 Found 841 hits with Last Name = 'xin' and Initial = 'm'
Found 841 hits with Last Name = 'xin' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50609173
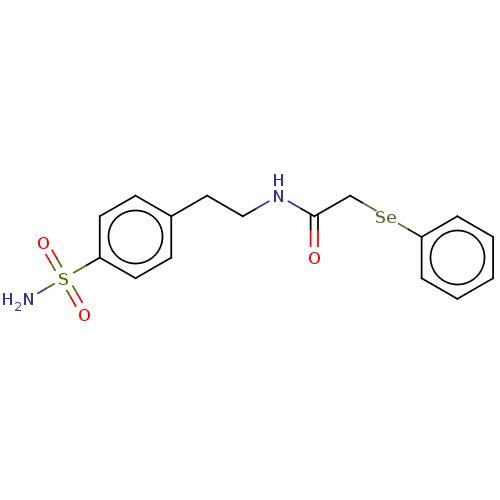
(CHEMBL5277717) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50609173

(CHEMBL5277717) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50609173

(CHEMBL5277717) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50609174
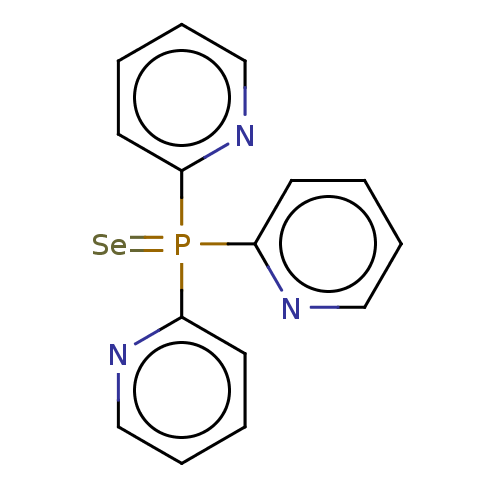
(CHEMBL5284662) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50609174

(CHEMBL5284662) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50609173

(CHEMBL5277717) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155308
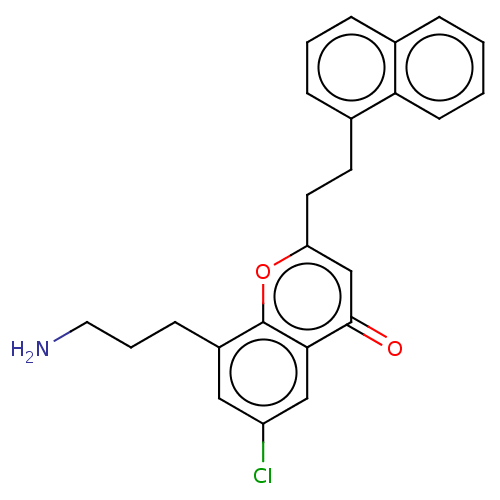
(CHEMBL3780791)Show SMILES Cl.Cl.NCCCc1cc(Cl)cc2c1oc(CCc1cccc3ccccc13)cc2=O Show InChI InChI=1S/C20H26N2O2/c23-19(14-7-2-1-3-8-14)18-11-6-12-22(18)20(24)17-13-15-9-4-5-10-16(15)21-17/h1-3,7-8,15-18,21H,4-6,9-13H2/t15-,16-,17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051567
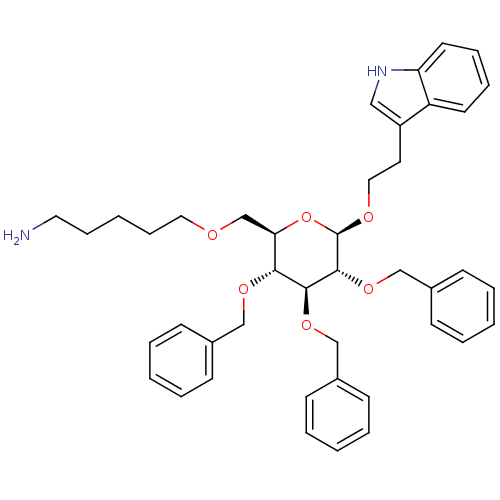
(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to sst4 receptor (unknown origin) |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155306
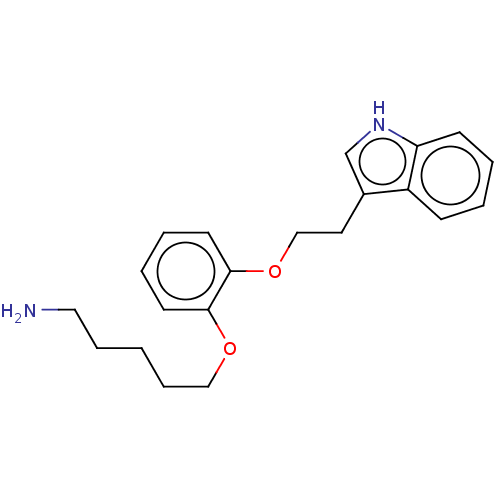
(CHEMBL3781875)Show InChI InChI=1S/C17H21N3O2S/c1-17(2,3)18-10-14(21)20-9-8-12(20)15(22)16-19-11-6-4-5-7-13(11)23-16/h4-7,12,18H,8-10H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to human sst4 receptor |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155305
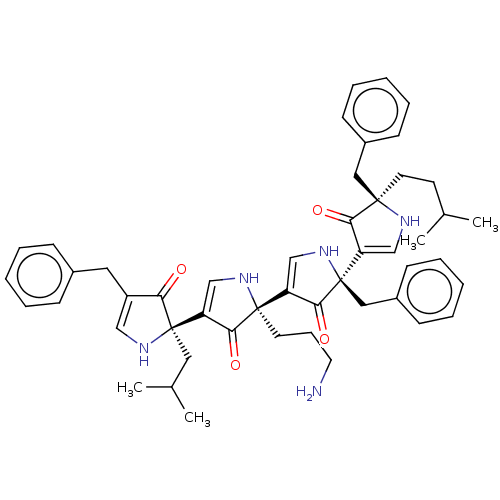
(CHEMBL3782021)Show SMILES CC(C)CC[C@]1(Cc2ccccc2)NC=C(C1=O)[C@@]1(Cc2ccccc2)NC=C(C1=O)[C@]1(CCCN)NC=C(C1=O)[C@@]1(CC(C)C)NC=C(Cc2ccccc2)C1=O |r,c:15,30,41,t:52| Show InChI InChI=1S/C49H57N5O4/c1-33(2)21-23-46(27-36-17-10-6-11-18-36)43(56)39(30-51-46)49(28-37-19-12-7-13-20-37)45(58)40(31-54-49)47(22-14-24-50)44(57)41(32-53-47)48(26-34(3)4)42(55)38(29-52-48)25-35-15-8-5-9-16-35/h5-13,15-20,29-34,51-54H,14,21-28,50H2,1-4H3/t46-,47+,48-,49-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-SRIF14 from human sst4 receptor |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155308

