 Found 2724 hits with Last Name = 'xiang' and Initial = 'z'
Found 2724 hits with Last Name = 'xiang' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M4 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M4 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089414
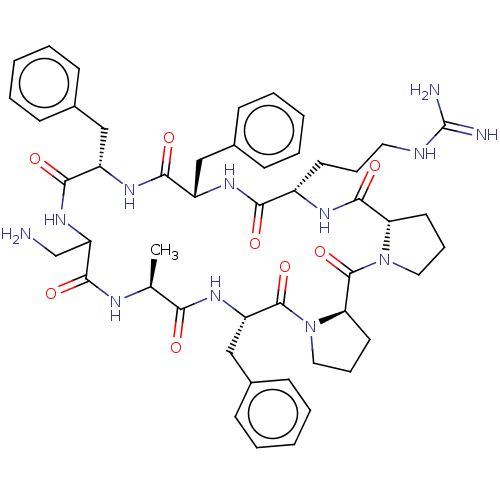
(CHEMBL3577996)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)C(CN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C49H64N12O8/c1-30-41(62)58-37(28-33-18-9-4-10-19-33)47(68)61-25-13-22-40(61)48(69)60-24-12-21-39(60)46(67)55-34(20-11-23-53-49(51)52)42(63)56-35(26-31-14-5-2-6-15-31)43(64)57-36(27-32-16-7-3-8-17-32)44(65)59-38(29-50)45(66)54-30/h2-10,14-19,30,34-40H,11-13,20-29,50H2,1H3,(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,58,62)(H,59,65)(H4,51,52,53)/t30-,34-,35-,36-,37-,38?,39-,40+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from rat recombinant muscarinic M5 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting |
Nat Chem Biol 4: 42-50 (2007)
Article DOI: 10.1038/nchembio.2007.55
BindingDB Entry DOI: 10.7270/Q2D50N55 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089415
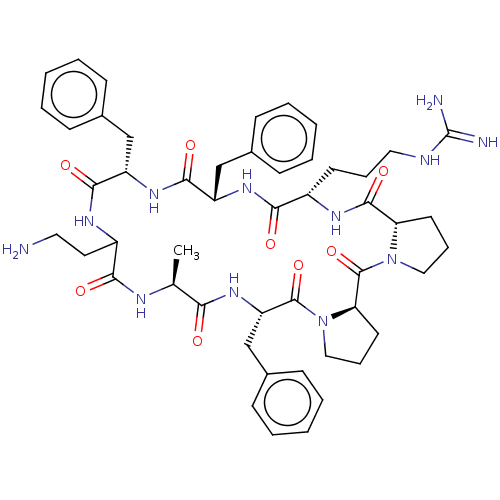
(CHEMBL3577997)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)C(CCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C50H66N12O8/c1-31-42(63)60-39(30-34-18-9-4-10-19-34)48(69)62-27-13-22-41(62)49(70)61-26-12-21-40(61)47(68)57-35(20-11-25-54-50(52)53)44(65)58-38(29-33-16-7-3-8-17-33)46(67)59-37(28-32-14-5-2-6-15-32)45(66)56-36(23-24-51)43(64)55-31/h2-10,14-19,31,35-41H,11-13,20-30,51H2,1H3,(H,55,64)(H,56,66)(H,57,68)(H,58,65)(H,59,67)(H,60,63)(H4,52,53,54)/t31-,35-,36?,37-,38-,39-,40-,41+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089418
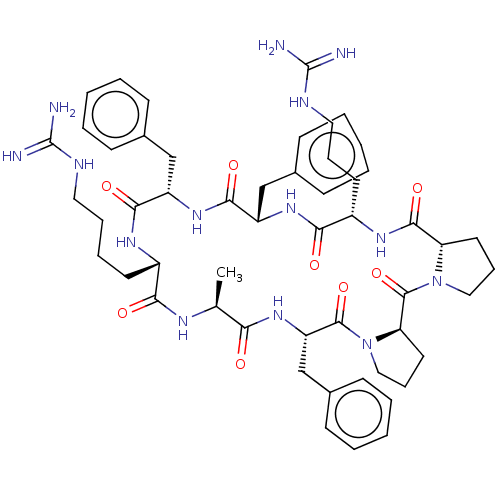
(CHEMBL3578000)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CCCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C53H72N14O8/c1-33-44(68)65-41(32-36-20-9-4-10-21-36)50(74)67-29-15-25-43(67)51(75)66-28-14-24-42(66)49(73)62-38(23-13-27-59-53(56)57)46(70)63-40(31-35-18-7-3-8-19-35)48(72)64-39(30-34-16-5-2-6-17-34)47(71)61-37(45(69)60-33)22-11-12-26-58-52(54)55/h2-10,16-21,33,37-43H,11-15,22-32H2,1H3,(H,60,69)(H,61,71)(H,62,73)(H,63,70)(H,64,72)(H,65,68)(H4,54,55,58)(H4,56,57,59)/t33-,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089413
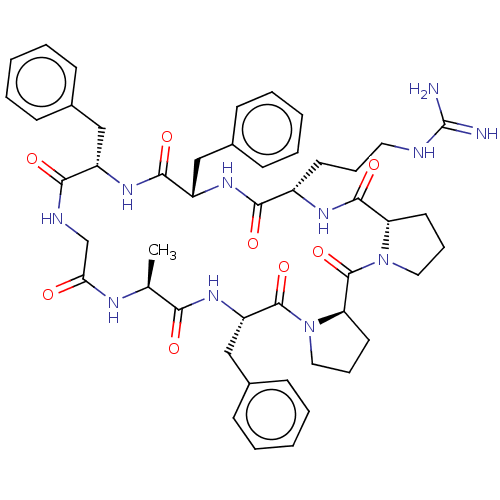
(CHEMBL3577995)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C48H61N11O8/c1-30-41(61)57-37(28-33-18-9-4-10-19-33)46(66)59-25-13-22-39(59)47(67)58-24-12-21-38(58)45(65)54-34(20-11-23-51-48(49)50)43(63)56-36(27-32-16-7-3-8-17-32)44(64)55-35(26-31-14-5-2-6-15-31)42(62)52-29-40(60)53-30/h2-10,14-19,30,34-39H,11-13,20-29H2,1H3,(H,52,62)(H,53,60)(H,54,65)(H,55,64)(H,56,63)(H,57,61)(H4,49,50,51)/t30-,34-,35-,36-,37-,38-,39+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089416
(CHEMBL3577998)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)C(CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C51H68N12O8/c1-32-43(64)61-40(31-35-19-9-4-10-20-35)49(70)63-28-14-24-42(63)50(71)62-27-13-23-41(62)48(69)58-37(22-12-26-55-51(53)54)45(66)59-39(30-34-17-7-3-8-18-34)47(68)60-38(29-33-15-5-2-6-16-33)46(67)57-36(21-11-25-52)44(65)56-32/h2-10,15-20,32,36-42H,11-14,21-31,52H2,1H3,(H,56,65)(H,57,67)(H,58,69)(H,59,66)(H,60,68)(H,61,64)(H4,53,54,55)/t32-,36?,37-,38-,39-,40-,41-,42+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089417

