

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50111903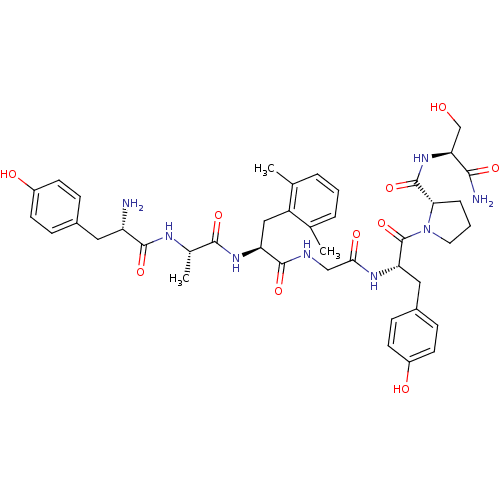 ((S)-1-[(S)-2-{2-[(S)-2-{(S)-2-[(S)-2-Amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.000540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor mu 1 using [3H]-DAMGO in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50111905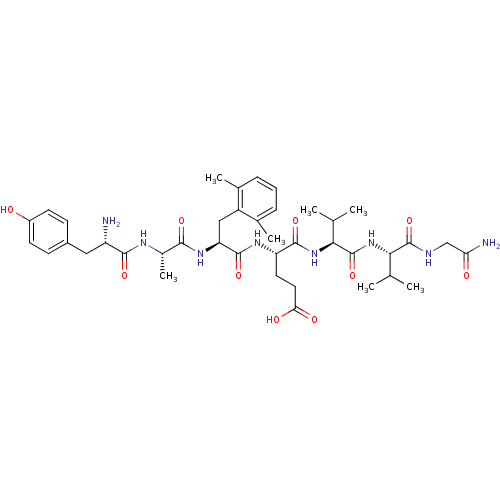 ((S)-4-[(S)-2-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 using [3H]-DT in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468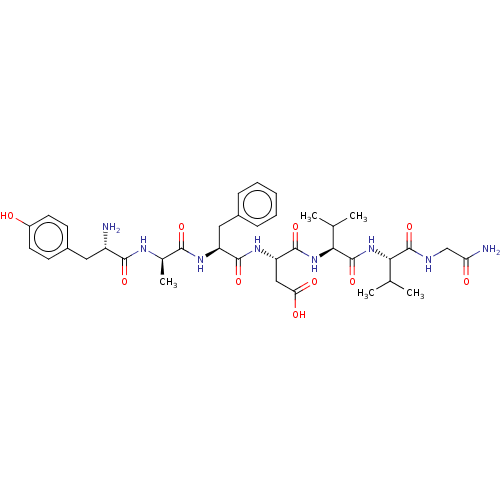 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 using [3H]-DT in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096716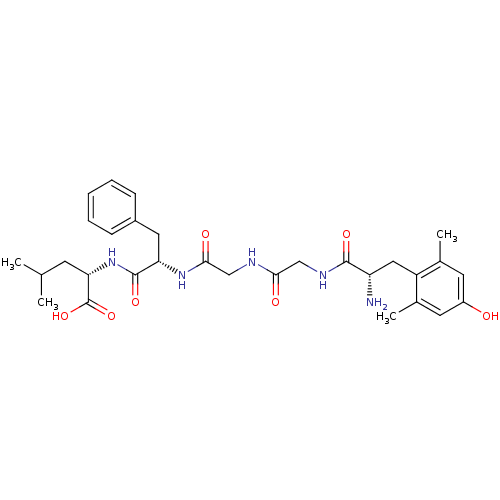 (CHEMBL100480 | Enkephalin derivative) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor mu 1 in rat brain synaptosomes using [3H]-DAMGO as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419354 (GR205171A | VOFOPITANT DIHYDROCHLORIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 19: 6430-46 (2011) Article DOI: 10.1016/j.bmc.2011.08.070 BindingDB Entry DOI: 10.7270/Q228081R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096719 ((S)-2-[(R)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor mu 1 in rat brain synaptosomes using [3H]-DAMGO as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096716 (CHEMBL100480 | Enkephalin derivative) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor delta 1 in rat brain synaptosomes using [3H]-deltorphin II as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17428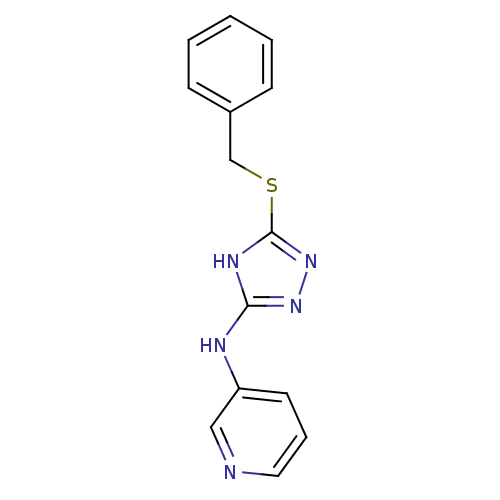 (1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17355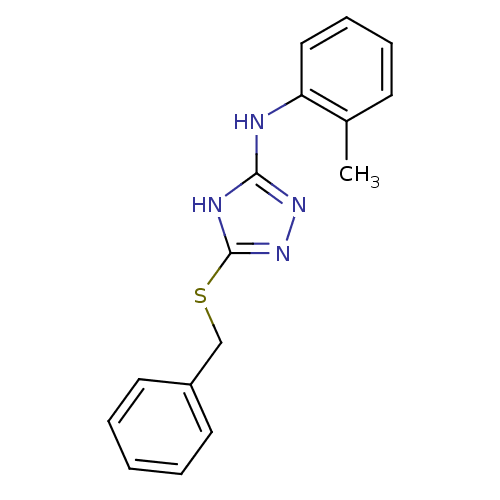 (1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17388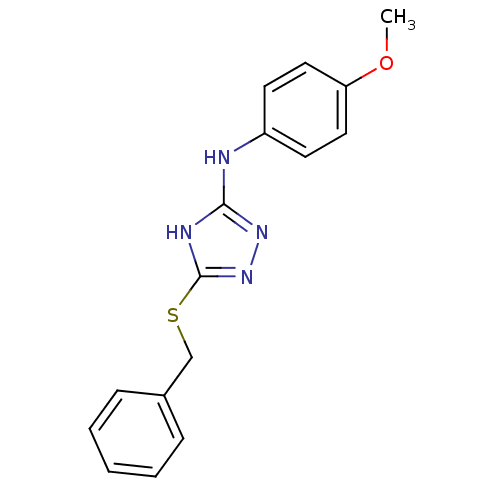 (1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17365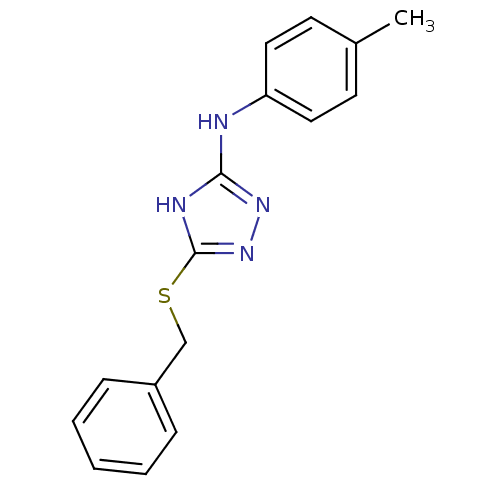 (1,2,4-Triazole Compound, 23 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059841 ((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor mu 1 using [3H]-DAMGO in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17390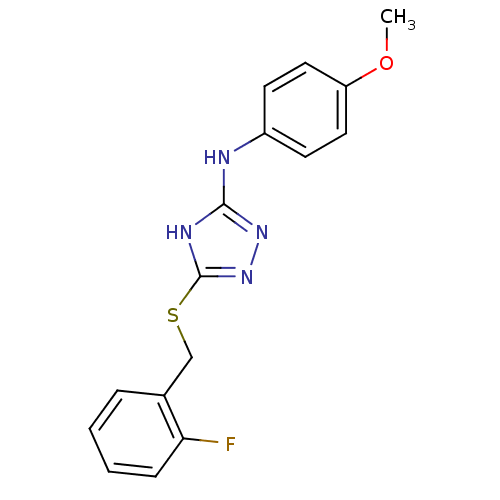 (1,2,4-Triazole Compound, 48 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17362 (1,2,4-Triazole Compound, 20 | 5-[(3-methylbut-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096719 ((S)-2-[(R)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor delta 1 in rat brain synaptosomes using [3H]-deltorphin II as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17395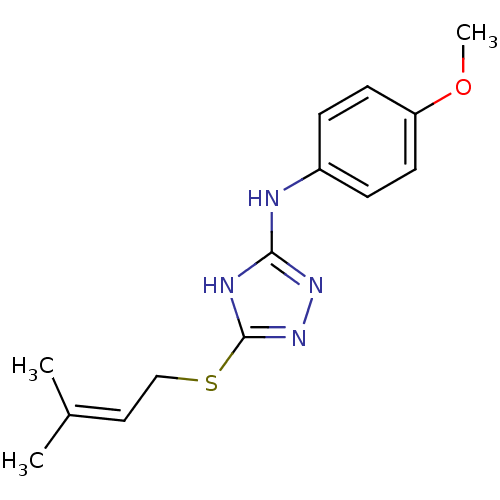 (1,2,4-Triazole Compound, 53 | N-(4-methoxyphenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17358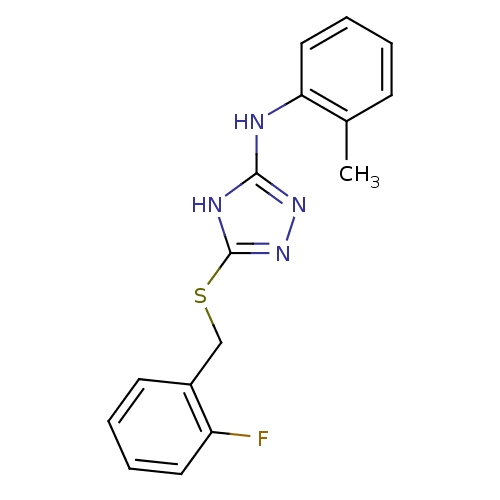 (1,2,4-Triazole Compound, 16 