(CHEMBL3780791)Show SMILES Cl.Cl.NCCCc1cc(Cl)cc2c1oc(CCc1cccc3ccccc13)cc2=O Show InChI InChI=1S/C20H26N2O2/c23-19(14-7-2-1-3-8-14)18-11-6-12-22(18)20(24)17-13-15-9-4-5-10-16(15)21-17/h1-3,7-8,15-18,21H,4-6,9-13H2/t15-,16-,17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to sst2 receptor (unknown origin) |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155306

(CHEMBL3781875)Show InChI InChI=1S/C17H21N3O2S/c1-17(2,3)18-10-14(21)20-9-8-12(20)15(22)16-19-11-6-4-5-7-13(11)23-16/h4-7,12,18H,8-10H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 receptor |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155309
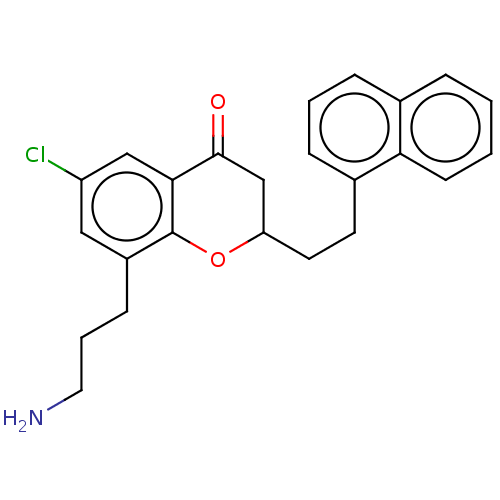
(CHEMBL3780496)Show SMILES NCCCc1cc(Cl)cc2C(=O)CC(CCc3cccc4ccccc34)Oc12 Show InChI InChI=1S/C18H24N2O2S/c21-17(13-7-9-23-11-13)16-6-3-8-20(16)18(22)15-10-12-4-1-2-5-14(12)19-15/h7,9,11-12,14-16,19H,1-6,8,10H2/t12-,14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155309

(CHEMBL3780496)Show SMILES NCCCc1cc(Cl)cc2C(=O)CC(CCc3cccc4ccccc34)Oc12 Show InChI InChI=1S/C18H24N2O2S/c21-17(13-7-9-23-11-13)16-6-3-8-20(16)18(22)15-10-12-4-1-2-5-14(12)19-15/h7,9,11-12,14-16,19H,1-6,8,10H2/t12-,14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50609172
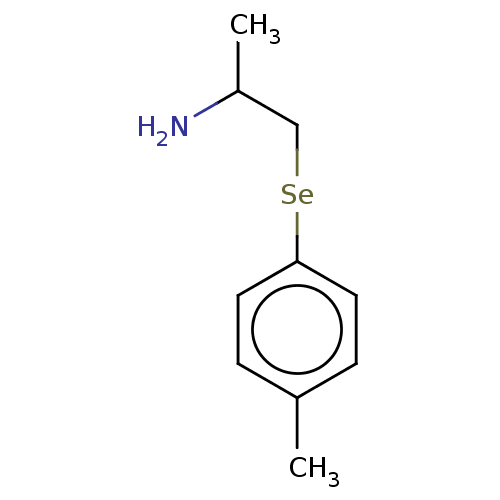
(CHEMBL5278584) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502319
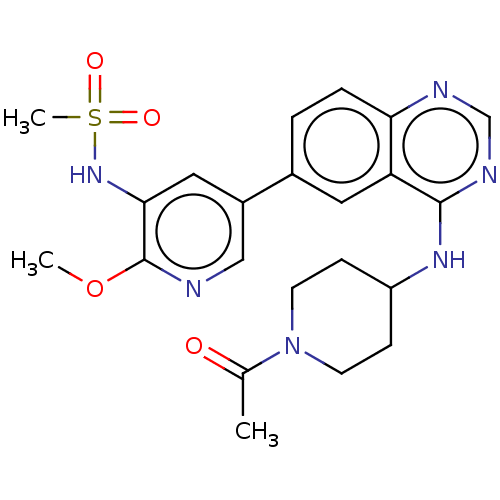
(CHEMBL4515169)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(NC3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C22H26N6O4S/c1-14(29)28-8-6-17(7-9-28)26-21-18-10-15(4-5-19(18)24-13-25-21)16-11-20(27-33(3,30)31)22(32-2)23-12-16/h4-5,10-13,17,27H,6-9H2,1-3H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502315
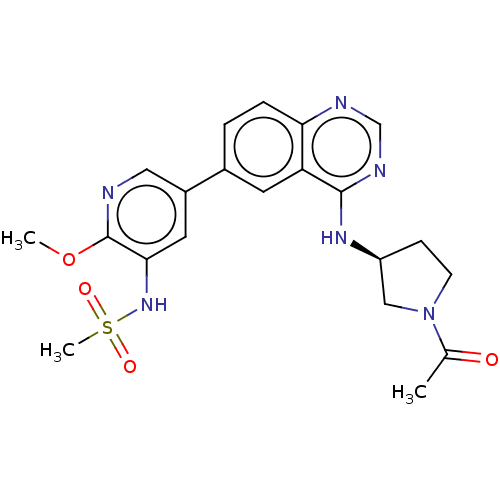
