

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561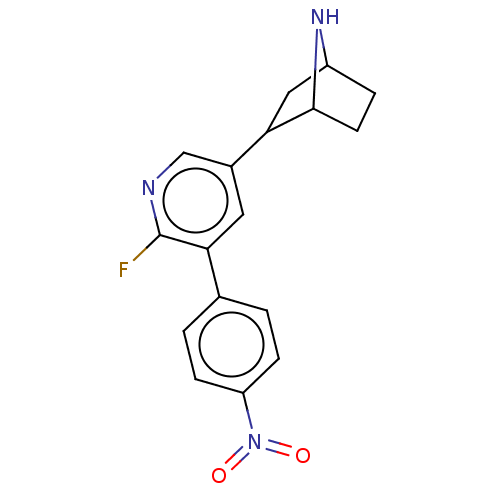 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127165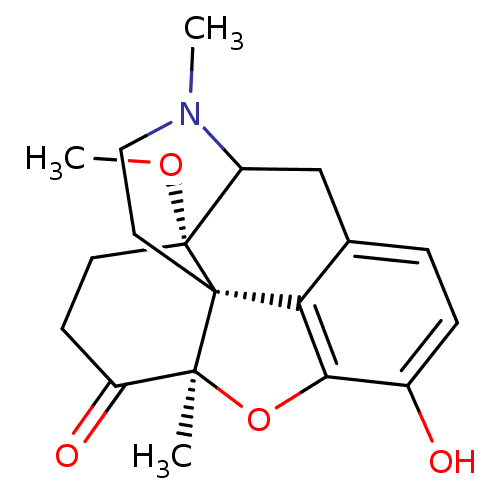 (10-hydroxy-17-methoxy-4,13-dimethyl-(13R,17S)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50395218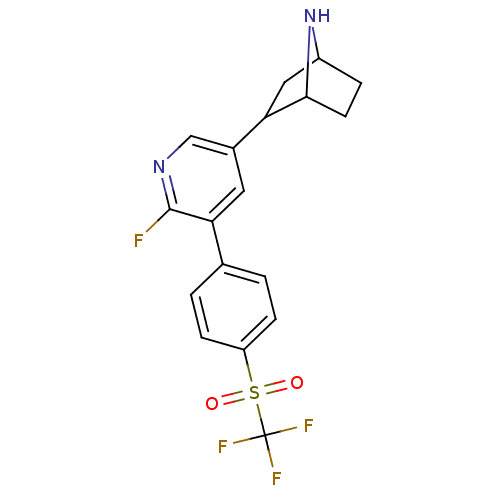 (CHEMBL2164666 | US9150581, RTI-7527-192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]p-CI-DPDPE in C6 glioma cells expressing the cloned Opioid receptor delta 1 | Bioorg Med Chem Lett 10: 2449-51 (2001) BindingDB Entry DOI: 10.7270/Q20K2939 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253328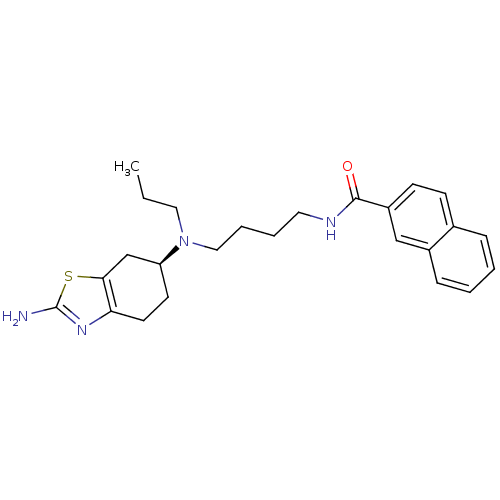 ((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50248793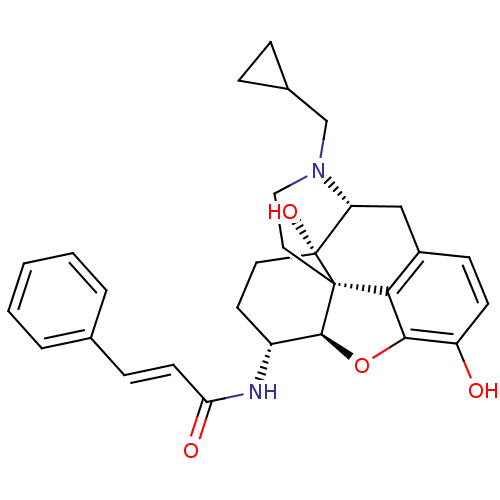 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against [3H]- DAMGO labeled mu opioid receptor in guinea-pig brain homogenate. | Bioorg Med Chem Lett 6: 167-172 (1996) Article DOI: 10.1016/0960-894X(95)00583-F BindingDB Entry DOI: 10.7270/Q2QJ7H83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM86805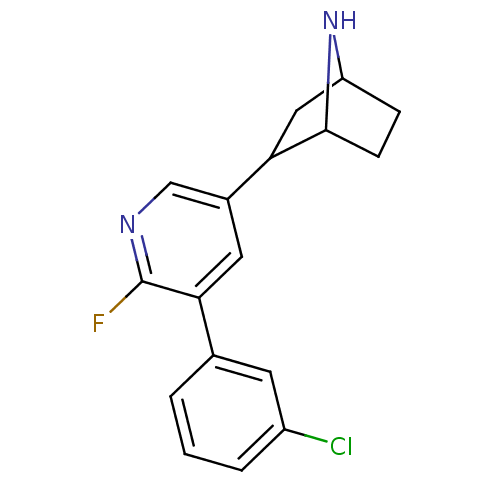 (CAS_45263788 | NSC_45263788 | US9150581, RTI-7527-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50027230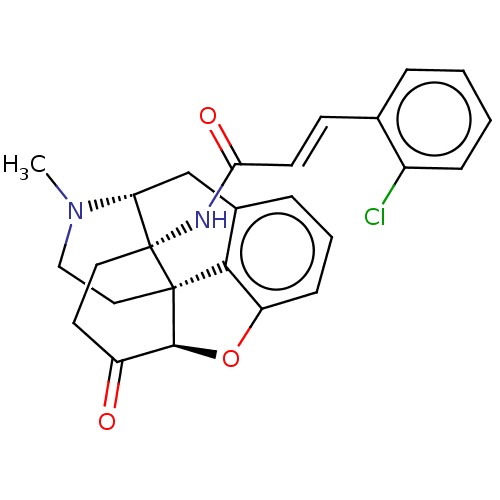 (CHEMBL2113666) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]Cl-DPDPE from