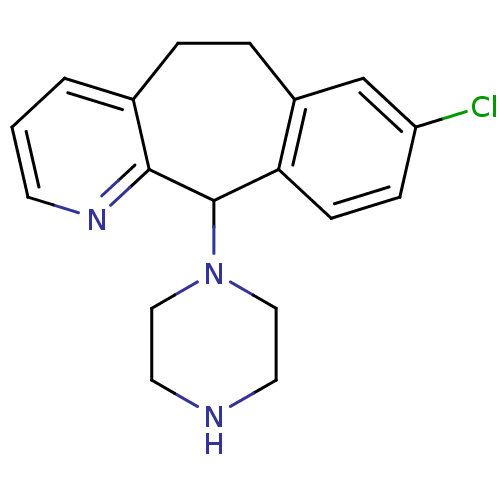 (8-Chloro-11-piperazin-1-yl-6,11-dihydro-5H-benzo[5...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051012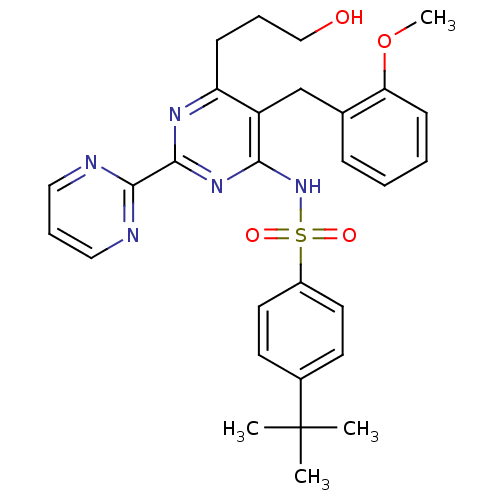 (4-tert-Butyl-N-[6-(3-hydroxy-propyl)-5-(2-methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073184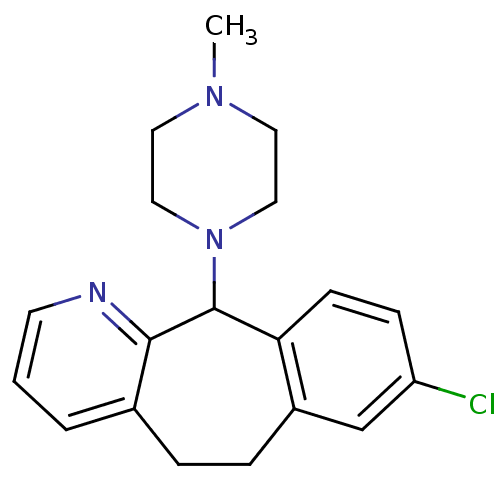 (8-Chloro-11-(4-methyl-piperazin-1-yl)-6,11-dihydro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18793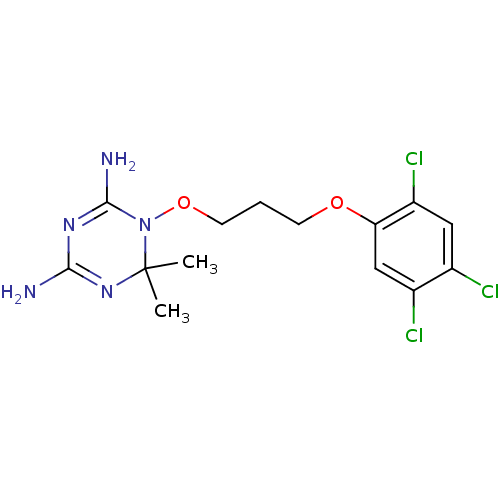 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50261110 (CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of high affinity TRAP form human platelet PAR1 | Bioorg Med Chem Lett 20: 6676-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.009 BindingDB Entry DOI: 10.7270/Q2KS6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12287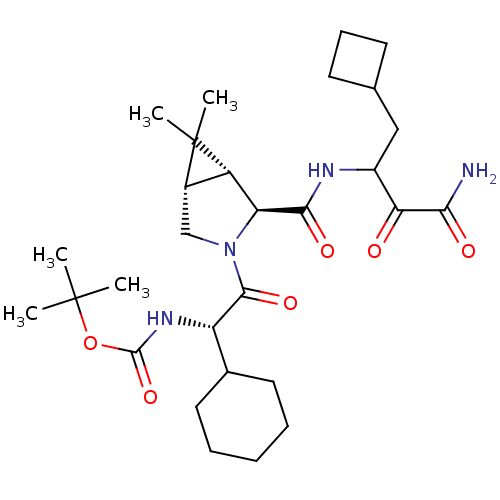 (SCH 491762 | tert-butyl N-[(1S)-2-[(1R,2S,5S)-2-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 6074-86 (2006) Article DOI: 10.1021/jm060325b BindingDB Entry DOI: 10.7270/Q2DF6PF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (RAT) | BDBM50281832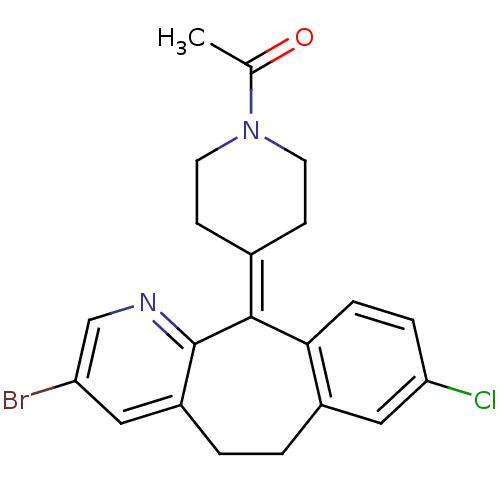 (1-[4-(3-Bromo-8-chloro-5,6-dihydro-benzo[5,6]cyclo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]-pyrilamine from H1 receptor in rat brain membrane. | Bioorg Med Chem Lett 3: 1073-1078 (1993) Article DOI: 10.1016/S0960-894X(00)80290-5 BindingDB Entry DOI: 