(CHEMBL4449091)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(N[C@H]3CCN(C3)C(C)=O)c2c1 |r| Show InChI InChI=1S/C21H24N6O4S/c1-13(28)27-7-6-16(11-27)25-20-17-8-14(4-5-18(17)23-12-24-20)15-9-19(26-32(3,29)30)21(31-2)22-10-15/h4-5,8-10,12,16,26H,6-7,11H2,1-3H3,(H,23,24,25)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem 23: 6059-68 (2015)
Article DOI: 10.1016/j.bmc.2015.05.043
BindingDB Entry DOI: 10.7270/Q2445P7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50510261
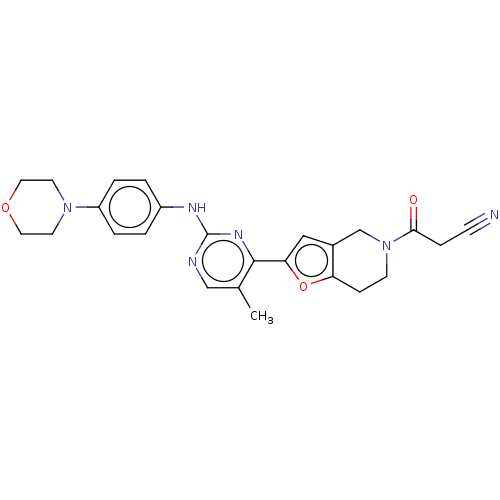
(CHEMBL4590082)Show SMILES Cc1cnc(Nc2ccc(cc2)N2CCOCC2)nc1-c1cc2CN(CCc2o1)C(=O)CC#N Show InChI InChI=1S/C25H26N6O3/c1-17-15-27-25(28-19-2-4-20(5-3-19)30-10-12-33-13-11-30)29-24(17)22-14-18-16-31(23(32)6-8-26)9-7-21(18)34-22/h2-5,14-15H,6-7,9-13,16H2,1H3,(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using TK-substrate-biotin as substrate preincubated for 5 mins followed by substrate addition and measured by 30 ... |
Bioorg Med Chem 27: 2592-2597 (2019)
Article DOI: 10.1016/j.bmc.2019.03.048
BindingDB Entry DOI: 10.7270/Q25D8W5B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM1774

(US10894048, Ref. Ex Comp. 1 | US8476284, 13)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shaanxi University of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by FRET assay |
Eur J Med Chem 151: 315-326 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.062
BindingDB Entry DOI: 10.7270/Q2JS9SZ0 |
More data for this
Ligand-Target Pair | |
Sonic hedgehog protein
(Mus musculus (Mouse)) | BDBM50443885

(CHEMBL3091556)Show SMILES Cc1cccc(C)c1NC(=O)c1ccc(Nc2ncc(c(n2)-c2ccc(OC(F)(F)F)cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C27H20F6N4O2/c1-15-4-3-5-16(2)22(15)36-24(38)18-6-10-19(11-7-18)35-25-34-14-21(26(28,29)30)23(37-25)17-8-12-20(13-9-17)39-27(31,32)33/h3-14H,1-2H3,(H,36,38)(H,34,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Simcere Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SHH signaling pathway in mouse NIH3T3 cells measured after 48 hrs by Gli-luciferase reporter assay |
Bioorg Med Chem Lett 23: 6777-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.022