human recombinant DOR expressed in CHO cells | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50127167 (10-hydroxy-17-(3-phenylpropoxy)-4-tetrahydro-2-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor kappa 1 was determined in human CHO cells using [3H]U-69593 | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50395220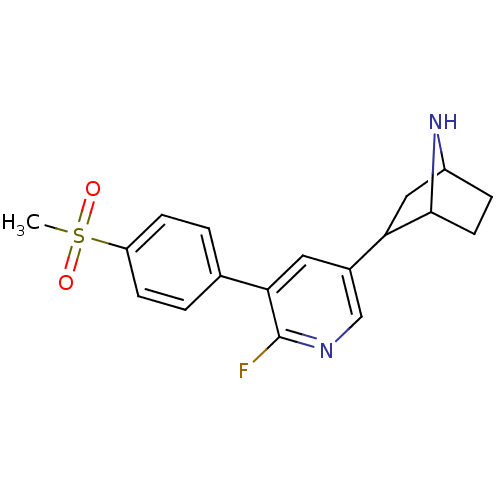 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127167 (10-hydroxy-17-(3-phenylpropoxy)-4-tetrahydro-2-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor delta 1 was determined in C6 rat glioma cells using [3H]Ile5,6 deltorphin II | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127162 (10-hydroxy-17-(3-phenylpropoxy)-4-propyl-(13R,17S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50287928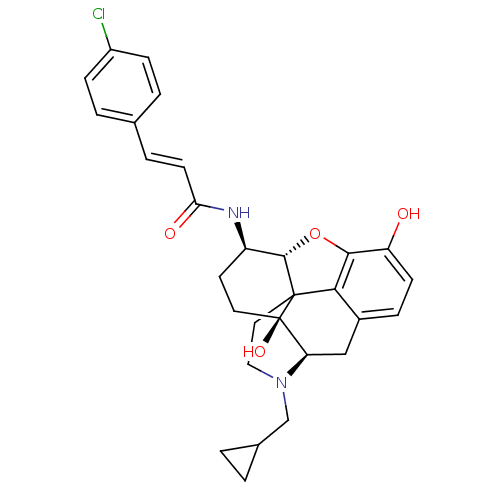 (1N-[4-cyclopropylmethyl-10,17-dihydroxy-(5R,13R,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against [3H]- DAMGO labeled mu opioid receptor in guinea-pig brain homogenate. | Bioorg Med Chem Lett 6: 167-172 (1996) Article DOI: 10.1016/0960-894X(95)00583-F BindingDB Entry DOI: 10.7270/Q2QJ7H83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50127163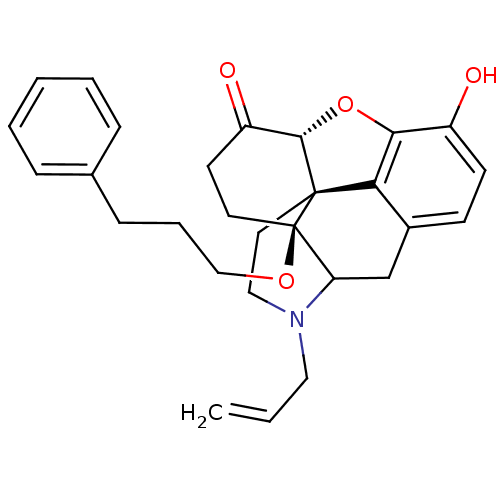 (4-allyl-10-hydroxy-17-(3-phenylpropoxy)-(13R,17S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor kappa 1 was determined in human CHO cells using [3H]U-69593 | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50193546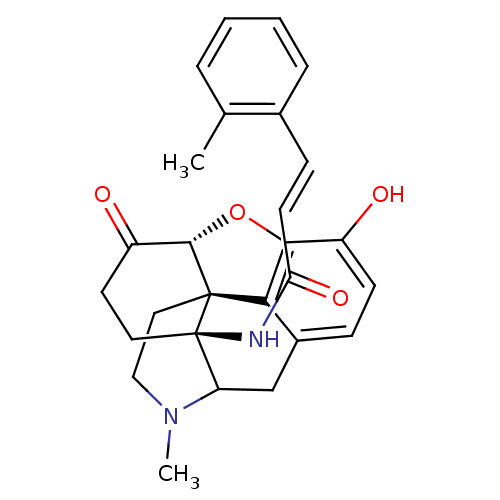 (14beta-(2'-methylcinnamoylamino)-7,8-dihydromorphi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]Cl-DPDPE from human recombinant DOR expressed in CHO cells | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50395217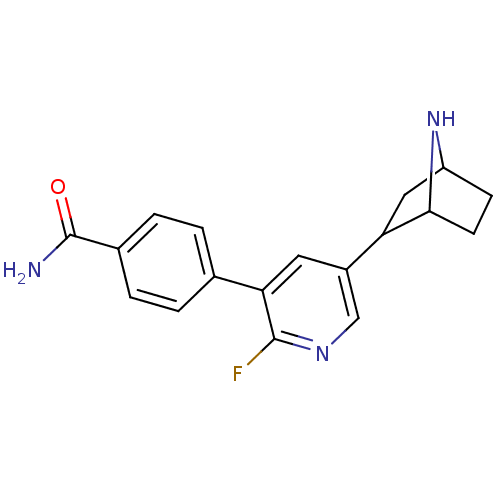 (CHEMBL2164667 | US9150581, RTI-7527-168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027230 (CHEMBL2113666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant MOR expressed in CHO cells | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50171007 (4-[5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-2-fluoro-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50193536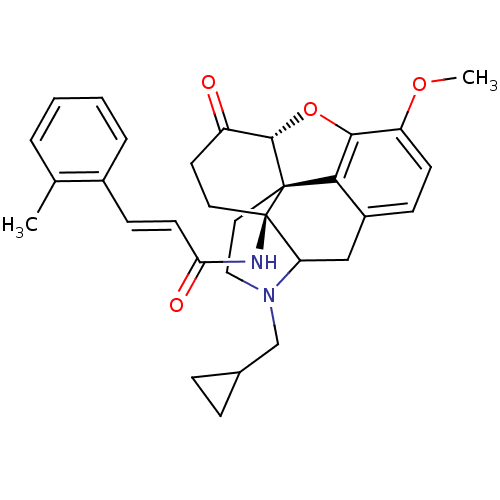 (N-cyclopropylmethyl-14beta-(2'-methylcinnamoylamin...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Binding affinity to MOR in guinea pig brain membrane | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50033649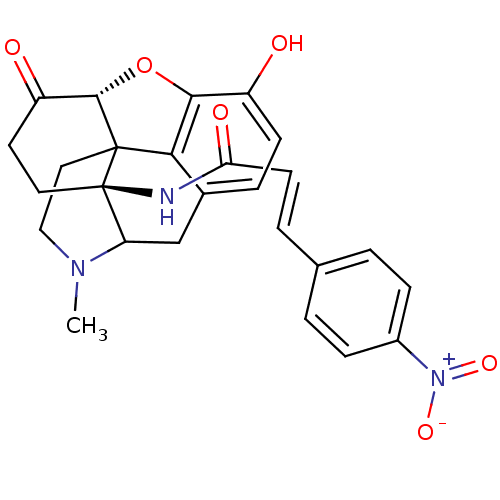 (1N-[10-hydroxy-4-methyl-14-oxo-(13R,17S)-12-oxa-4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Stimulation of [35S]GTPgammaS binding to human recombinant MOR | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127163 (4-allyl-10-hydroxy-17-(3-phenylpropoxy)-(13R,17S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127167 (10-hydroxy-17-(3-phenylpropoxy)-4-tetrahydro-2-fur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50287927 (1N-[4-cyclopropylmethyl-10,17-dihydroxy-(5R,13R,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against [3H]- DAMGO labeled mu opioid receptor in guinea-pig brain homogenate. | Bioorg Med Chem Lett 6: 167-172 (1996) Article DOI: 10.1016/0960-894X(95)00583-F BindingDB Entry DOI: 10.7270/Q2QJ7H83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50398951 (CHEMBL2179258) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in guinea pig brain membrane | J Med Chem 55: 9868-74 (2012) Article DOI: 10.1021/jm301096s BindingDB Entry DOI: 10.7270/Q2M909TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50287928 (1N-[4-cyclopropylmethyl-10,17-dihydroxy-(5R,13R,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against [3H]- U-69593 labeled kappa1 opioid receptor in guinea-pig brain homogenate. | Bioorg Med Chem Lett 6: 167-172 (1996) Article DOI: 10.1016/0960-894X(95)00583-F BindingDB Entry DOI: 10.7270/Q2QJ7H83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50248793 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against [3H]- U-69593 labeled kappa1 opioid receptor in guinea-pig brain homogenate. | Bioorg Med Chem Lett 6: 167-172 (1996) Article DOI: 10.1016/0960-894X(95)00583-F BindingDB Entry DOI: 