10.7270/Q2445MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50035483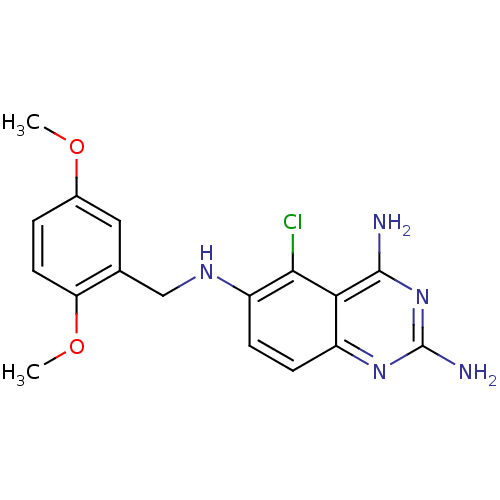 (5-Chloro-N*6*-(2,5-dimethoxy-benzyl)-quinazoline-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50329617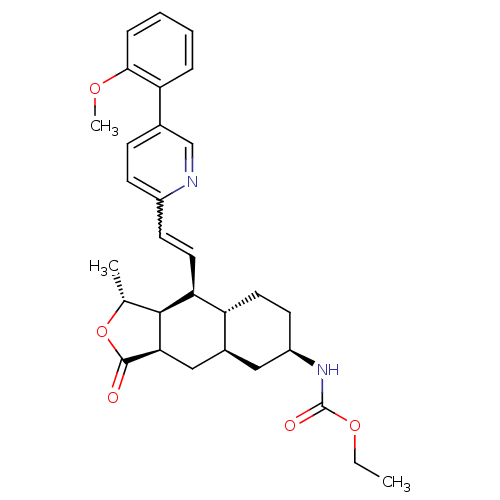 (CHEMBL1270636 | ethyl(1R,3aR,4aR,6R,8aR,9S,9aS)-9-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of high affinity TRAP form human platelet PAR1 | Bioorg Med Chem Lett 20: 6676-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.009 BindingDB Entry DOI: 10.7270/Q2KS6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12309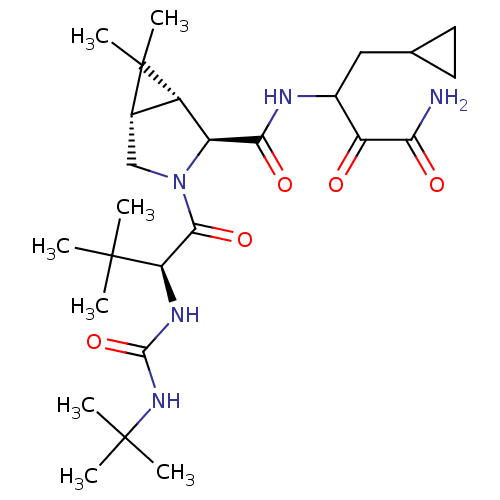 ((1R,5S)-N-[3-amino-1-(cyclopropylmethyl)-2,3-dioxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 6074-86 (2006) Article DOI: 10.1021/jm060325b BindingDB Entry DOI: 10.7270/Q2DF6PF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Max-like protein X (Homo sapiens (Human)) | BDBM50388975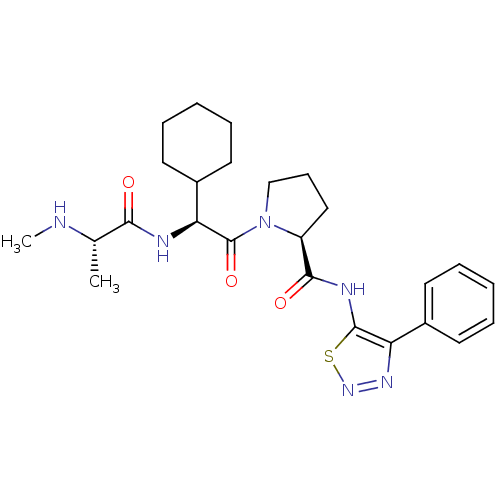 (CHEMBL2063869) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 5-FAM-conjugated AVP-diPhe-FAM from MLXBIR3SG after 30 mins by fluorescence polarization-based competition assay | J Med Chem 55: 4101-13 (2012) Article DOI: 10.1021/jm300060k BindingDB Entry DOI: 10.7270/Q2HQ410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12311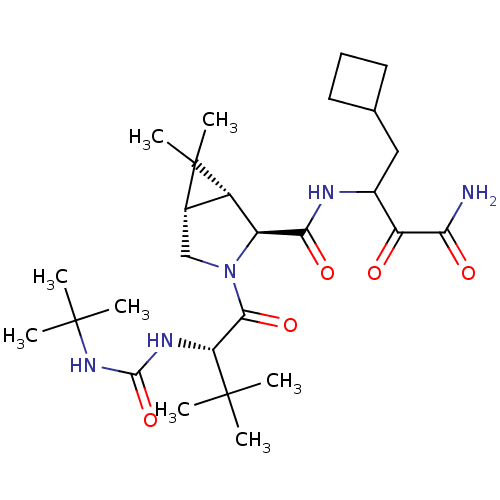 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 6074-86 (2006) Article DOI: 10.1021/jm060325b BindingDB Entry DOI: 