(CHEMBL3577999)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C52H70N12O8/c1-33-44(65)62-41(32-36-20-9-4-10-21-36)50(71)64-29-15-25-43(64)51(72)63-28-14-24-42(63)49(70)59-38(23-13-27-56-52(54)55)46(67)60-40(31-35-18-7-3-8-19-35)48(69)61-39(30-34-16-5-2-6-17-34)47(68)58-37(45(66)57-33)22-11-12-26-53/h2-10,16-21,33,37-43H,11-15,22-32,53H2,1H3,(H,57,66)(H,58,68)(H,59,70)(H,60,67)(H,61,69)(H,62,65)(H4,54,55,56)/t33-,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089409
(CHEMBL3577991)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)C(CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C51H66N12O9/c1-31-43(65)61-39(29-34-18-9-4-10-19-34)49(71)63-27-13-22-41(63)50(72)62-26-12-21-40(62)48(70)58-35(20-11-25-55-51(53)54)44(66)59-37(28-33-16-7-3-8-17-33)47(69)57-36(24-23-32-14-5-2-6-15-32)45(67)60-38(30-42(52)64)46(68)56-31/h2-10,14-19,31,35-41H,11-13,20-30H2,1H3,(H2,52,64)(H,56,68)(H,57,69)(H,58,70)(H,59,66)(H,60,67)(H,61,65)(H4,53,54,55)/t31-,35-,36?,37-,38-,39-,40-,41+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089411
(CHEMBL3577993)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)C(Cc1cccc3ccccc13)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-16-6-3-7-17-34)52(74)66-27-13-24-44(66)53(75)65-26-12-23-43(65)51(73)60-38(22-11-25-58-54(56)57)47(69)61-39(28-33-14-4-2-5-15-33)49(71)62-40(50(72)63-41(31-45(55)67)48(70)59-32)30-36-20-10-19-35-18-8-9-21-37(35)36/h2-10,14-21,32,38-44H,11-13,22-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,69)(H,62,71)(H,63,72)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089404

(CHEMBL3577986)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(Cc1cccc3ccccc13)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-16-6-3-7-17-34)52(74)66-27-13-24-44(66)53(75)65-26-12-23-43(65)51(73)60-38(22-11-25-58-54(56)57)47(69)62-40(30-36-20-10-19-35-18-8-9-21-37(35)36)50(72)61-39(28-33-14-4-2-5-15-33)49(71)63-41(31-45(55)67)48(70)59-32/h2-10,14-21,32,38-44H,11-13,22-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,72)(H,62,69)(H,63,71)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089398

(CHEMBL3577981)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C50H64N12O9/c1-30-42(64)60-38(28-33-18-9-4-10-19-33)48(70)62-25-13-22-40(62)49(71)61-24-12-21-39(61)47(69)56-34(20-11-23-54-50(52)53)43(65)57-35(26-31-14-5-2-6-15-31)45(67)58-36(27-32-16-7-3-8-17-32)46(68)59-37(29-41(51)63)44(66)55-30/h2-10,14-19,30,34-40H,11-13,20-29H2,1H3,(H2,51,63)(H,55,66)(H,56,69)(H,57,65)(H,58,67)(H,59,68)(H,60,64)(H4,52,53,54)/t30-,34-,35-,36-,37-,38-,39-,40+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089413

(CHEMBL3577995)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C48H61N11O8/c1-30-41(61)57-37(28-33-18-9-4-10-19-33)46(66)59-25-13-22-39(59)47(67)58-24-12-21-38(58)45(65)54-34(20-11-23-51-48(49)50)43(63)56-36(27-32-16-7-3-8-17-32)44(64)55-35(26-31-14-5-2-6-15-31)42(62)52-29-40(60)53-30/h2-10,14-19,30,34-39H,11-13,20-29H2,1H3,(H,52,62)(H,53,60)(H,54,65)(H,55,64)(H,56,63)(H,57,61)(H4,49,50,51)/t30-,34-,35-,36-,37-,38-,39+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445876
(CHEMBL3105692)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2cc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C27H21Cl2NO4S2/c1-2-36(33,34)20-12-10-17(11-13-20)14-24(31)30-25-16-22(18-6-5-7-19(28)15-18)27(35-25)26(32)21-8-3-4-9-23(21)29/h3-13,15-16H,2,14H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 25-[26,27-3H]hydroxycholesterol from RORgammat receptor ligand binding domain (unknown origin) after 60 mins |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445875