10.7270/Q2QJ7H83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 52: 1553-7 (2009) Article DOI: 10.1021/jm8012272 BindingDB Entry DOI: 10.7270/Q2G44R6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant MOR expressed in CHO cells | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50193546 (14beta-(2'-methylcinnamoylamino)-7,8-dihydromorphi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant MOR expressed in CHO cells | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50193546 (14beta-(2'-methylcinnamoylamino)-7,8-dihydromorphi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human recombinant KOR expressed in CHO cells | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50287929 (1N-[4-cyclopropylmethyl-10,17-dihydroxy-(5R,13R,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against [3H]- DAMGO labeled mu opioid receptor in guinea-pig brain homogenate. | Bioorg Med Chem Lett 6: 167-172 (1996) Article DOI: 10.1016/0960-894X(95)00583-F BindingDB Entry DOI: 10.7270/Q2QJ7H83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50398952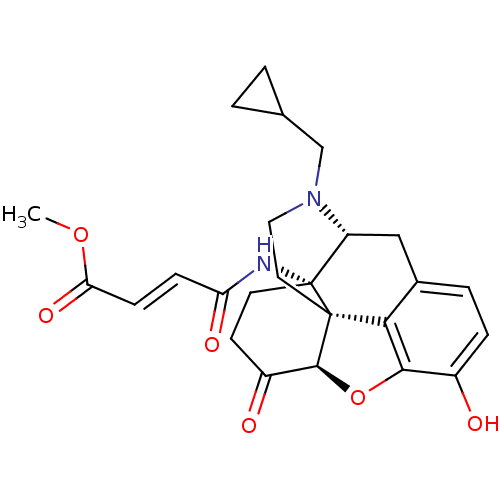 (CHEMBL2179264) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in guinea pig brain membrane | J Med Chem 55: 9868-74 (2012) Article DOI: 10.1021/jm301096s BindingDB Entry DOI: 10.7270/Q2M909TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM86806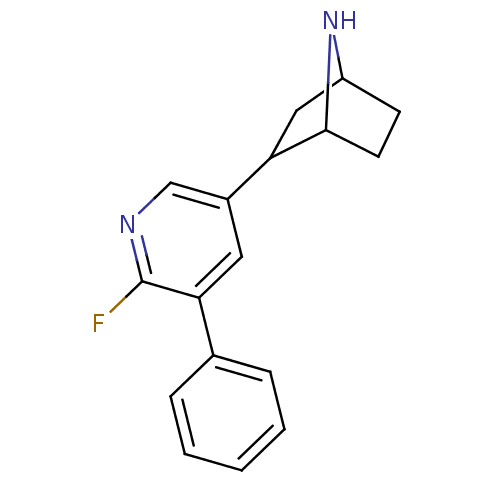 (CAS_45263762 | NSC_45263762 | US9150581, RTI-7527-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM86806 (CAS_45263762 | NSC_45263762 | US9150581, RTI-7527-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127170 (4-cyclobutylmethyl-10-hydroxy-17-(3-phenylpropoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50052754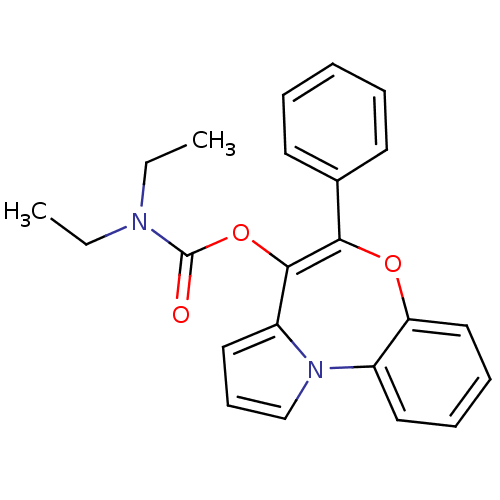 (CHEMBL115740 | Diethyl-carbamic acid 5-phenyl-6-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Binding affinity against peripheral-type benzodiazepine receptor (PBR) from rat cortex homogenate using [3H]-PK 11195 as radioligand | J Med Chem 39: 3435-50 (1996) Article DOI: 10.1021/jm960251b BindingDB Entry DOI: 10.7270/Q23B60SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127163 (4-allyl-10-hydroxy-17-(3-phenylpropoxy)-(13R,17S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor delta 1 was determined in C6 rat glioma cells using [3H]Ile5,6 deltorphin II | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM86806 (CAS_45263762 | NSC_45263762 | US9150581, RTI-7527-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50041240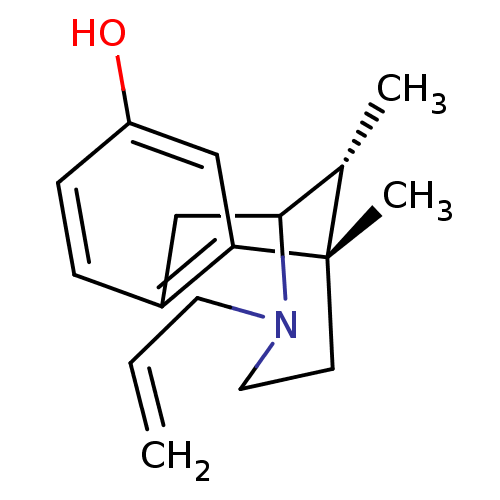 ((6S,11R)-3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195678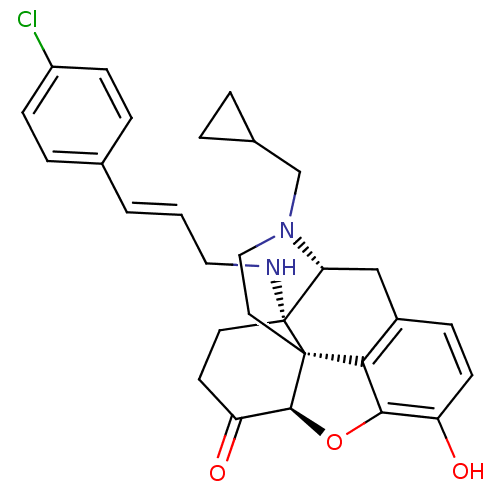 ((1R,5R,13R,17S)-17-{[(2E)-3-(4-chlorophenyl)prop-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 6926-30 (2009) Article