10.7270/Q2DF6PF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50041617 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin B receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50329616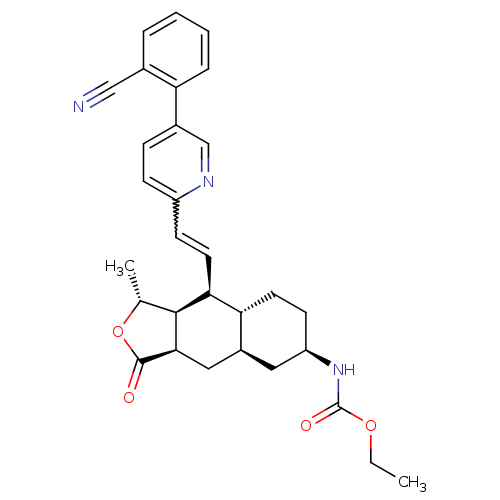 (CHEMBL1270537 | ethyl(1R,3aR,4aR,6R,8aR,9S,9aS)-9-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of high affinity TRAP form human platelet PAR1 | Bioorg Med Chem Lett 20: 6676-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.009 BindingDB Entry DOI: 10.7270/Q2KS6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065425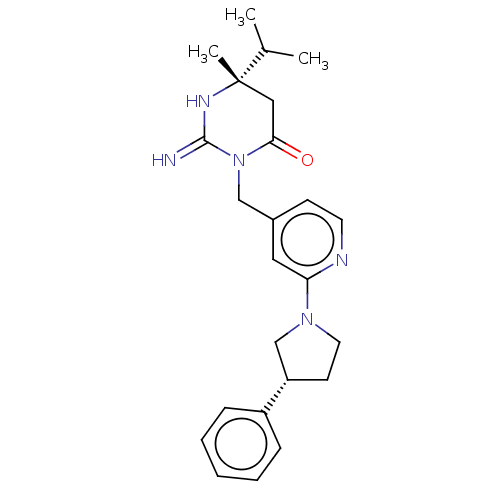 (CHEMBL3401347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Max-like protein X (Homo sapiens (Human)) | BDBM50388974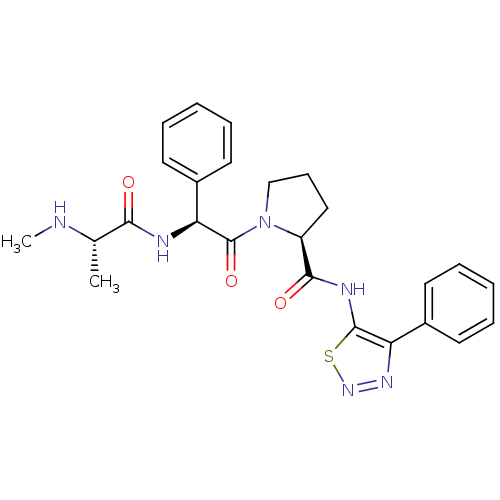 (CHEMBL2063868) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 5-FAM-conjugated AVP-diPhe-FAM from MLXBIR3SG after 30 mins by fluorescence polarization-based competition assay | J Med Chem 55: 4101-13 (2012) Article DOI: 10.1021/jm300060k BindingDB Entry DOI: 10.7270/Q2HQ410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12307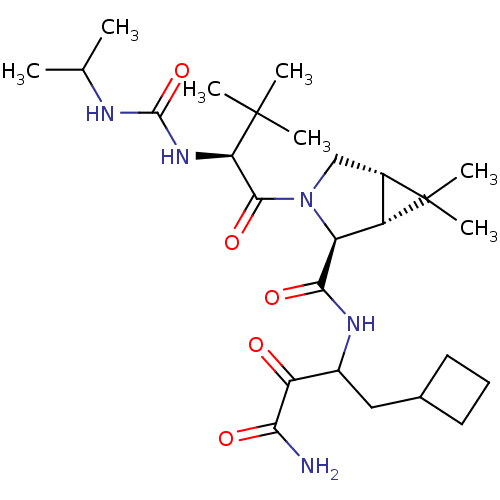 ((1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 6074-86 (2006) Article DOI: 10.1021/jm060325b BindingDB Entry DOI: 10.7270/Q2DF6PF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50388975 (CHEMBL2063869) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 5-FAM-conjugated AVP-diPhe-FAM