BindingDB Entry DOI: 10.7270/Q2R49S7W |
More data for this
Ligand-Target Pair | |
Sonic hedgehog protein
(Mus musculus (Mouse)) | BDBM50443912

(CHEMBL3091466)Show SMILES Cc1cccc(C)c1NC(=O)c1ccc(Nc2ncc(C)c(n2)-c2ccncc2)cc1 Show InChI InChI=1S/C25H23N5O/c1-16-5-4-6-17(2)22(16)29-24(31)20-7-9-21(10-8-20)28-25-27-15-18(3)23(30-25)19-11-13-26-14-12-19/h4-15H,1-3H3,(H,29,31)(H,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Simcere Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SHH signaling pathway in mouse NIH3T3 cells measured after 48 hrs by Gli-luciferase reporter assay |
Bioorg Med Chem Lett 23: 6777-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.022
BindingDB Entry DOI: 10.7270/Q2R49S7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50525693
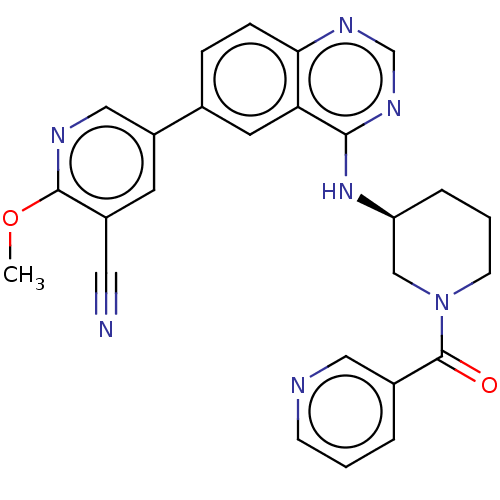
(CHEMBL4468088)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(N[C@H]3CCCN(C3)C(=O)c3cccnc3)c2c1 |r| Show InChI InChI=1S/C26H23N7O2/c1-35-25-19(12-27)10-20(14-29-25)17-6-7-23-22(11-17)24(31-16-30-23)32-21-5-3-9-33(15-21)26(34)18-4-2-8-28-13-18/h2,4,6-8,10-11,13-14,16,21H,3,5,9,15H2,1H3,(H,30,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K delta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins in presence of ATP by Kinase-Glo Plus reage... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.051
BindingDB Entry DOI: 10.7270/Q2MC93GX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50463838
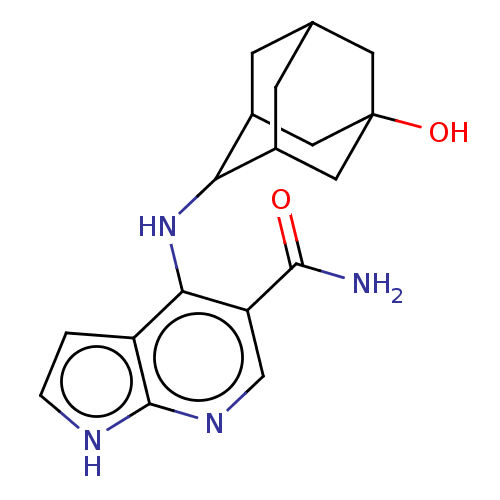
(CHEMBL4238926)Show SMILES NC(=O)c1cnc2[nH]ccc2c1NC1C2CC3CC1CC(O)(C3)C2 |TLB:13:14:22:17.18.19,12:13:22.16.17:19,THB:15:16:19:23.14.13,15:14:22.16.17:19,13:18:22:23.15.14,(62.43,-6.04,;61.1,-6.81,;61.1,-8.35,;59.76,-6.04,;59.75,-4.49,;58.42,-3.73,;57.08,-4.5,;55.61,-4.03,;54.71,-5.28,;55.62,-6.53,;57.09,-6.04,;58.42,-6.82,;58.42,-8.36,;57.14,-9.21,;57.12,-10.75,;56.11,-12.03,;54.69,-11.48,;54.69,-9.88,;55.72,-8.64,;54.38,-9.11,;54.39,-10.61,;53.04,-9.84,;53.19,-11.89,;55.71,-11.09,)| Show InChI InChI=1S/C18H22N4O2/c19-16(23)13-8-21-17-12(1-2-20-17)15(13)22-14-10-3-9-4-11(14)7-18(24,5-9)6-10/h1-2,8-11,14,24H,3-7H2,(H2,19,23)(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 kinase-domain using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr by ELISA |