(CHEMBL3105671)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2cc(c(s2)C(=O)c2cccc(F)c2)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C27H21ClFNO4S2/c1-2-36(33,34)22-11-9-17(10-12-22)13-24(31)30-25-16-23(18-5-3-7-20(28)14-18)27(35-25)26(32)19-6-4-8-21(29)15-19/h3-12,14-16H,2,13H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 25-[26,27-3H]hydroxycholesterol from RORgammat receptor ligand binding domain (unknown origin) after 60 mins |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877
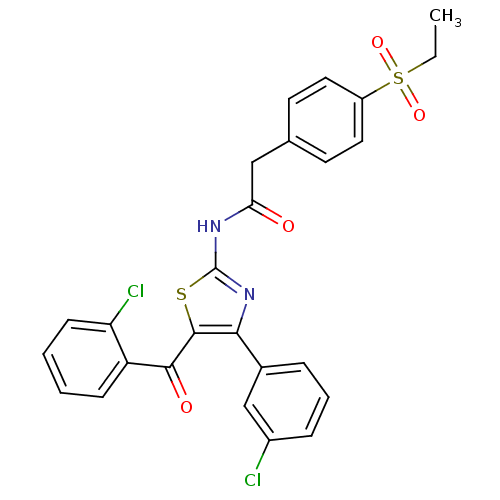
(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 25-[26,27-3H]hydroxycholesterol from RORgammat receptor ligand binding domain (unknown origin) after 60 mins |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 3
(Mus musculus) | BDBM50115373
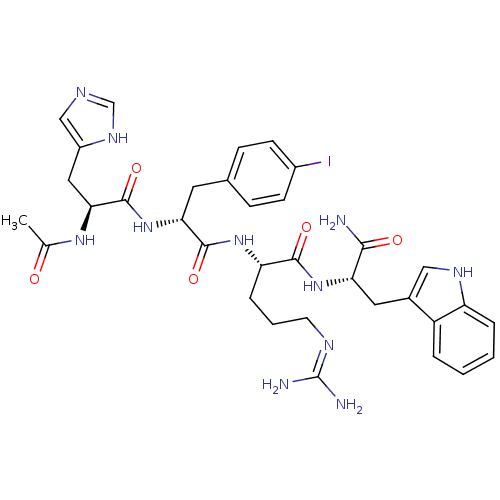
(2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc(I)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:4.3,14.14,26.27,wD:37.38,(23.38,-8.66,;23.38,-10.2,;22.06,-10.96,;24.72,-10.97,;26.05,-10.21,;26.05,-8.66,;27.39,-7.89,;28.79,-8.52,;29.83,-7.38,;29.06,-6.04,;27.55,-6.36,;27.39,-10.97,;27.39,-12.51,;28.72,-10.21,;30.06,-10.97,;30.06,-12.51,;31.39,-13.29,;32.73,-12.51,;34.05,-13.29,;34.05,-14.83,;35.38,-15.6,;32.72,-15.59,;31.39,-14.82,;31.39,-10.21,;31.39,-8.66,;32.72,-10.97,;34.05,-10.2,;34.05,-8.67,;35.38,-7.89,;35.38,-6.36,;36.72,-5.59,;36.72,-4.06,;35.39,-3.28,;38.06,-3.28,;35.38,-10.97,;35.38,-12.52,;36.72,-10.21,;38.06,-10.98,;38.06,-12.52,;39.39,-13.3,;40.8,-12.67,;41.82,-13.82,;41.06,-15.15,;41.52,-16.62,;40.5,-17.76,;38.99,-17.44,;38.51,-15.97,;39.55,-14.83,;39.39,-10.22,;40.73,-10.98,;39.39,-8.68,)| Show InChI InChI=1S/C34H42IN11O5/c1-19(47)43-29(15-23-17-39-18-42-23)33(51)46-28(13-20-8-10-22(35)11-9-20)32(50)44-26(7-4-12-40-34(37)38)31(49)45-27(30(36)48)14-21-16-41-25-6-3-2-5-24(21)25/h2-3,5-6,8-11,16-18,26-29,41H,4,7,12-15H2,1H3,(H2,36,48)(H,39,42)(H,43,47)(H,44,50)(H,45,49)(H,46,51)(H4,37,38,40)/t26-,27-,28+,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding constant (Ki) at mouse Melanocortin-3 receptor |
J Med Chem 45: 3073-81 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9CHF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089412
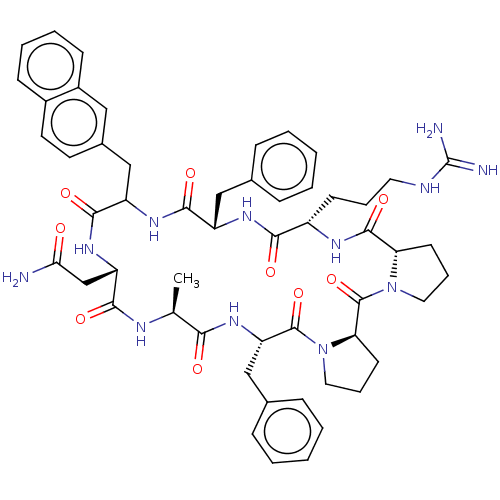
(CHEMBL3577994)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)C(Cc1ccc3ccccc3c1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-15-6-3-7-16-34)52(74)66-26-12-21-44(66)53(75)65-25-11-20-43(65)51(73)60-38(19-10-24-58-54(56)57)47(69)61-39(28-33-13-4-2-5-14-33)49(71)62-40(50(72)63-41(31-45(55)67)48(70)59-32)30-35-22-23-36-17-8-9-18-37(36)27-35/h2-9,13-18,22-23,27,32,38-44H,10-12,19-21,24-26,28-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,69)(H,62,71)(H,63,72)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089422

(CHEMBL3577978)Show SMILES [H][C@]12CSSC[C@@]([H])(NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@@H](NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N2)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C82H108N22O19S4/c1-43-68(111)95-55(33-46-15-7-4-8-16-46)73(116)103-63-41-126-125-40-62(77(120)94-54(22-14-32-90-82(87)88)69(112)96-56(34-47-17-9-5-10-18-47)71(114)97-57(35-48-19-11-6-12-20-48)72(115)100-60(38-65(83)109)70(113)91-43)102-75(118)59(37-50-25-29-52(108)30-26-50)99-79(122)64(104-80(123)66(44(2)105)92-45(3)106)42-127-124-39-61(76(119)93-53(67(84)110)21-13-31-89-81(85)86)101-74(117)58(98-78(63)121)36-49-23-27-51(107)28-24-49/h4-12,15-20,23-30,43-44,53-64,66,105,107-108H,13-14,21-22,31-42H2,1-3H3,(H2,83,109)(H2,84,110)(H,91,113)(H,92,106)(H,93,119)(H,94,120)(H,95,111)(H,96,112)(H,97,114)(H,98,121)(H,99,122)(H,100,115)(H,101,117)(H,102,118)(H,103,116)(H,104,123)(H4,85,86,89)(H4,87,88,90)/t43-,44+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63+,64-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089405
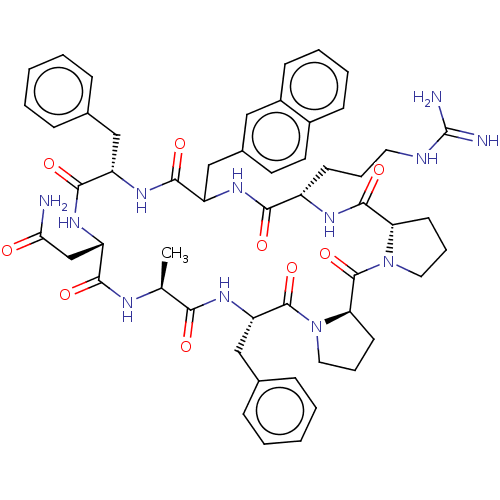
(CHEMBL3577987)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(Cc1ccc3ccccc3c1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-15-6-3-7-16-34)52(74)66-26-12-21-44(66)53(75)65-25-11-20-43(65)51(73)60-38(19-10-24-58-54(56)57)47(69)61-40(30-35-22-23-36-17-8-9-18-37(36)27-35)50(72)62-39(28-33-13-4-2-5-14-33)49(71)63-41(31-45(55)67)48(70)59-32/h2-9,13-18,22-23,27,32,38-44H,10-12,19-21,24-26,28-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,69)(H,62,72)(H,63,71)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089417

