

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium-transporting ATPase subunit beta (Sus scrofa (Pig)) | BDBM50073332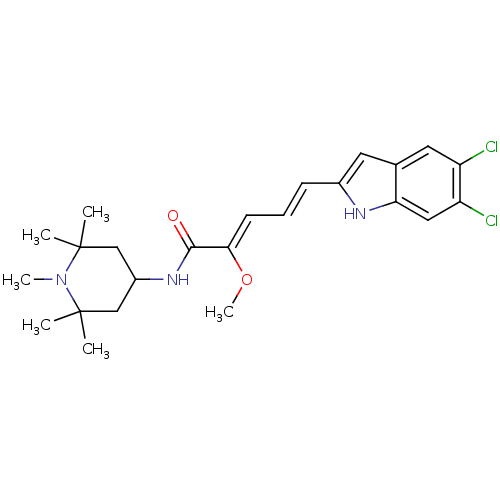 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Research Unit Curated by ChEMBL | Assay Description Compound was measured for the inhibition of bafilomycin-sensitive ATPase activity in partially purified membrane preparation from chicken osteoclast ... | Bioorg Med Chem Lett 8: 3621-6 (1999) BindingDB Entry DOI: 10.7270/Q2R49PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064180 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064182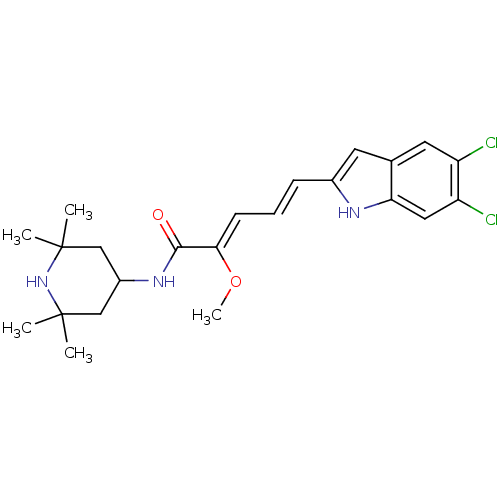 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit B, kidney isoform (Homo sapiens (Human)) | BDBM50064181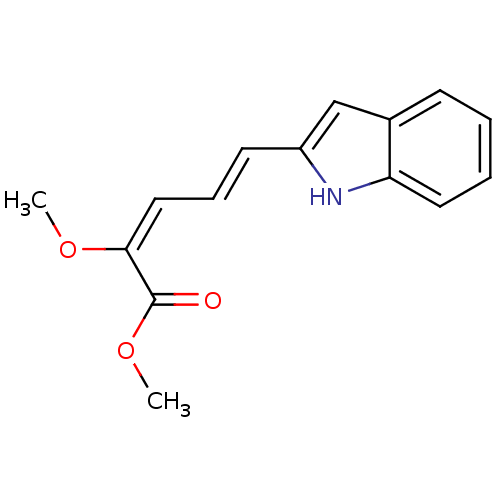 ((2E,4E)-5-(1H-Indol-2-yl)-2-methoxy-penta-2,4-dien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from human kidney cortex(hK) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064184 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064186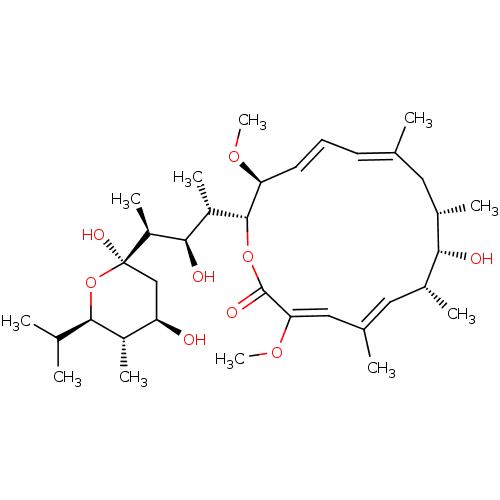 ((3Z,5E,7R,8S,9S,11E,13E,15S,16R)-16-{(2S,3R,4S)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064181 ((2E,4E)-5-(1H-Indol-2-yl)-2-methoxy-penta-2,4-dien...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064181 ((2E,4E)-5-(1H-Indol-2-yl)-2-methoxy-penta-2,4-dien...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken adrenal glands(cAG) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit B, kidney isoform (Homo sapiens (Human)) | BDBM50064180 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from human kidney cortex(hK) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit B, kidney isoform (Homo sapiens (Human)) | BDBM50064182 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from human kidney cortex(hK) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase subunit beta (Sus scrofa (Pig)) | BDBM50073331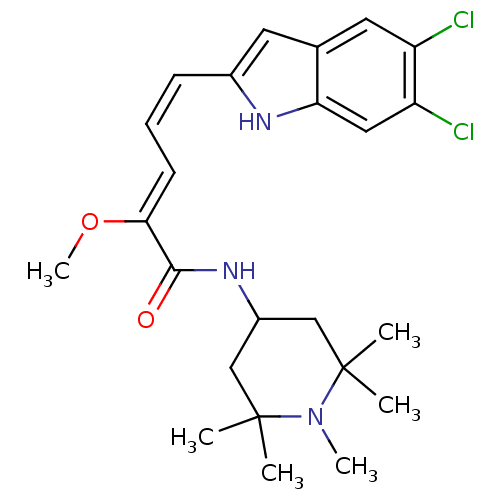 ((2Z,4Z)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 663 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Research Unit Curated by ChEMBL | Assay Description Compound was measured for the inhibition of bafilomycin-sensitive ATPase activity in partially purified membrane preparation from chicken osteoclast ... | Bioorg Med Chem Lett 