from cIAP1 BIR3 domain after 30 mins by fluorescence polarization-based competition assay | J Med Chem 55: 4101-13 (2012) Article DOI: 10.1021/jm300060k BindingDB Entry DOI: 10.7270/Q2HQ410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065393 (CHEMBL3401344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50329618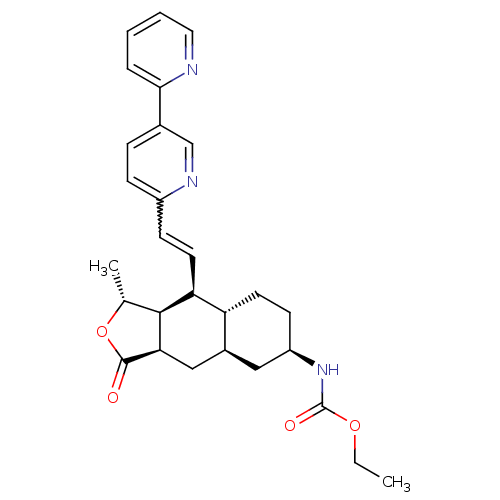 (CHEMBL1270738 | ethyl(1R,3aR,4aR,6R,8aR,9S,9aS)-9-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of high affinity TRAP form human platelet PAR1 | Bioorg Med Chem Lett 20: 6676-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.009 BindingDB Entry DOI: 10.7270/Q2KS6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50061467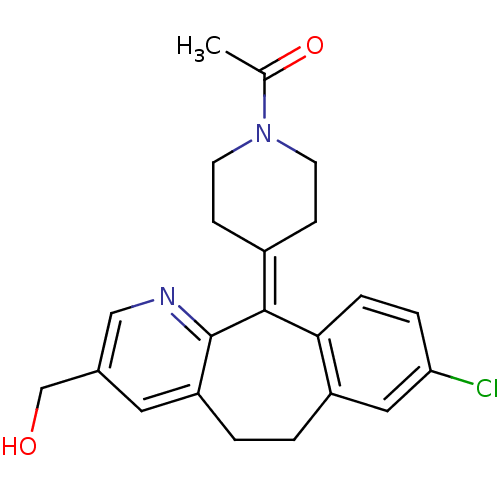 (1-[4-(8-Chloro-3-hydroxymethyl-5,6-dihydro-benzo[5...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]-pyrilamine from H1 receptor in rat brain membrane. | Bioorg Med Chem Lett 3: 1073-1078 (1993) Article DOI: 10.1016/S0960-894X(00)80290-5 BindingDB Entry DOI: 10.7270/Q2445MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50007464 (4-(8-Chloro-5,6-dihydro-benzo[5,6]cyclohepta[1,2-b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor in rat brain membranes was evaluated using [3H]-pyrilamine as radioligand | J Med Chem 34: 457-61 (1991) BindingDB Entry DOI: 10.7270/Q2BV7FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12286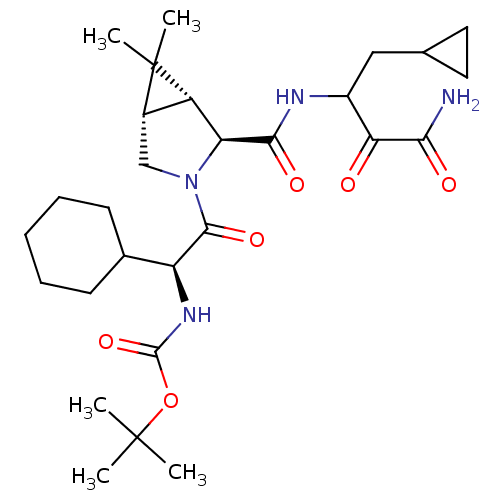 (1,1-Dimethylethyl-[1(S)-cyclohexyl-2-[(1R,5S)-2(S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 49: 6074-86 (2006) Article DOI: 10.1021/jm060325b BindingDB Entry DOI: 10.7270/Q2DF6PF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50388975 (CHEMBL2063869) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 5-FAM-conjugated AVP-diPhe-FAM from XIAP BIR3 domain after 30 mins by fluorescence polarization-based competition assay | J Med Chem 55: 4101-13 (2012) Article DOI: 10.1021/jm300060k BindingDB Entry DOI: 10.7270/Q2HQ410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Max-like protein X (Homo sapiens (Human)) | BDBM50388969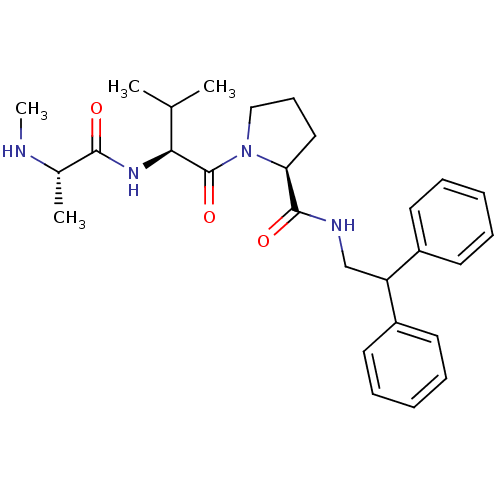 (CHEMBL2063862) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 5-FAM-conjugated AVP-diPhe-FAM from MLXBIR3SG after 30 mins by fluorescence polarization-based competition assay | J Med Chem 55: 4101-13 (2012) Article DOI: 10.1021/jm300060k BindingDB Entry DOI: 10.7270/Q2HQ410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Max-like protein X (Homo sapiens (Human)) | BDBM50388970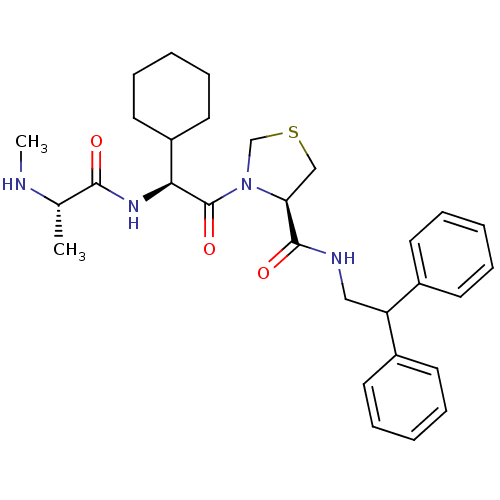 (CHEMBL2063863) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 5-FAM-conjugated AVP-diPhe-FAM from MLXBIR3SG after 30 mins by fluorescence polarization-based competition assay | J Med Chem 55: 4101-13 (2012) Article DOI: 10.1021/jm300060k BindingDB Entry DOI: 10.7270/Q2HQ410F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2533 total ) | Next | Last >> |