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17430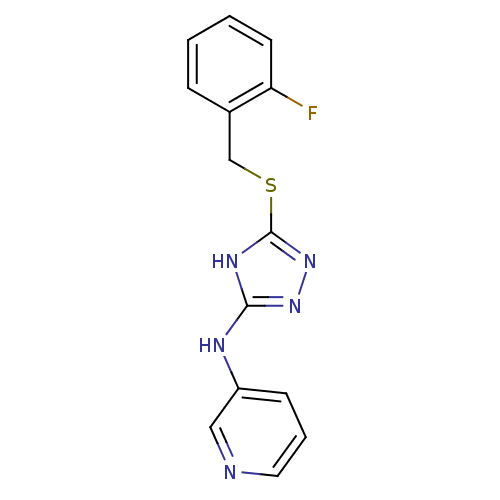 (1,2,4-Triazole Compound, 88 | N-(5-{[(2-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17411 (1,2,4-Triazole Compound, 69 | methyl 4-[(5-{[(2-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17377 (1,2,4-Triazole Compound, 35 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17352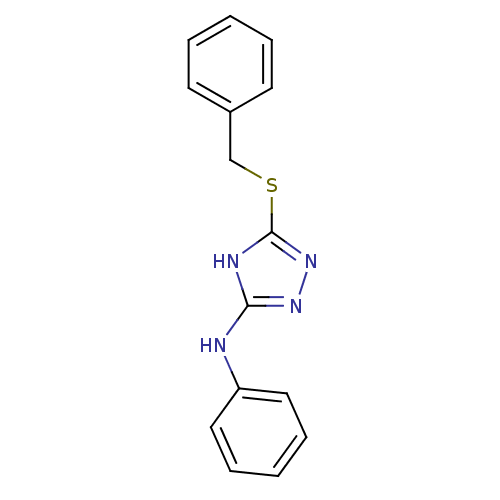 (1,2,4-Triazole Compound, 6 | 5-(benzylsulfanyl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17431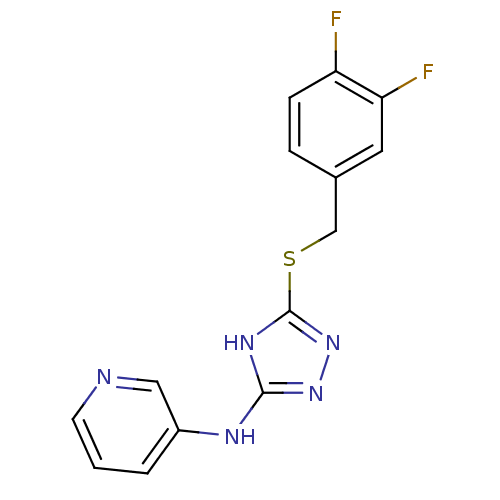 (1,2,4-Triazole Compound, 89 | N-(5-{[(3,4-difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17416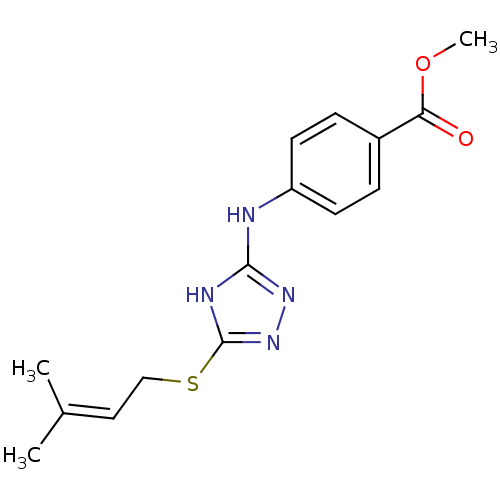 (1,2,4-Triazole Compound, 74 | methyl 4-({5-[(3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523555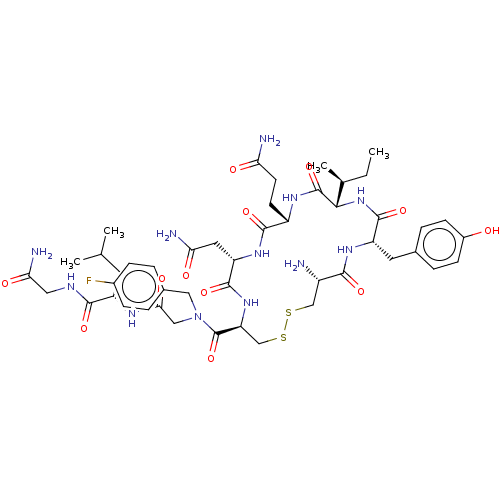 (CHEMBL4474284) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17385 (1,2,4-Triazole Compound, 43 