

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010542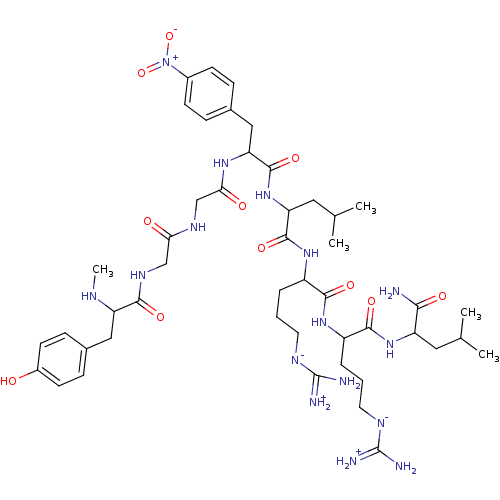 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010532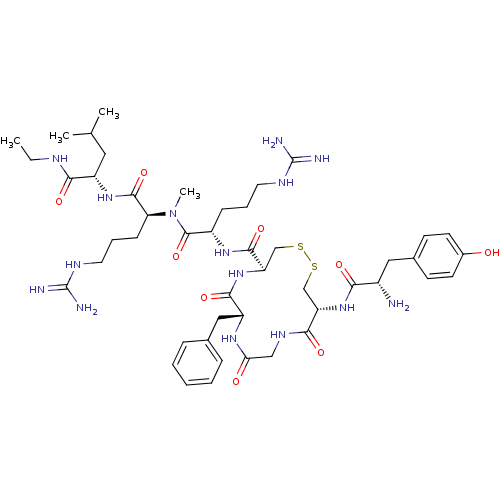 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010542 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010538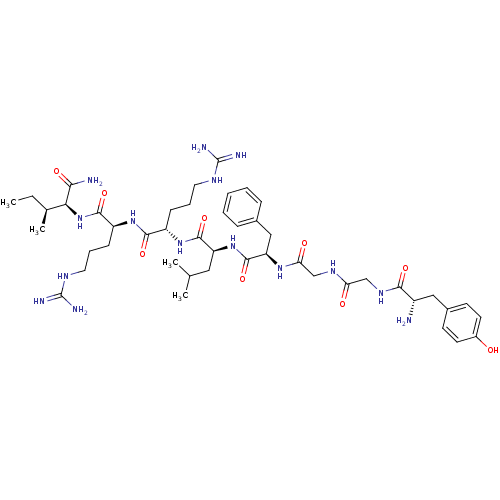 (2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010537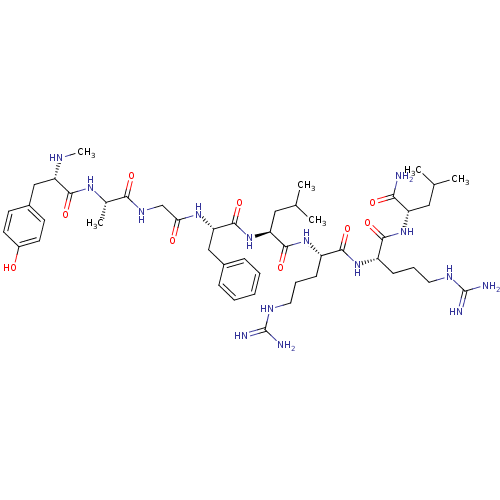 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010535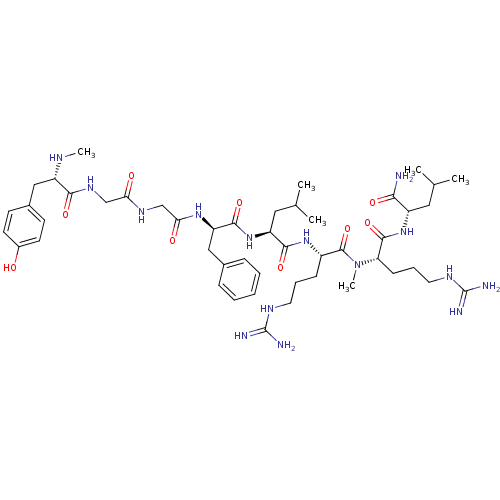 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010536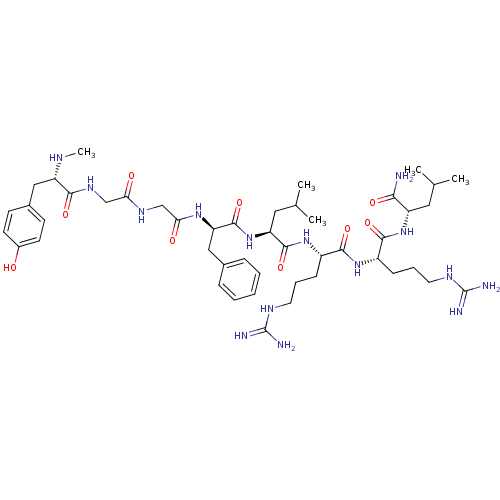 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010532 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010545 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010534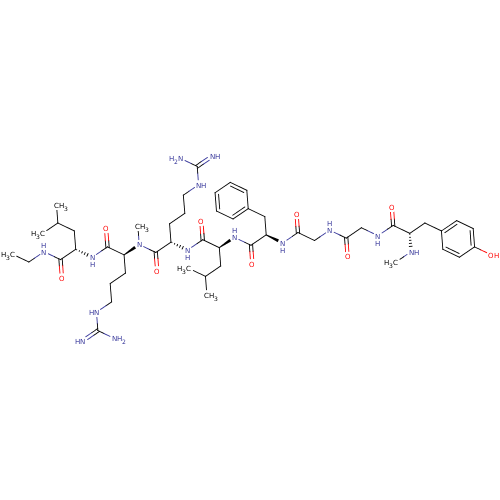 (2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010536 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010532 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010535 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010545 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010534 (2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010538 (2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50010537 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010545 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010538 (2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010534 (2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010542 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010537 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010536 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50010535 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010532 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50010532 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50010532 