8: 3621-6 (1999) BindingDB Entry DOI: 10.7270/Q2R49PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064182 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken adrenal glands(cAG) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064180 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken adrenal glands(cAG) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit B, kidney isoform (Homo sapiens (Human)) | BDBM50064184 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from human kidney cortex(hK) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064183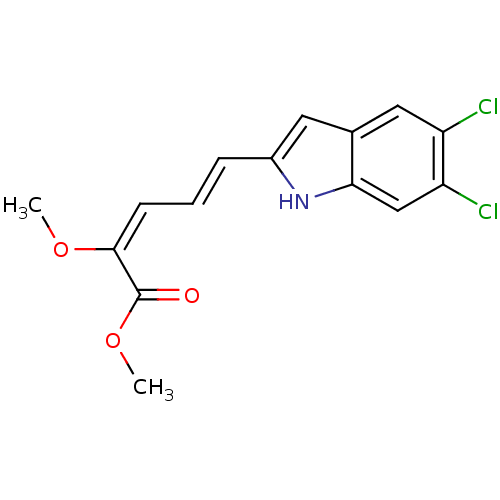 ((2E,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts (cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium-transporting ATPase subunit beta (Sus scrofa (Pig)) | BDBM50073333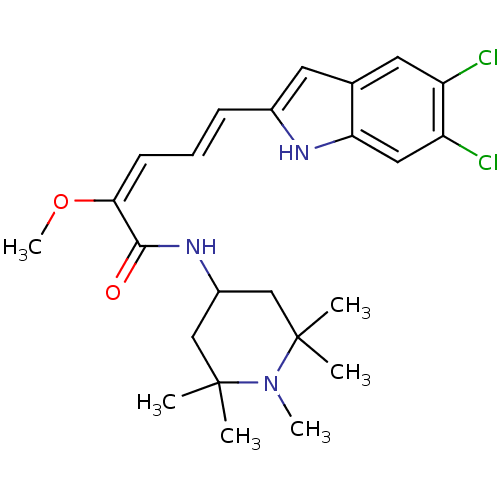 ((2E,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Research Unit Curated by ChEMBL | Assay Description Compound was measured for the inhibition of bafilomycin-sensitive ATPase activity in partially purified membrane preparation from chicken osteoclast ... | Bioorg Med Chem Lett 8: 3621-6 (1999) BindingDB Entry DOI: 10.7270/Q2R49PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064184 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken adrenal glands(cAG) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064185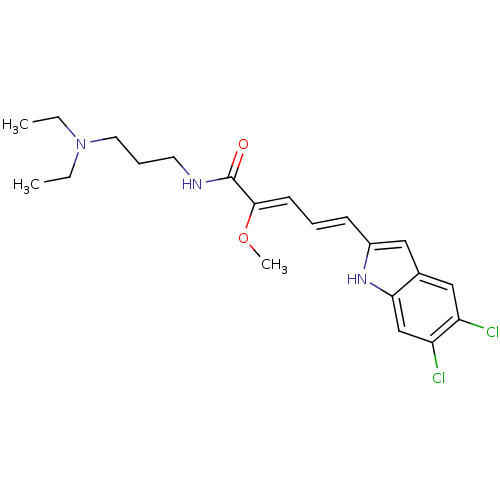 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken adrenal glands(cAG) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064185 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken adrenal glands(cAG) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit B, kidney isoform (Homo sapiens (Human)) | BDBM50064185 ((2Z,4E)-5-(5,6-Dichloro-1H-indol-2-yl)-2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from human kidney cortex(hK) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064437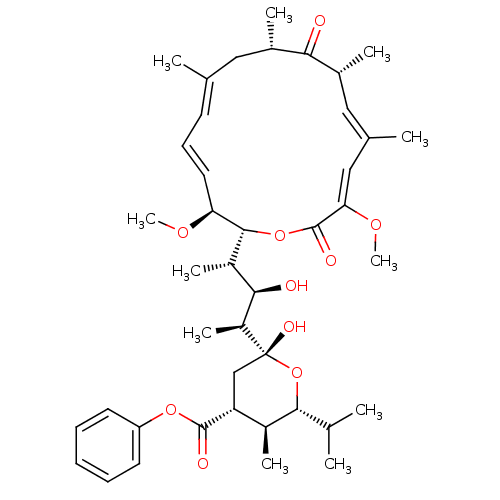 (2-[3-(3,15-Dimethoxy-7,9,11,13-tetramethyl-10,16-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from chicken osteoclasts (cOc) | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064438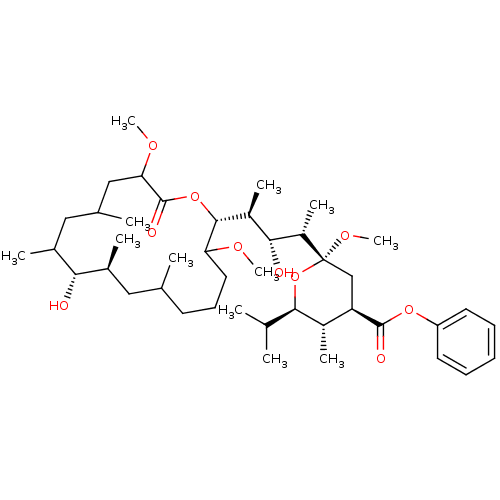 ((2R,4R,5S,6R)-2-[(1S,2R,3S)-2-Hydroxy-3-((2R,9S,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from chicken osteoclasts (cOc). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064440 ((2S,3R,4S)-4-((4E,6E,12E,14Z)-(2R,3S,9S,11R)-3,15-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from bovine chromaffin granules (bCG). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064438 ((2R,4R,5S,6R)-2-[(1S,2R,3S)-2-Hydroxy-3-((2R,9S,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from bovine chromaffin granules (bCG). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064439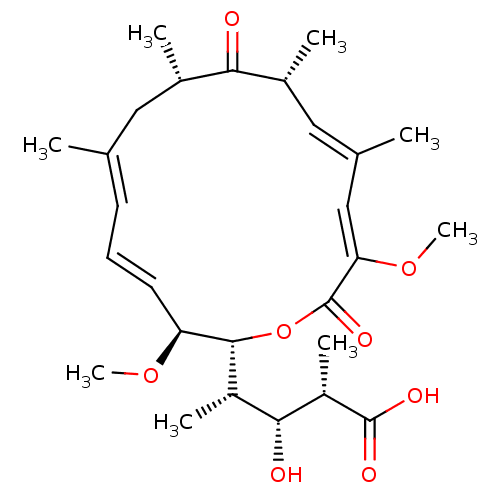 ((2S,3R,4S)-4-((4E,6E,12E,14Z)-(2R,3S,9S,11R)-3,15-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from bovine chromaffin granules (bCG). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064440 ((2S,3R,4S)-4-((4E,6E,12E,14Z)-(2R,3S,9S,11R)-3,15-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from chicken osteoclasts (cOc). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064441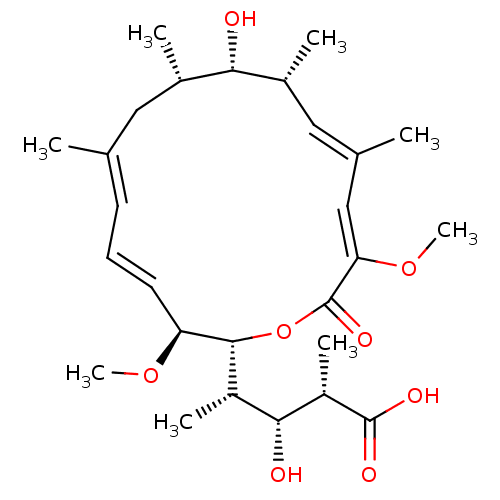 ((2S,3R,4S)-3-Hydroxy-4-((4E,6E,12E,14Z)-(2R,3S,9S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from chicken osteoclasts (cOc). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064437 (2-[3-(3,15-Dimethoxy-7,9,11,13-tetramethyl-10,16-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from bovine chromaffin granules (bCG) | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064441 ((2S,3R,4S)-3-Hydroxy-4-((4E,6E,12E,14Z)-(2R,3S,9S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from bovine chromaffin granules (bCG). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase subunit S1 (Homo sapiens (Human)) | BDBM50064439 ((2S,3R,4S)-4-((4E,6E,12E,14Z)-(2R,3S,9S,11R)-3,15-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham SpA Curated by ChEMBL | Assay Description Inhibition of V-ATPase-driven proton transport in membrane vesicles derived from chicken osteoclasts (cOc). | J Med Chem 41: 1883-93 (1998) Article DOI: 10.1021/jm9707838 BindingDB Entry DOI: 10.7270/Q2TM7987 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| V-type proton ATPase 116 kDa subunit a 1 (Gallus gallus) | BDBM50064181 ((2E,4E)-5-(1H-Indol-2-yl)-2-methoxy-penta-2,4-dien...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc) | J Med Chem 41: 1568-73 (1998) Article DOI: 10.1021/jm9800144 BindingDB Entry DOI: 10.7270/Q2CJ8CM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||