Bioorg Med Chem 27: 2592-2597 (2019)
Article DOI: 10.1016/j.bmc.2019.03.048
BindingDB Entry DOI: 10.7270/Q25D8W5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502318
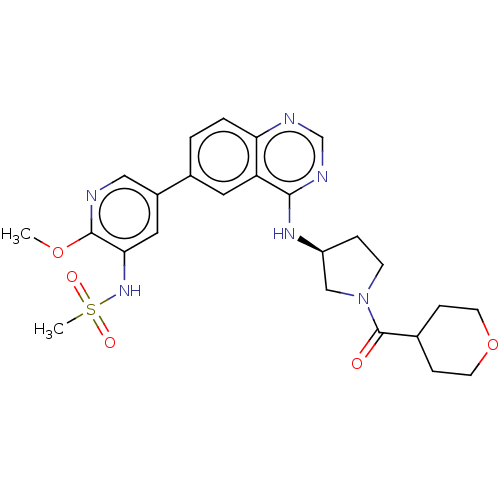
(CHEMBL4542688)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2c1 |r| Show InChI InChI=1S/C25H30N6O5S/c1-35-24-22(30-37(2,33)34)12-18(13-26-24)17-3-4-21-20(11-17)23(28-15-27-21)29-19-5-8-31(14-19)25(32)16-6-9-36-10-7-16/h3-4,11-13,15-16,19,30H,5-10,14H2,1-2H3,(H,27,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50161162
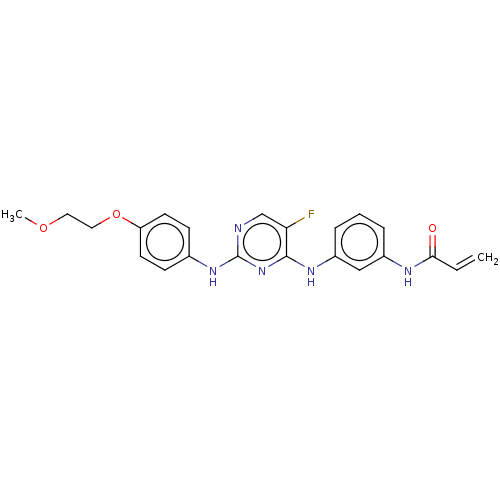
(AVL-292 | CC-292 | Spebrutinib | US10596172, Compo...)Show SMILES COCCOc1ccc(Nc2ncc(F)c(Nc3cccc(NC(=O)C=C)c3)n2)cc1 Show InChI InChI=1S/C19H18ClN3O/c20-13-9-11-7-8-21-14-6-5-10-3-1-2-4-12(10)16(14)15(11)18-17(13)22-19(24)23-18/h1-4,9,14,16,21H,5-8H2,(H2,22,23,24)/t14-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Shaanxi University of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Eur J Med Chem 151: 315-326 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.062
BindingDB Entry DOI: 10.7270/Q2JS9SZ0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50201907

(CHEMBL3940537)Show SMILES Cc1cnc(Nc2ccc(cc2)N2CCOCC2)nc1-c1cc2CN(CCc2o1)S(C)(=O)=O Show InChI InChI=1S/C23H27N5O4S/c1-16-14-24-23(25-18-3-5-19(6-4-18)27-9-11-31-12-10-27)26-22(16)21-13-17-15-28(33(2,29)30)8-7-20(17)32-21/h3-6,13-14H,7-12,15H2,1-2H3,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant human JAK2 expressed in baculovirus using TK-substrate-biotin as substrate preincubated for 5 mins followed by s... |
Bioorg Med Chem 25: 75-83 (2017)
Article DOI: 10.1016/j.bmc.2016.10.011
BindingDB Entry DOI: 10.7270/Q21Z46CH |
More data for this
Ligand-Target Pair | |