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990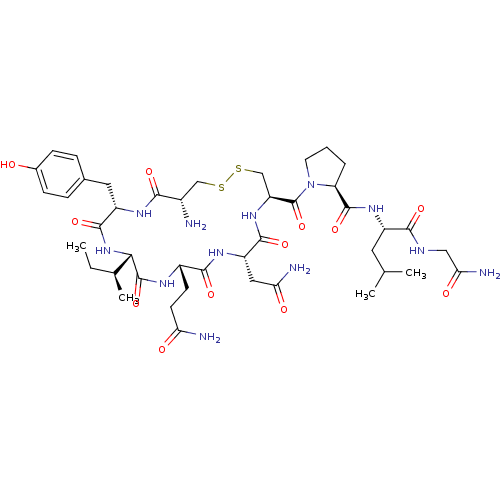 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17380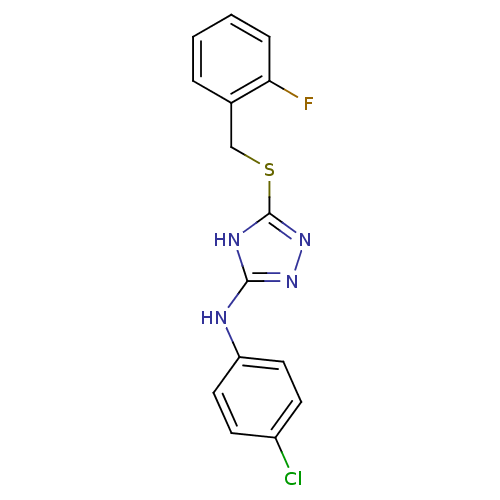 (1,2,4-Triazole Compound, 38 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17368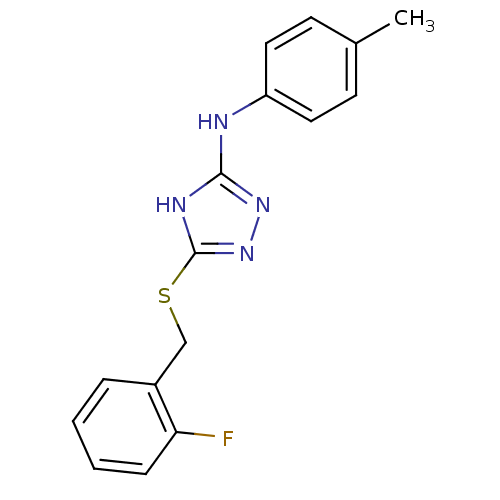 (1,2,4-Triazole Compound, 26 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17408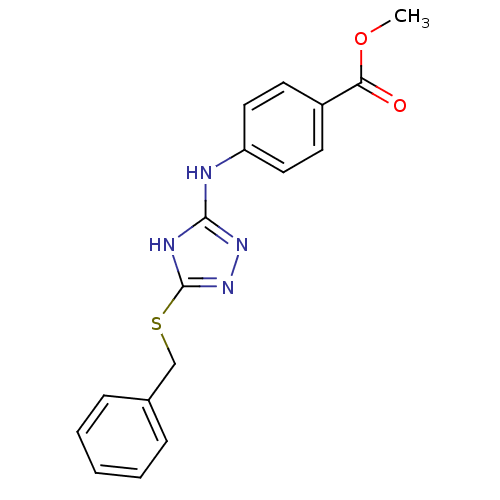 (1,2,4-Triazole Compound, 66 | methyl 4-{[5-(benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17354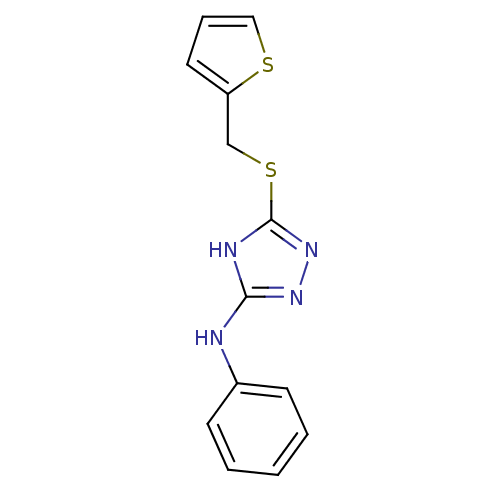 (1,2,4-Triazole Compound, 12 | N-phenyl-5-[(thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.75 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17418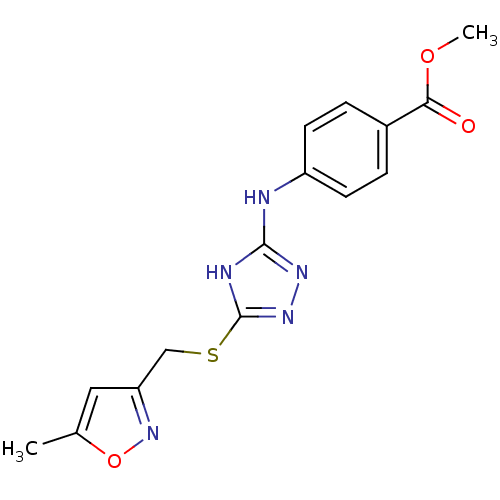 (1,2,4-Triazole Compound, 76 | methyl 4-[(5-{[(5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by PDSP Ki Database | J Pharmacol Exp Ther 299: 