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor delta 1 of mouse vas deferens (MVD). | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010538 (2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010534 (2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010545 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010535 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9409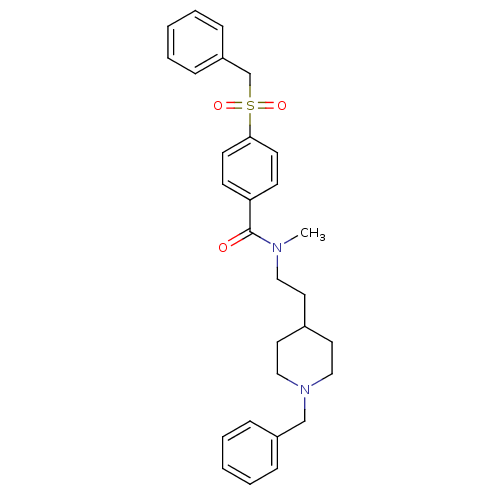 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9409 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was determined in vitro and ex vivo for anti-AChE activity in rat brain | Bioorg Med Chem Lett 2: 871-876 (1992) Article DOI: 10.1016/S0960-894X(00)80547-8 BindingDB Entry DOI: 10.7270/Q21R6QDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010540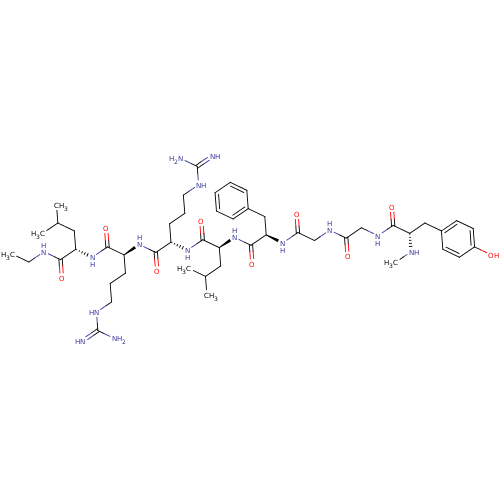 (2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50010546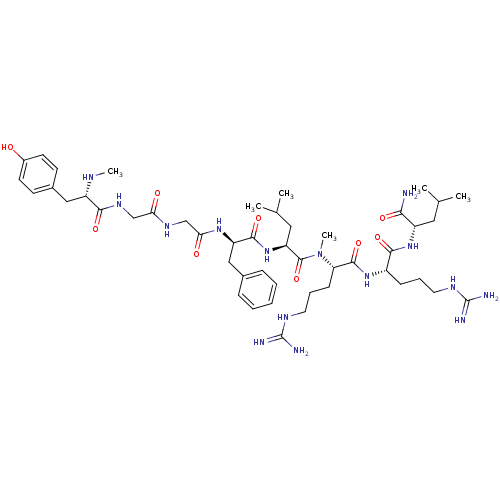 (2-{5-Guanidino-2-[5-guanidino-2-({2-[2-(2-{2-[3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004016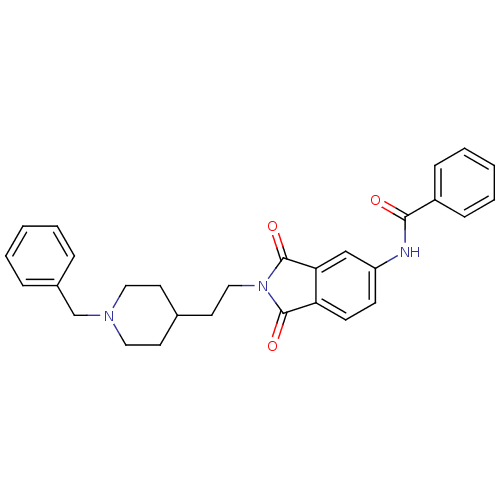 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004016 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010542 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010536 (2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50029933 (2-[3-(1-Benzyl-piperidin-4-yl)-propyl]-5,6-dimetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50029944 (5,6-Dimethoxy-2-[1-(3-methyl-benzyl)-piperidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004007 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1,3-dioxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50010535 (2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004001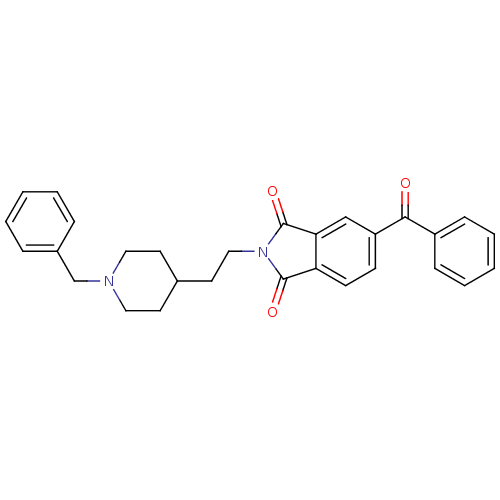 (5-Benzoyl-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 376 total ) | Next | Last >> |