Sonic hedgehog protein
(Mus musculus (Mouse)) | BDBM50443889

(CHEMBL3091552)Show SMILES CCCc1cc(nc(Nc2ccc(cc2)C(=O)Nc2c(C)cccc2C)n1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H27F3N4O2/c1-4-6-23-17-25(20-11-15-24(16-12-20)38-29(30,31)32)35-28(34-23)33-22-13-9-21(10-14-22)27(37)36-26-18(2)7-5-8-19(26)3/h5,7-17H,4,6H2,1-3H3,(H,36,37)(H,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Simcere Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SHH signaling pathway in mouse NIH3T3 cells measured after 48 hrs by Gli-luciferase reporter assay |
Bioorg Med Chem Lett 23: 6777-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.022
BindingDB Entry DOI: 10.7270/Q2R49S7W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458067
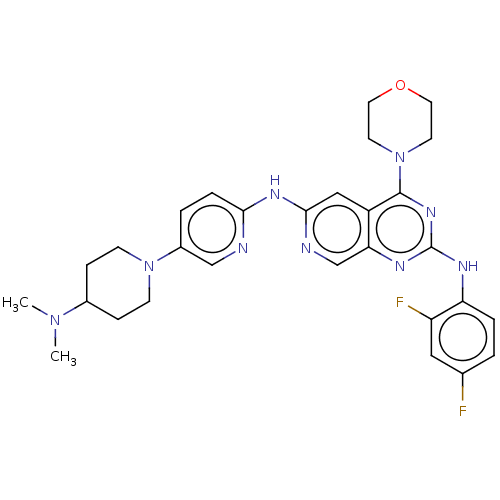
(CHEMBL4215080)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4F)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H33F2N9O/c1-38(2)20-7-9-39(10-8-20)21-4-6-26(32-17-21)36-27-16-22-25(18-33-27)35-29(34-24-5-3-19(30)15-23(24)31)37-28(22)40-11-13-41-14-12-40/h3-6,15-18,20H,7-14H2,1-2H3,(H,32,33,36)(H,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458066
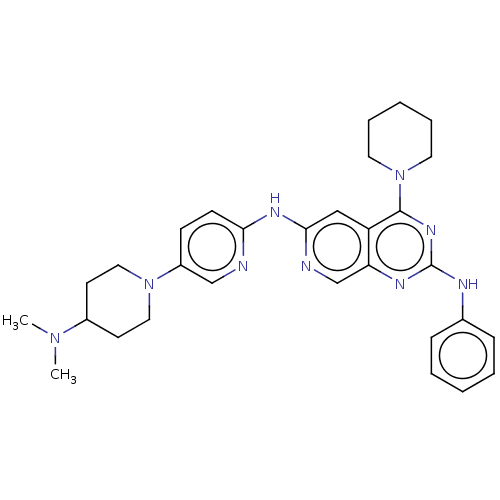
(CHEMBL4203016)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C30H37N9/c1-37(2)23-13-17-38(18-14-23)24-11-12-27(31-20-24)35-28-19-25-26(21-32-28)34-30(33-22-9-5-3-6-10-22)36-29(25)39-15-7-4-8-16-39/h3,5-6,9-12,19-21,23H,4,7-8,13-18H2,1-2H3,(H,31,32,35)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502321
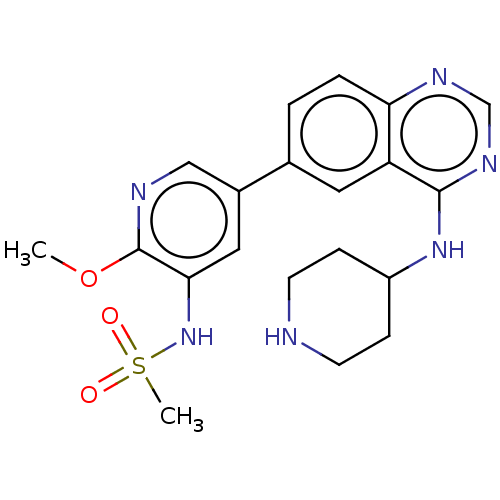
(CHEMBL4461362)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(NC3CCNCC3)c2c1 Show InChI InChI=1S/C20H24N6O3S/c1-29-20-18(26-30(2,27)28)10-14(11-22-20)13-3-4-17-16(9-13)19(24-12-23-17)25-15-5-7-21-8-6-15/h3-4,9-12,15,21,26H,5-8H2,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502317
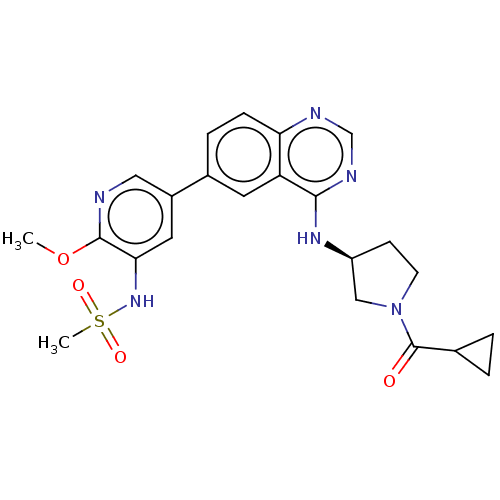