(CHEMBL3577999)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C52H70N12O8/c1-33-44(65)62-41(32-36-20-9-4-10-21-36)50(71)64-29-15-25-43(64)51(72)63-28-14-24-42(63)49(70)59-38(23-13-27-56-52(54)55)46(67)60-40(31-35-18-7-3-8-19-35)48(69)61-39(30-34-16-5-2-6-17-34)47(68)58-37(45(66)57-33)22-11-12-26-53/h2-10,16-21,33,37-43H,11-15,22-32,53H2,1H3,(H,57,66)(H,58,68)(H,59,70)(H,60,67)(H,61,69)(H,62,65)(H4,54,55,56)/t33-,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089415

(CHEMBL3577997)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)C(CCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C50H66N12O8/c1-31-42(63)60-39(30-34-18-9-4-10-19-34)48(69)62-27-13-22-41(62)49(70)61-26-12-21-40(61)47(68)57-35(20-11-25-54-50(52)53)44(65)58-38(29-33-16-7-3-8-17-33)46(67)59-37(28-32-14-5-2-6-15-32)45(66)56-36(23-24-51)43(64)55-31/h2-10,14-19,31,35-41H,11-13,20-30,51H2,1H3,(H,55,64)(H,56,66)(H,57,68)(H,58,65)(H,59,67)(H,60,63)(H4,52,53,54)/t31-,35-,36?,37-,38-,39-,40-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089402
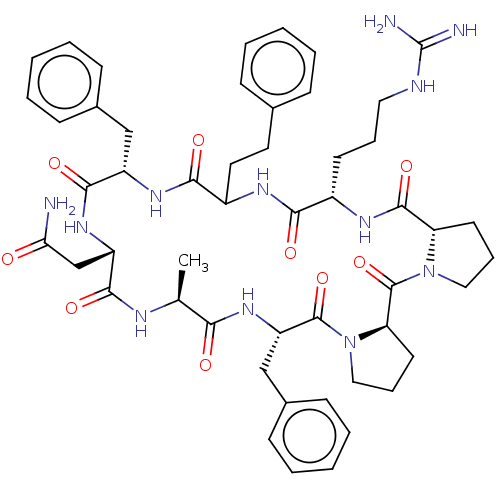
(CHEMBL3577985)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(CCc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C51H66N12O9/c1-31-43(65)61-39(29-34-18-9-4-10-19-34)49(71)63-27-13-22-41(63)50(72)62-26-12-21-40(62)48(70)58-35(20-11-25-55-51(53)54)44(66)57-36(24-23-32-14-5-2-6-15-32)45(67)59-37(28-33-16-7-3-8-17-33)47(69)60-38(30-42(52)64)46(68)56-31/h2-10,14-19,31,35-41H,11-13,20-30H2,1H3,(H2,52,64)(H,56,68)(H,57,66)(H,58,70)(H,59,67)(H,60,69)(H,61,65)(H4,53,54,55)/t31-,35-,36?,37-,38-,39-,40-,41+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089414