DOI: 10.1021/jm901074a BindingDB Entry DOI: 10.7270/Q2GH9J1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50249017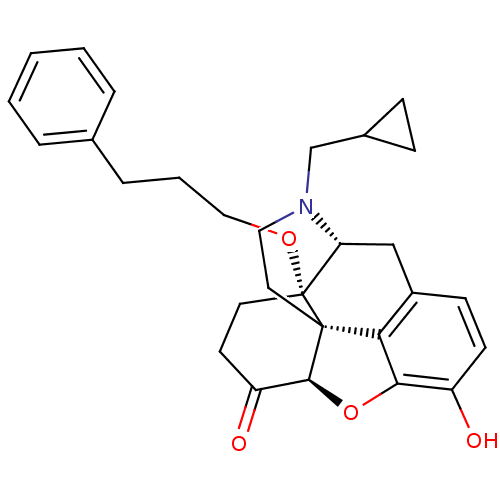 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 52: 1553-7 (2009) Article DOI: 10.1021/jm8012272 BindingDB Entry DOI: 10.7270/Q2G44R6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50193542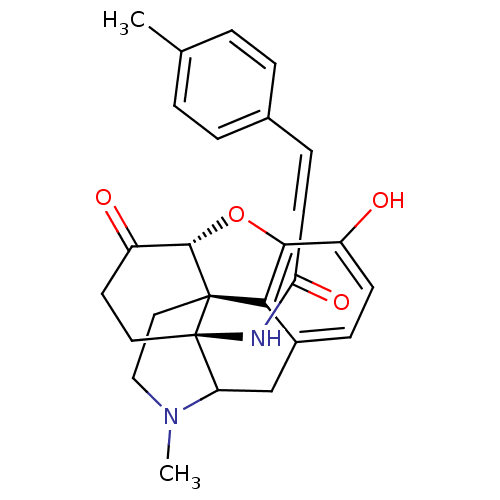 (17-methyl-4'-methyldihydromorphinone) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Stimulation of [35S]GTPgammaS binding to human recombinant MOR | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50400513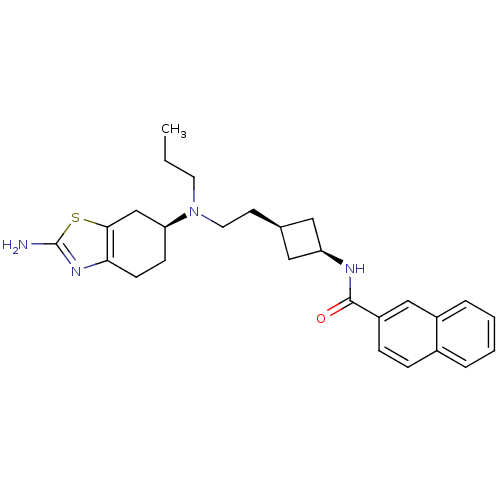 (CHEMBL2203404) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]R(+)-7-OH-DPAT from Sprague-Dawley rat dopamine D3 receptor after 90 mins | ACS Med Chem Lett 2: 620-625 (2011) Article DOI: 10.1021/ml200100t BindingDB Entry DOI: 10.7270/Q2028SQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50193544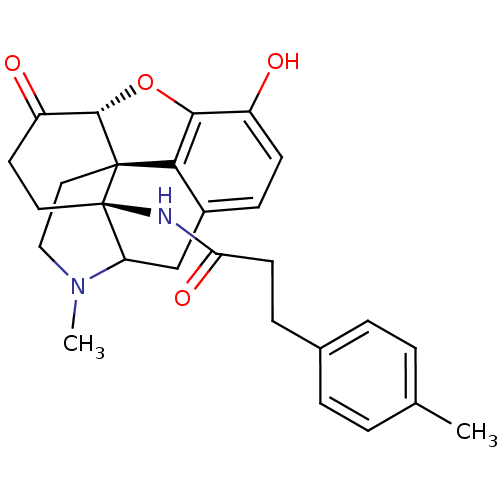 (N-[(1R,13R,17S)-10-hydroxy-4-methyl-14-oxo-12-oxa-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Stimulation of [35S]GTPgammaS binding to human recombinant MOR | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184563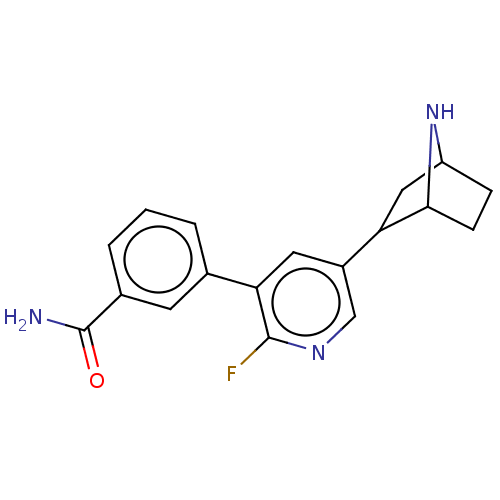 (US9150581, RTI-7527-169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 666 total ) | Next | Last >> |