(CHEMBL4471468)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(N[C@H]3CCN(C3)C(=O)C3CC3)c2c1 |r| Show InChI InChI=1S/C23H26N6O4S/c1-33-22-20(28-34(2,31)32)10-16(11-24-22)15-5-6-19-18(9-15)21(26-13-25-19)27-17-7-8-29(12-17)23(30)14-3-4-14/h5-6,9-11,13-14,17,28H,3-4,7-8,12H2,1-2H3,(H,25,26,27)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T790M double mutant (unknown origin) |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sonic hedgehog protein
(Mus musculus (Mouse)) | BDBM50443903
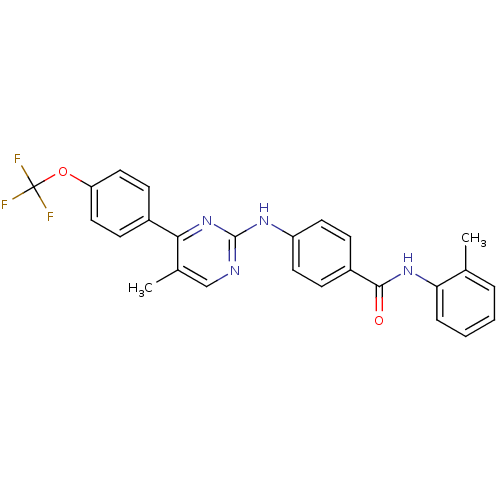
(CHEMBL3091565)Show SMILES Cc1ccccc1NC(=O)c1ccc(Nc2ncc(C)c(n2)-c2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C26H21F3N4O2/c1-16-5-3-4-6-22(16)32-24(34)19-7-11-20(12-8-19)31-25-30-15-17(2)23(33-25)18-9-13-21(14-10-18)35-26(27,28)29/h3-15H,1-2H3,(H,32,34)(H,30,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Simcere Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SHH signaling pathway in mouse NIH3T3 cells measured after 48 hrs by Gli-luciferase reporter assay |
Bioorg Med Chem Lett 23: 6777-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.022
BindingDB Entry DOI: 10.7270/Q2R49S7W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using TK-substrate-biotin as substrate preincubated for 5 mins followed by substrate addition and measured by 30 ... |
Bioorg Med Chem 27: 2592-2597 (2019)
Article DOI: 10.1016/j.bmc.2019.03.048
BindingDB Entry DOI: 10.7270/Q25D8W5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using TK-substrate-biotin as substrate preincubated for 5 mins followed by substrate addition and measured by 30 ... |
Bioorg Med Chem 27: 2592-2597 (2019)
Article DOI: 10.1016/j.bmc.2019.03.048
BindingDB Entry DOI: 10.7270/Q25D8W5B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458068
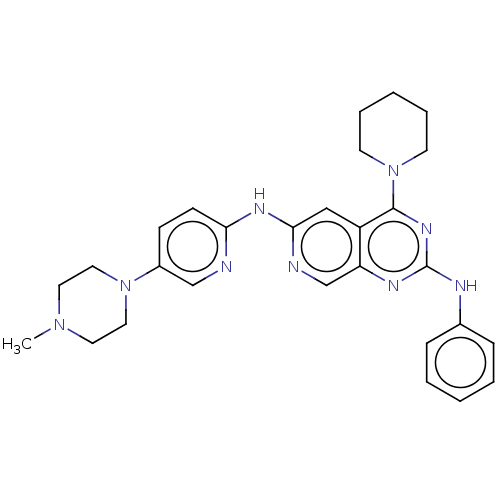
(CHEMBL4206716)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C28H33N9/c1-35-14-16-36(17-15-35)22-10-11-25(29-19-22)33-26-18-23-24(20-30-26)32-28(31-21-8-4-2-5-9-21)34-27(23)37-12-6-3-7-13-37/h2,4-5,8-11,18-20H,3,6-7,12-17H2,1H3,(H,29,30,33)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458062