(CHEMBL3577996)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)C(CN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C49H64N12O8/c1-30-41(62)58-37(28-33-18-9-4-10-19-33)47(68)61-25-13-22-40(61)48(69)60-24-12-21-39(60)46(67)55-34(20-11-23-53-49(51)52)42(63)56-35(26-31-14-5-2-6-15-31)43(64)57-36(27-32-16-7-3-8-17-32)44(65)59-38(29-50)45(66)54-30/h2-10,14-19,30,34-40H,11-13,20-29,50H2,1H3,(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,58,62)(H,59,65)(H4,51,52,53)/t30-,34-,35-,36-,37-,38?,39-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089406
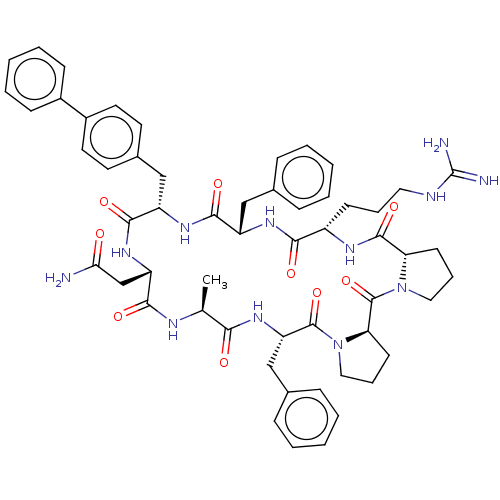
(CHEMBL3577988)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C56H68N12O9/c1-34-48(70)66-44(32-36-16-7-3-8-17-36)54(76)68-29-13-22-46(68)55(77)67-28-12-21-45(67)53(75)62-40(20-11-27-60-56(58)59)49(71)63-41(30-35-14-5-2-6-15-35)51(73)64-42(52(74)65-43(33-47(57)69)50(72)61-34)31-37-23-25-39(26-24-37)38-18-9-4-10-19-38/h2-10,14-19,23-26,34,40-46H,11-13,20-22,27-33H2,1H3,(H2,57,69)(H,61,72)(H,62,75)(H,63,71)(H,64,73)(H,65,74)(H,66,70)(H4,58,59,60)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089424
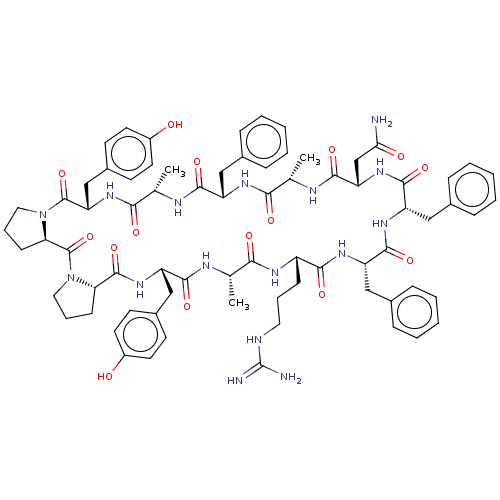
(CHEMBL3577979)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC2=O |r| Show InChI InChI=1S/C74H92N16O15/c1-42-62(94)82-52(22-13-33-78-74(76)77)65(97)84-55(37-46-18-9-5-10-19-46)69(101)85-56(38-47-20-11-6-12-21-47)70(102)86-57(41-61(75)93)68(100)81-43(2)63(95)83-53(36-45-16-7-4-8-17-45)66(98)80-44(3)64(96)88-58(40-49-27-31-51(92)32-28-49)72(104)90-35-15-24-60(90)73(105)89-34-14-23-59(89)71(103)87-54(67(99)79-42)39-48-25-29-50(91)30-26-48/h4-12,16-21,25-32,42-44,52-60,91-92H,13-15,22-24,33-41H2,1-3H3,(H2,75,93)(H,79,99)(H,80,98)(H,81,100)(H,82,94)(H,83,95)(H,84,97)(H,85,101)(H,86,102)(H,87,103)(H,88,96)(H4,76,77,78)/t42-,43-,44-,52-,53-,54-,55-,56-,57-,58-,59-,60+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089399
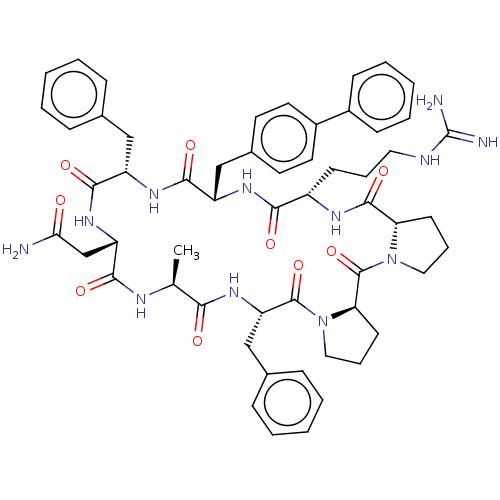
(CHEMBL3577982)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C56H68N12O9/c1-34-48(70)66-44(32-36-16-7-3-8-17-36)54(76)68-29-13-22-46(68)55(77)67-28-12-21-45(67)53(75)62-40(20-11-27-60-56(58)59)49(71)63-42(31-37-23-25-39(26-24-37)38-18-9-4-10-19-38)51(73)64-41(30-35-14-5-2-6-15-35)52(74)65-43(33-47(57)69)50(72)61-34/h2-10,14-19,23-26,34,40-46H,11-13,20-22,27-33H2,1H3,(H2,57,69)(H,61,72)(H,62,75)(H,63,71)(H,64,73)(H,65,74)(H,66,70)(H4,58,59,60)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089421
(CHEMBL3577977)Show SMILES C[C@@H](O)[C@H](NC(C)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C84H114N22O21S2/c1-43-70(115)97-57(35-48-17-9-6-10-18-48)75(120)104-64(79(124)100-61(39-52-27-31-54(112)32-28-52)77(122)105-67(45(3)108)80(125)95-55(69(86)114)23-15-33-91-83(87)88)42-129-128-41-63(103-76(121)60(38-51-25-29-53(111)30-26-51)102-81(126)68(46(4)109)106-82(127)66(44(2)107)94-47(5)110)78(123)96-56(24-16-34-92-84(89)90)71(116)98-58(36-49-19-11-7-12-20-49)73(118)99-59(37-50-21-13-8-14-22-50)74(119)101-62(40-65(85)113)72(117)93-43/h6-14,17-22,25-32,43-46,55-64,66-68,107-109,111-112H,15-16,23-24,33-42H2,1-5H3,(H2,85,113)(H2,86,114)(H,93,117)(H,94,110)(H,95,125)(H,96,123)(H,97,115)(H,98,116)(H,99,118)(H,100,124)(H,101,119)(H,102,126)(H,103,121)(H,104,120)(H,105,122)(H,106,127)(H4,87,88,91)(H4,89,90,92)/t43-,44+,45+,46+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,66-,67-,68-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089416

(CHEMBL3577998)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)C(CCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C51H68N12O8/c1-32-43(64)61-40(31-35-19-9-4-10-20-35)49(70)63-28-14-24-42(63)50(71)62-27-13-23-41(62)48(69)58-37(22-12-26-55-51(53)54)45(66)59-39(30-34-17-7-3-8-18-34)47(68)60-38(29-33-15-5-2-6-16-33)46(67)57-36(21-11-25-52)44(65)56-32/h2-10,15-20,32,36-42H,11-14,21-31,52H2,1H3,(H,56,65)(H,57,67)(H,58,69)(H,59,66)(H,60,68)(H,61,64)(H4,53,54,55)/t32-,36?,37-,38-,39-,40-,41-,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089418

(CHEMBL3578000)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CCCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C53H72N14O8/c1-33-44(68)65-41(32-36-20-9-4-10-21-36)50(74)67-29-15-25-43(67)51(75)66-28-14-24-42(66)49(73)62-38(23-13-27-59-53(56)57)46(70)63-40(31-35-18-7-3-8-19-35)48(72)64-39(30-34-16-5-2-6-17-34)47(71)61-37(45(69)60-33)22-11-12-26-58-52(54)55/h2-10,16-21,33,37-43H,11-15,22-32H2,1H3,(H,60,69)(H,61,71)(H,62,73)(H,63,70)(H,64,72)(H,65,68)(H4,54,55,58)(H4,56,57,59)/t33-,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50115362

(2-{2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C38H45N11O5/c1-22(50)46-33(18-27-20-42-21-45-27)37(54)49-32(16-23-12-13-24-7-2-3-8-25(24)15-23)36(53)47-30(11-6-14-43-38(40)41)35(52)48-31(34(39)51)17-26-19-44-29-10-5-4-9-28(26)29/h2-5,7-10,12-13,15,19-21,30-33,44H,6,11,14,16-18H2,1H3,(H2,39,51)(H,42,45)(H,46,50)(H,47,53)(H,48,52)(H,49,54)(H4,40,41,43)/t30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Binding constant (Ki) at mouse Melanocortin-3 receptor |
J Med Chem 45: 3073-81 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9CHF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089420
(CHEMBL3577976)Show SMILES C[C@@H](O)[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C82H110N22O19S2/c1-43(68(111)95-55(67(84)110)23-15-33-89-81(85)86)91-72(115)57(38-51-25-29-53(107)30-26-51)101-79(122)64-42-125-124-41-63(103-77(120)59(39-52-27-31-54(108)32-28-52)98-70(113)45(3)93-80(123)66(46(4)105)94-47(5)106)78(121)96-56(24-16-34-90-82(87)88)71(114)99-60(36-49-19-11-7-12-20-49)74(117)100-61(37-50-21-13-8-14-22-50)75(118)102-62(40-65(83)109)73(116)92-44(2)69(112)97-58(76(119)104-64)35-48-17-9-6-10-18-48/h6-14,17-22,25-32,43-46,55-64,66,105,107-108H,15-16,23-24,33-42H2,1-5H3,(H2,83,109)(H2,84,110)(H,91,115)(H,92,116)(H,93,123)(H,94,106)(H,95,111)(H,96,121)(H,97,112)(H,98,113)(H,99,114)(H,100,117)(H,101,122)(H,102,118)(H,103,120)(H,104,119)(H4,85,86,89)(H4,87,88,90)/t43-,44-,45-,46+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445878
(CHEMBL3105815)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(cs2)-c2cc(Cl)ccc2Cl)cc1 Show InChI InChI=1S/C19H16Cl2N2O3S2/c1-2-28(25,26)14-6-3-12(4-7-14)9-18(24)23-19-22-17(11-27-19)15-10-13(20)5-8-16(15)21/h3-8,10-11H,2,9H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 25-[26,27-3H]hydroxycholesterol from RORgammat receptor ligand binding domain (unknown origin) after 60 mins |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089398