908-14 (2001) BindingDB Entry DOI: 10.7270/Q2JS9P1N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17414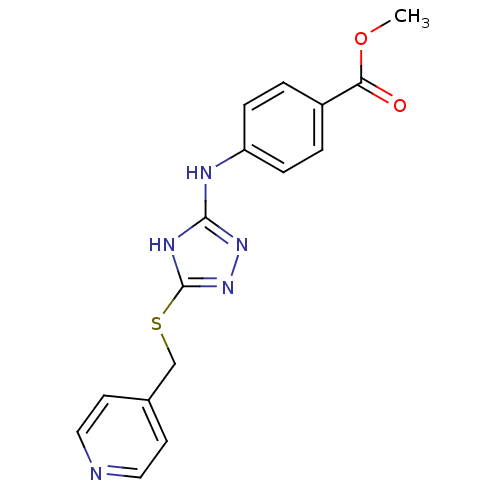 (1,2,4-Triazole Compound, 72 | methyl 4-({5-[(pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17413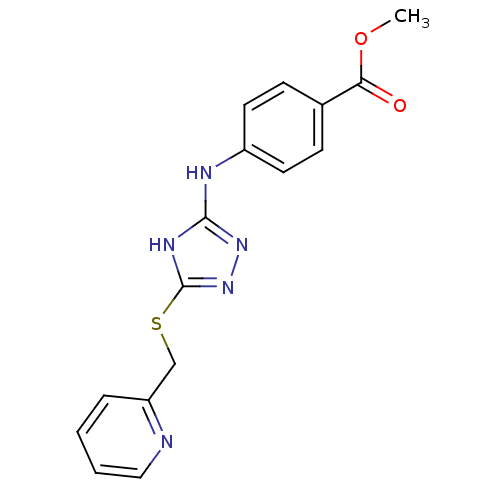 (1,2,4-Triazole Compound, 71 | methyl 4-({5-[(pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17405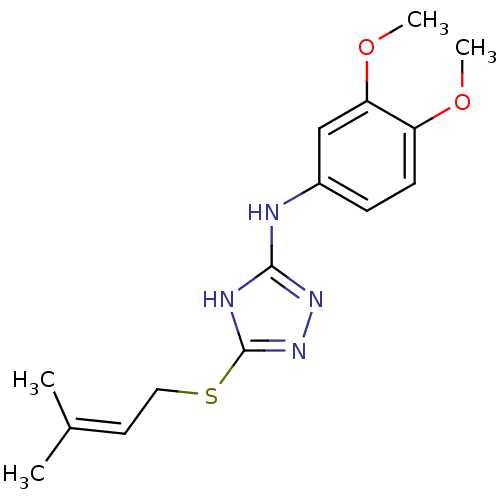 (1,2,4-Triazole Compound, 63 | N-(3,4-dimethoxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523556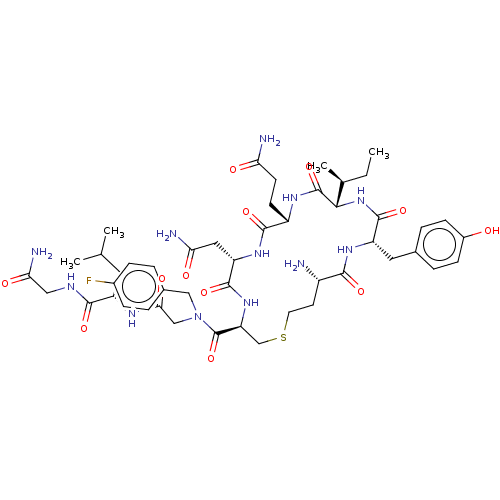 (CHEMBL4458988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17442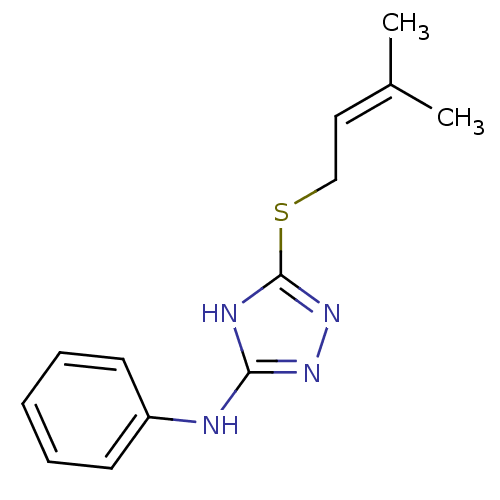 (1,2,4-Triazole Compound, 101 | 5-[(3-methylbut-2-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17446 ((3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044686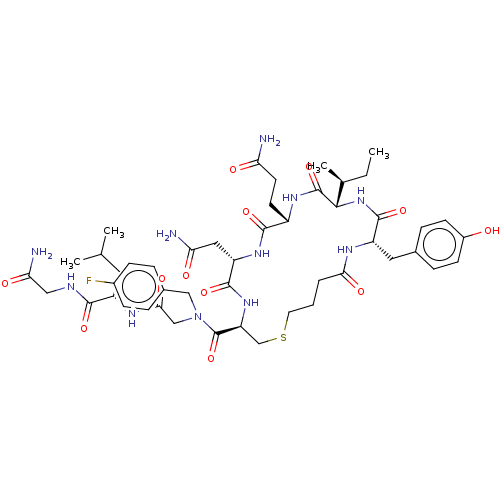 (CHEMBL3354594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17373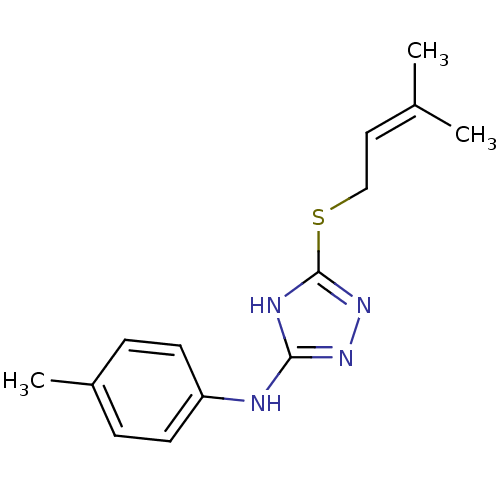 (1,2,4-Triazole Compound, 31 | 5-[(3-methylbut-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096720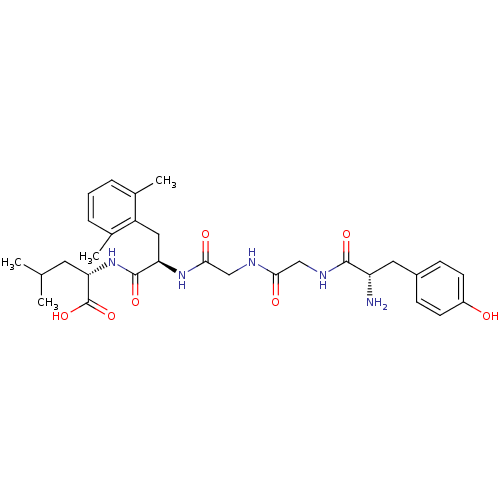 ((S)-2-[(R)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor mu 1 in rat brain synaptosomes using [3H]-DAMGO as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17400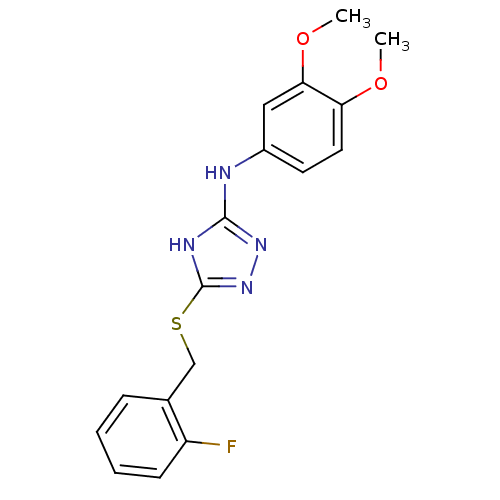 (1,2,4-Triazole Compound, 58 | N-(3,4-dimethoxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17412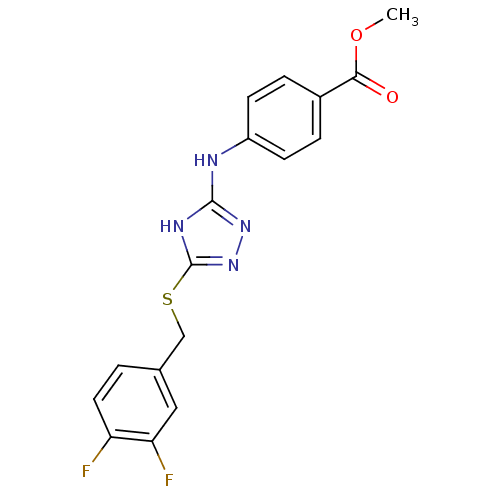 (1,2,4-Triazole Compound, 70 | methyl 4-[(5-{[(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044742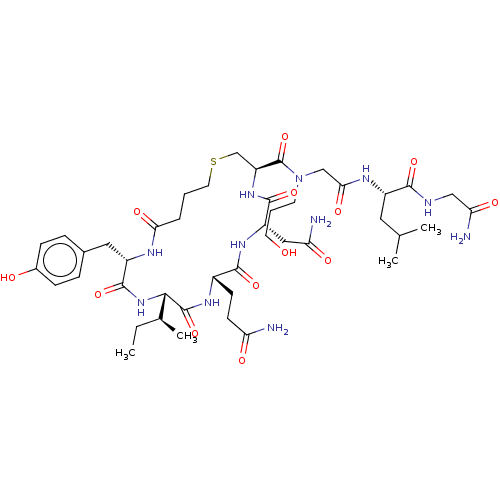 (CHEMBL3354579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465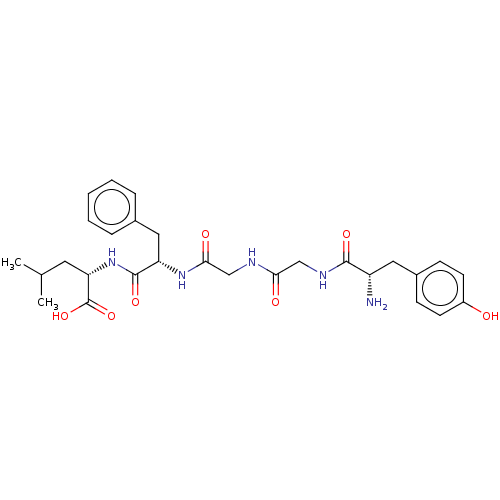 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor delta 1 in rat brain synaptosomes using [3H]-deltorphin II as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523557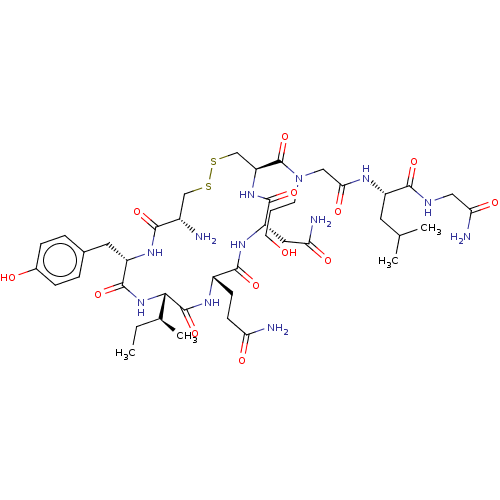 (CHEMBL4583231) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22913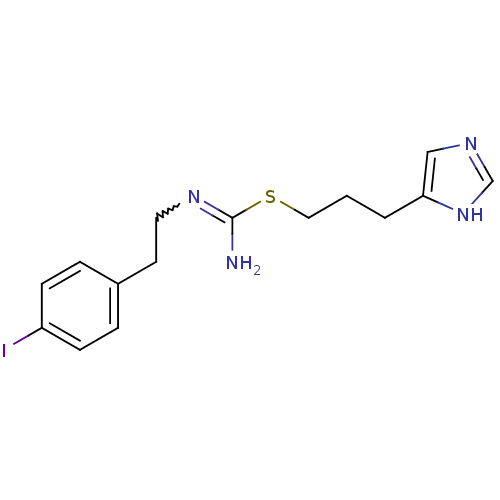 (CHEMBL1237146 | CHEMBL498770 | Iodophenpropit | {[...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by PDSP Ki Database | J Pharmacol Exp Ther 299: 908-14 (2001) BindingDB Entry DOI: 10.7270/Q2JS9P1N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17381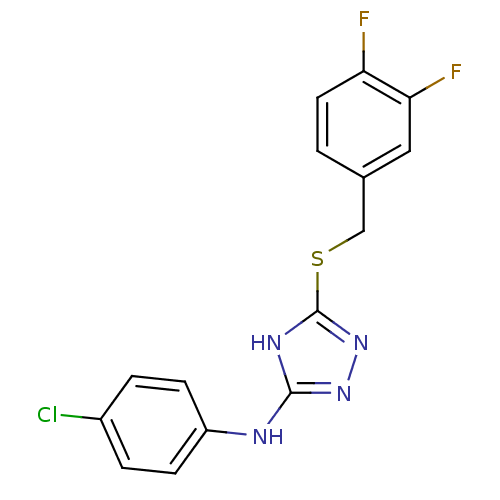 (1,2,4-Triazole Compound, 39 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17436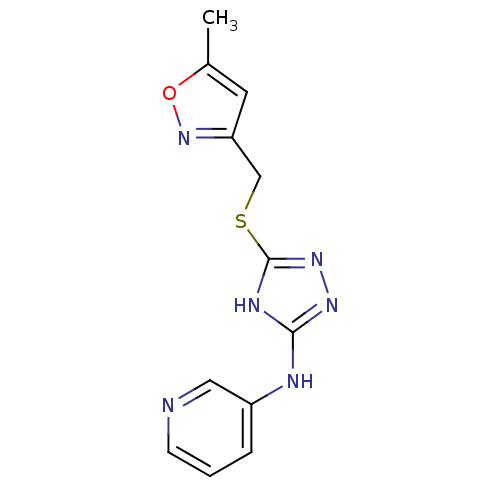 (1,2,4-Triazole Compound, 94 | N-(5-{[(5-methyl-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17396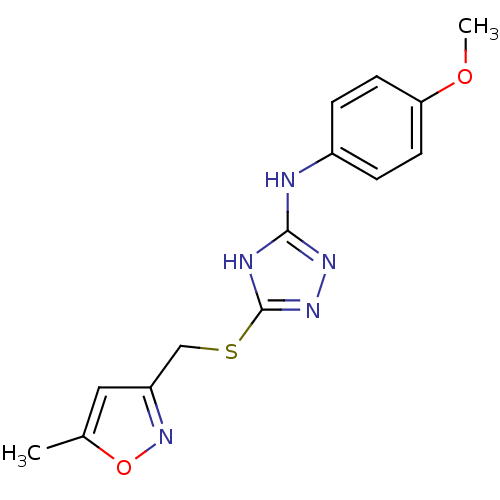 (1,2,4-Triazole Compound, 54 | N-(4-methoxyphenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2052 total ) | Next | Last >> |