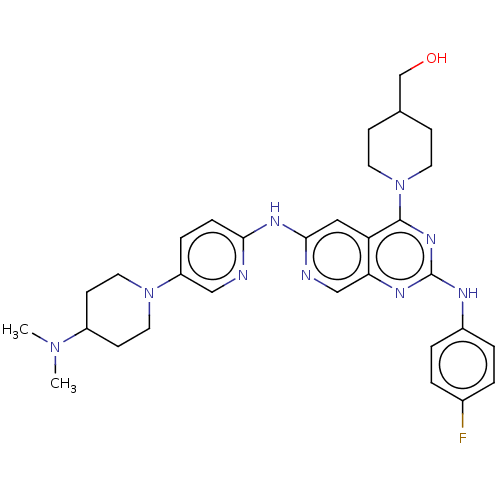
(CHEMBL4209019)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4)nc3cn2)N2CCC(CO)CC2)nc1 Show InChI InChI=1S/C31H38FN9O/c1-39(2)24-11-15-40(16-12-24)25-7-8-28(33-18-25)37-29-17-26-27(19-34-29)36-31(35-23-5-3-22(32)4-6-23)38-30(26)41-13-9-21(20-42)10-14-41/h3-8,17-19,21,24,42H,9-16,20H2,1-2H3,(H,33,34,37)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458068

(CHEMBL4206716)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C28H33N9/c1-35-14-16-36(17-15-35)22-10-11-25(29-19-22)33-26-18-23-24(20-30-26)32-28(31-21-8-4-2-5-9-21)34-27(23)37-12-6-3-7-13-37/h2,4-5,8-11,18-20H,3,6-7,12-17H2,1H3,(H,29,30,33)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Sonic hedgehog protein
(Mus musculus (Mouse)) | BDBM50443896
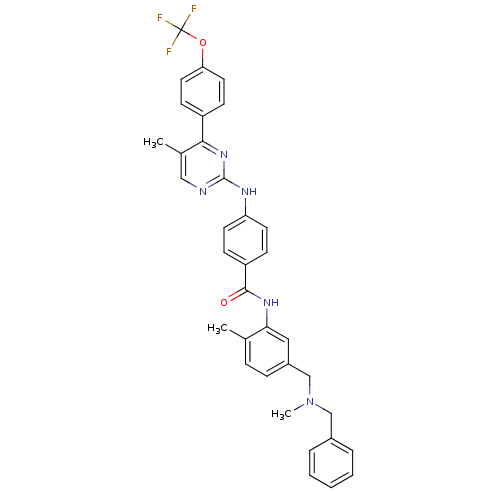
(CHEMBL3091572)Show SMILES CN(Cc1ccccc1)Cc1ccc(C)c(NC(=O)c2ccc(Nc3ncc(C)c(n3)-c3ccc(OC(F)(F)F)cc3)cc2)c1 Show InChI InChI=1S/C35H32F3N5O2/c1-23-9-10-26(22-43(3)21-25-7-5-4-6-8-25)19-31(23)41-33(44)28-11-15-29(16-12-28)40-34-39-20-24(2)32(42-34)27-13-17-30(18-14-27)45-35(36,37)38/h4-20H,21-22H2,1-3H3,(H,41,44)(H,39,40,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Simcere Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SHH signaling pathway in mouse NIH3T3 cells measured after 48 hrs by Gli-luciferase reporter assay |
Bioorg Med Chem Lett 23: 6777-83 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.022
BindingDB Entry DOI: 10.7270/Q2R49S7W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR-2 (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-3
(Homo sapiens (Human)) | BDBM50461172
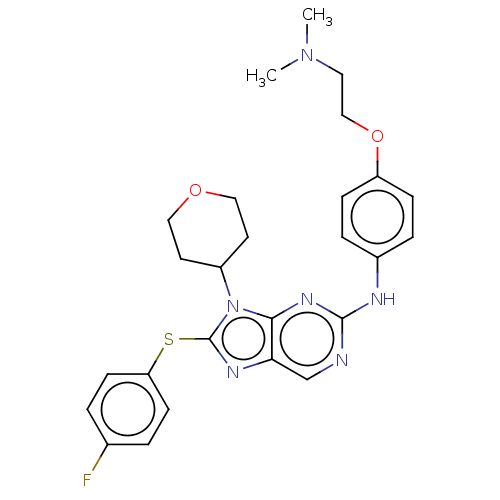
(CHEMBL4228518)Show SMILES CN(C)CCOc1ccc(Nc2ncc3nc(Sc4ccc(F)cc4)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C26H29FN6O2S/c1-32(2)13-16-35-21-7-5-19(6-8-21)29-25-28-17-23-24(31-25)33(20-11-14-34-15-12-20)26(30-23)36-22-9-3-18(27)4-10-22/h3-10,17,20H,11-16H2,1-2H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data