(CHEMBL3577981)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C50H64N12O9/c1-30-42(64)60-38(28-33-18-9-4-10-19-33)48(70)62-25-13-22-40(62)49(71)61-24-12-21-39(61)47(69)56-34(20-11-23-54-50(52)53)43(65)57-35(26-31-14-5-2-6-15-31)45(67)58-36(27-32-16-7-3-8-17-32)46(68)59-37(29-41(51)63)44(66)55-30/h2-10,14-19,30,34-40H,11-13,20-29H2,1H3,(H2,51,63)(H,55,66)(H,56,69)(H,57,65)(H,58,67)(H,59,68)(H,60,64)(H4,52,53,54)/t30-,34-,35-,36-,37-,38-,39-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089422

(CHEMBL3577978)Show SMILES [H][C@]12CSSC[C@@]([H])(NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@@H](NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N2)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C82H108N22O19S4/c1-43-68(111)95-55(33-46-15-7-4-8-16-46)73(116)103-63-41-126-125-40-62(77(120)94-54(22-14-32-90-82(87)88)69(112)96-56(34-47-17-9-5-10-18-47)71(114)97-57(35-48-19-11-6-12-20-48)72(115)100-60(38-65(83)109)70(113)91-43)102-75(118)59(37-50-25-29-52(108)30-26-50)99-79(122)64(104-80(123)66(44(2)105)92-45(3)106)42-127-124-39-61(76(119)93-53(67(84)110)21-13-31-89-81(85)86)101-74(117)58(98-78(63)121)36-49-23-27-51(107)28-24-49/h4-12,15-20,23-30,43-44,53-64,66,105,107-108H,13-14,21-22,31-42H2,1-3H3,(H2,83,109)(H2,84,110)(H,91,113)(H,92,106)(H,93,119)(H,94,120)(H,95,111)(H,96,112)(H,97,114)(H,98,121)(H,99,122)(H,100,115)(H,101,117)(H,102,118)(H,103,116)(H,104,123)(H4,85,86,89)(H4,87,88,90)/t43-,44+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63+,64-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089399

(CHEMBL3577982)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C56H68N12O9/c1-34-48(70)66-44(32-36-16-7-3-8-17-36)54(76)68-29-13-22-46(68)55(77)67-28-12-21-45(67)53(75)62-40(20-11-27-60-56(58)59)49(71)63-42(31-37-23-25-39(26-24-37)38-18-9-4-10-19-38)51(73)64-41(30-35-14-5-2-6-15-35)52(74)65-43(33-47(57)69)50(72)61-34/h2-10,14-19,23-26,34,40-46H,11-13,20-22,27-33H2,1H3,(H2,57,69)(H,61,72)(H,62,75)(H,63,71)(H,64,73)(H,65,74)(H,66,70)(H4,58,59,60)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089421

(CHEMBL3577977)Show SMILES C[C@@H](O)[C@H](NC(C)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C84H114N22O21S2/c1-43-70(115)97-57(35-48-17-9-6-10-18-48)75(120)104-64(79(124)100-61(39-52-27-31-54(112)32-28-52)77(122)105-67(45(3)108)80(125)95-55(69(86)114)23-15-33-91-83(87)88)42-129-128-41-63(103-76(121)60(38-51-25-29-53(111)30-26-51)102-81(126)68(46(4)109)106-82(127)66(44(2)107)94-47(5)110)78(123)96-56(24-16-34-92-84(89)90)71(116)98-58(36-49-19-11-7-12-20-49)73(118)99-59(37-50-21-13-8-14-22-50)74(119)101-62(40-65(85)113)72(117)93-43/h6-14,17-22,25-32,43-46,55-64,66-68,107-109,111-112H,15-16,23-24,33-42H2,1-5H3,(H2,85,113)(H2,86,114)(H,93,117)(H,94,110)(H,95,125)(H,96,123)(H,97,115)(H,98,116)(H,99,118)(H,100,124)(H,101,119)(H,102,126)(H,103,121)(H,104,120)(H,105,122)(H,106,127)(H4,87,88,91)(H4,89,90,92)/t43-,44+,45+,46+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,66-,67-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089404

(CHEMBL3577986)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(Cc1cccc3ccccc13)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-16-6-3-7-17-34)52(74)66-27-13-24-44(66)53(75)65-26-12-23-43(65)51(73)60-38(22-11-25-58-54(56)57)47(69)62-40(30-36-20-10-19-35-18-8-9-21-37(35)36)50(72)61-39(28-33-14-4-2-5-15-33)49(71)63-41(31-45(55)67)48(70)59-32/h2-10,14-21,32,38-44H,11-13,22-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,72)(H,62,69)(H,63,71)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089420

(CHEMBL3577976)Show SMILES C[C@@H](O)[C@H](NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C82H110N22O19S2/c1-43(68(111)95-55(67(84)110)23-15-33-89-81(85)86)91-72(115)57(38-51-25-29-53(107)30-26-51)101-79(122)64-42-125-124-41-63(103-77(120)59(39-52-27-31-54(108)32-28-52)98-70(113)45(3)93-80(123)66(46(4)105)94-47(5)106)78(121)96-56(24-16-34-90-82(87)88)71(114)99-60(36-49-19-11-7-12-20-49)74(117)100-61(37-50-21-13-8-14-22-50)75(118)102-62(40-65(83)109)73(116)92-44(2)69(112)97-58(76(119)104-64)35-48-17-9-6-10-18-48/h6-14,17-22,25-32,43-46,55-64,66,105,107-108H,15-16,23-24,33-42H2,1-5H3,(H2,83,109)(H2,84,110)(H,91,115)(H,92,116)(H,93,123)(H,94,106)(H,95,111)(H,96,121)(H,97,112)(H,98,113)(H,99,114)(H,100,117)(H,101,122)(H,102,118)(H,103,120)(H,104,119)(H4,85,86,89)(H4,87,88,90)/t43-,44-,45-,46+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089405

(CHEMBL3577987)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(Cc1ccc3ccccc3c1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-15-6-3-7-16-34)52(74)66-26-12-21-44(66)53(75)65-25-11-20-43(65)51(73)60-38(19-10-24-58-54(56)57)47(69)61-40(30-35-22-23-36-17-8-9-18-37(36)27-35)50(72)62-39(28-33-13-4-2-5-14-33)49(71)63-41(31-45(55)67)48(70)59-32/h2-9,13-18,22-23,27,32,38-44H,10-12,19-21,24-26,28-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,69)(H,62,72)(H,63,71)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50089408
(CHEMBL3577990)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O)c1ccccc1 |r| Show InChI InChI=1S/C49H62N12O9/c1-29-41(63)58-36(27-31-16-7-3-8-17-31)47(69)61-25-13-22-38(61)48(70)60-24-12-21-37(60)45(67)55-33(20-11-23-53-49(51)52)42(64)56-34(26-30-14-5-2-6-15-30)44(66)59-40(32-18-9-4-10-19-32)46(68)57-35(28-39(50)62)43(65)54-29/h2-10,14-19,29,33-38,40H,11-13,20-28H2,1H3,(H2,50,62)(H,54,65)(H,55,67)(H,56,64)(H,57,68)(H,58,63)(H,59,66)(H4,51,52,53)/t29-,33-,34-,35-,36-,37-,38+,40?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC4R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089424

(CHEMBL3577979)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC2=O |r| Show InChI InChI=1S/C74H92N16O15/c1-42-62(94)82-52(22-13-33-78-74(76)77)65(97)84-55(37-46-18-9-5-10-19-46)69(101)85-56(38-47-20-11-6-12-21-47)70(102)86-57(41-61(75)93)68(100)81-43(2)63(95)83-53(36-45-16-7-4-8-17-45)66(98)80-44(3)64(96)88-58(40-49-27-31-51(92)32-28-49)72(104)90-35-15-24-60(90)73(105)89-34-14-23-59(89)71(103)87-54(67(99)79-42)39-48-25-29-50(91)30-26-48/h4-12,16-21,25-32,42-44,52-60,91-92H,13-15,22-24,33-41H2,1-3H3,(H2,75,93)(H,79,99)(H,80,98)(H,81,100)(H,82,94)(H,83,95)(H,84,97)(H,85,101)(H,86,102)(H,87,103)(H,88,96)(H4,76,77,78)/t42-,43-,44-,52-,53-,54-,55-,56-,57-,58-,59-,60+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50089411

(CHEMBL3577993)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)C(Cc1cccc3ccccc13)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC2=O |r| Show InChI InChI=1S/C54H66N12O9/c1-32-46(68)64-42(29-34-16-6-3-7-17-34)52(74)66-27-13-24-44(66)53(75)65-26-12-23-43(65)51(73)60-38(22-11-25-58-54(56)57)47(69)61-39(28-33-14-4-2-5-15-33)49(71)62-40(50(72)63-41(31-45(55)67)48(70)59-32)30-36-20-10-19-35-18-8-9-21-37(35)36/h2-10,14-21,32,38-44H,11-13,22-31H2,1H3,(H2,55,67)(H,59,70)(H,60,73)(H,61,69)(H,62,71)(H,63,72)(H,64,68)(H4,56,57,58)/t32-,38-,39-,40?,41-,42-,43-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of MTII from mouse MC3R expressed in HEK293 cells |
J Med Chem 58: 4638-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00184
BindingDB Entry DOI: 10.7270/Q2T155C9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50114745
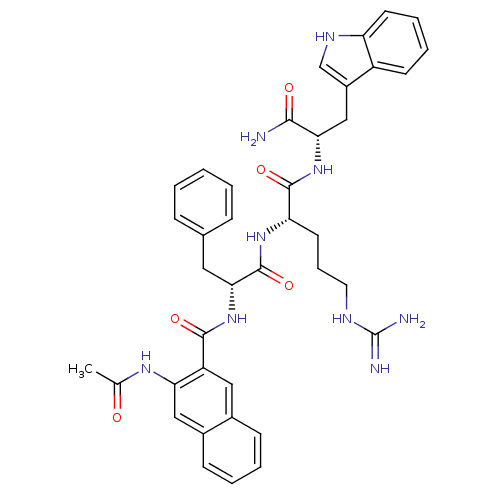
(1N-{3-[1-[4-amino(imino)methylamino-1-[1-carbamoyl...)Show SMILES CC(=O)Nc1cc2ccccc2cc1C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C39H43N9O5/c1-23(49)45-32-20-26-13-6-5-12-25(26)19-29(32)36(51)48-34(18-24-10-3-2-4-11-24)38(53)46-31(16-9-17-43-39(41)42)37(52)47-33(35(40)50)21-27-22-44-30-15-8-7-14-28(27)30/h2-8,10-15,19-20,22,31,33-34,44H,9,16-18,21H2,1H3,(H2,40,50)(H,45,49)(H,46,53)(H,47,52)(H,48,51)(H4,41,42,43)/t31-,33-,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Agonist activity of compound in mouse Melanocortin-3 receptor (mMC3R) |
J Med Chem 45: 2801-10 (2002)
BindingDB Entry DOI: 10.7270/Q27945DV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50033373
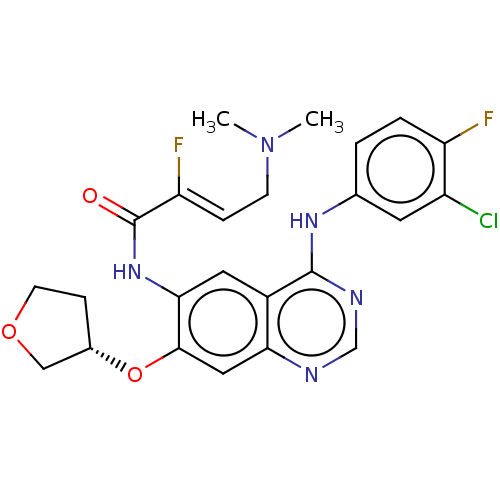
(CHEMBL3357641)Show SMILES CN(C)C\C=C(/F)C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H24ClF2N5O3/c1-32(2)7-5-19(27)24(33)31-21-10-16-20(11-22(21)35-15-6-8-34-12-15)28-13-29-23(16)30-14-3-4-18(26)17(25)9-14/h3-5,9-11,13,15H,6-8,12H2,1-2H3,(H,31,33)(H,28,29,30)/b19-5-/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) incubated for 5 mins by HTRF assay |
J Med Chem 57: 9889-900 (2014)
Article DOI: 10.1021/jm5014659
BindingDB Entry DOI: 10.7270/Q26W9CPC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50033374
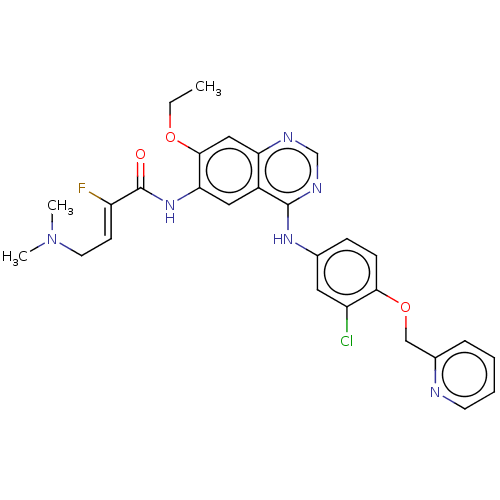
(CHEMBL3357637)Show SMILES CCOc1cc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)C(\F)=C\CN(C)C Show InChI InChI=1S/C28H28ClFN6O3/c1-4-38-26-15-23-20(14-24(26)35-28(37)22(30)10-12-36(2)3)27(33-17-32-23)34-18-8-9-25(21(29)13-18)39-16-19-7-5-6-11-31-19/h5-11,13-15,17H,4,12,16H2,1-3H3,(H,35,37)(H,32,33,34)/b22-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) incubated for 5 mins by HTRF assay |
J Med Chem 57: 9889-900 (2014)
Article DOI: 10.1021/jm5014659
BindingDB Entry DOI: 10.7270/Q26W9CPC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50033376
(CHEMBL3357634)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C(\F)=C\CN(C)C Show InChI InChI=1S/C21H20ClF2N5O2/c1-29(2)7-6-16(24)21(30)28-18-9-13-17(10-19(18)31-3)25-11-26-20(13)27-12-4-5-15(23)14(22)8-12/h4-6,8-11H,7H2,1-3H3,(H,28,30)(H,25,26,27)/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) incubated for 5 mins by HTRF assay |
J Med Chem 57: 9889-900 (2014)
Article DOI: 10.1021/jm5014659
BindingDB Entry DOI: 10.7270/Q26W9CPC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
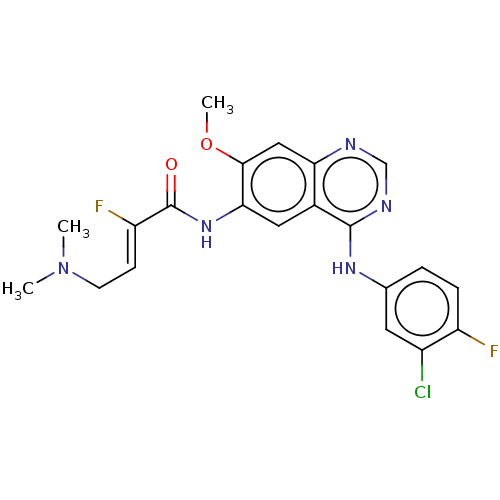
(Homo sapiens (Human)) | BDBM50033384
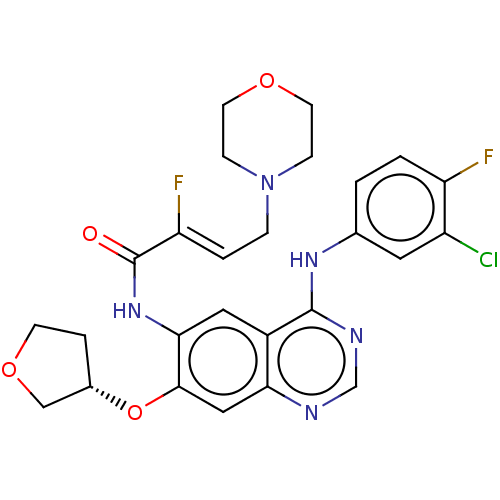
(CHEMBL3357639)Show SMILES F\C(=C/CN1CCOCC1)C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C26H26ClF2N5O4/c27-19-11-16(1-2-20(19)28)32-25-18-12-23(33-26(35)21(29)3-5-34-6-9-36-10-7-34)24(13-22(18)30-15-31-25)38-17-4-8-37-14-17/h1-3,11-13,15,17H,4-10,14H2,(H,33,35)(H,30,31,32)/b21-3-/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) incubated for 5 mins by HTRF assay |
J Med Chem 57: 9889-900 (2014)
Article DOI: 10.1021/jm5014659
BindingDB Entry DOI: 10.7270/Q26W9CPC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50144869

(CHEMBL411775 | Tyr-c[Asp-His-DPhe-Arg-Trp-Asn-Ala-...)Show SMILES C[C@@H]1NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](CC(=O)NC(NC(=O)[C@@H](Cc2ccccc2)NC1=O)C(=O)NN[C@@H](Cc1ccc(O)cc1)C(=O)C(O)=O)NN[C@H]1Cc2ccc(O)cc2NC1=O Show InChI InChI=1S/C73H85N21O17/c1-37-62(100)84-52(27-39-13-6-3-7-14-39)68(106)90-61(71(109)94-91-50(60(99)72(110)111)25-40-18-21-44(95)22-19-40)89-59(98)33-57(93-92-56-28-41-20-23-45(96)31-49(41)83-69(56)107)70(108)87-54(30-43-35-77-36-80-43)67(105)85-51(26-38-11-4-2-5-12-38)65(103)82-48(17-10-24-78-73(75)76)63(101)86-53(29-42-34-79-47-16-9-8-15-46(42)47)66(104)88-55(32-58(74)97)64(102)81-37/h2-9,11-16,18-23,31,34-37,48,50-57,61,79,91-93,95-96H,10,17,24-30,32-33H2,1H3,(H2,74,97)(H,77,80)(H,81,102)(H,82,103)(H,83,107)(H,84,100)(H,85,105)(H,86,101)(H,87,108)(H,88,104)(H,89,98)(H,90,106)(H,94,109)(H,110,111)(H4,75,76,78)/t37-,48+,50-,51+,52+,53-,54+,55+,56-,57+,61?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards melanocortin MC4 receptor by displacing radioligand [125I]-hAGRP(82-132) |
J Med Chem 47: 2194-207 (2004)
Article DOI: 10.1021/jm0303608
BindingDB Entry DOI: 10.7270/Q2474BM0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data