

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 407.3 BDBM11162 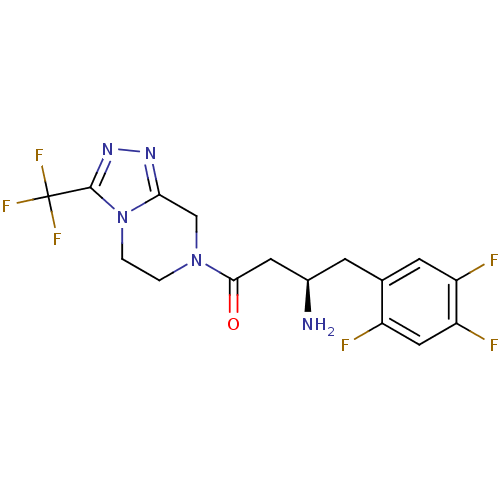 Purchase Purchase | Wt: 303.3 BDBM11695 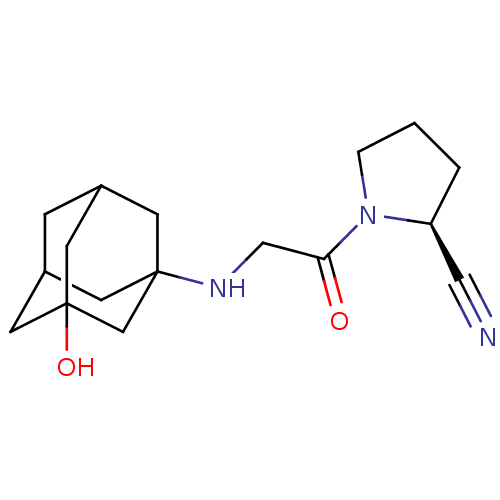 Purchase Purchase | Wt: 258.3 BDBM12192 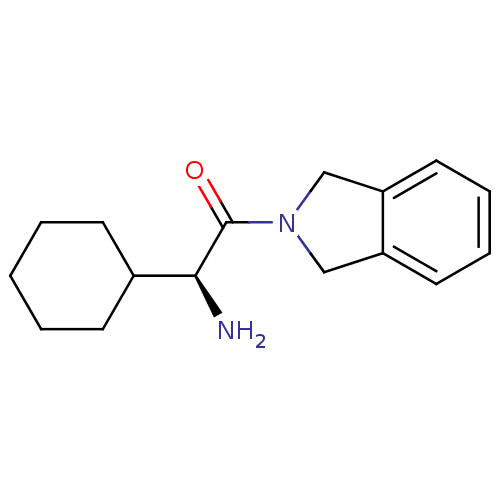 Purchase Purchase | Wt: 230.3 BDBM12188 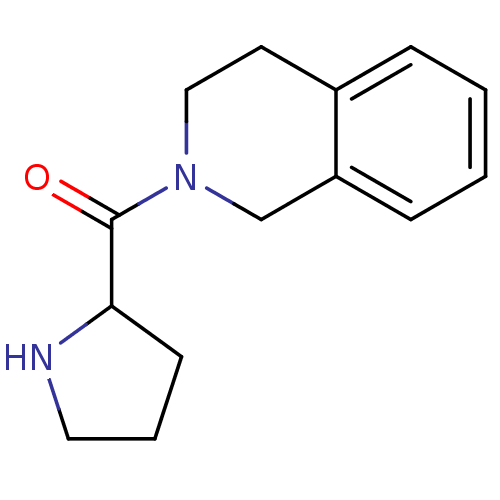 Purchase Purchase | Wt: 339.3 BDBM16285 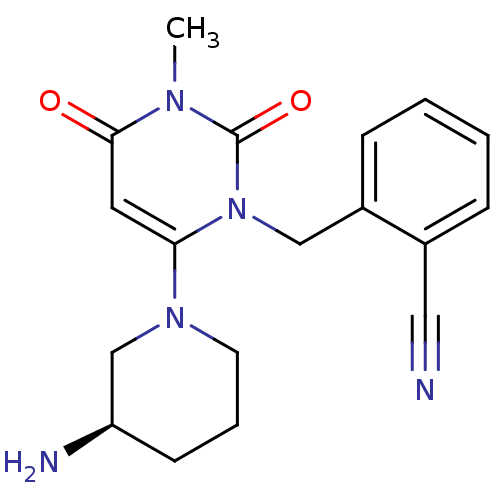 Purchase Purchase |
| Wt: 398.4 BDBM50003020 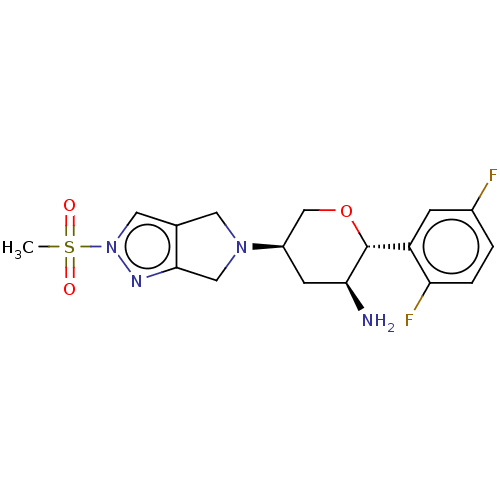 Purchase Purchase | Wt: 344.3 BDBM50009366  Purchase Purchase | Wt: 214.0 BDBM50050513 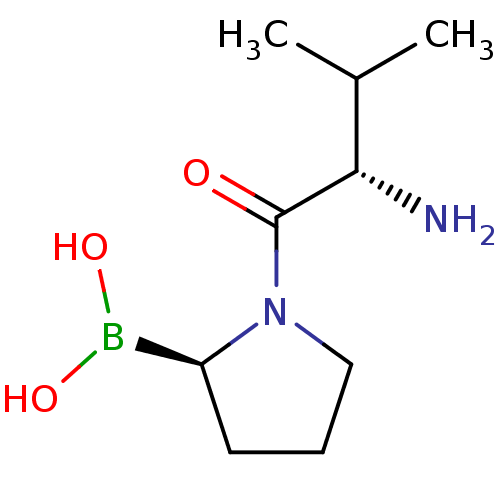 Purchase Purchase | Wt: 128.1 BDBM50118931  Purchase Purchase | Wt: 224.3 BDBM50118932 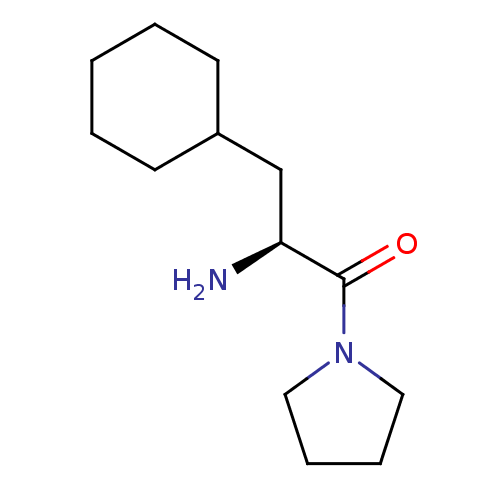 Purchase Purchase |
| Wt: 198.3 BDBM50118936  Purchase Purchase | Wt: 218.2 BDBM50118939  Purchase Purchase | Wt: 184.2 BDBM50118943 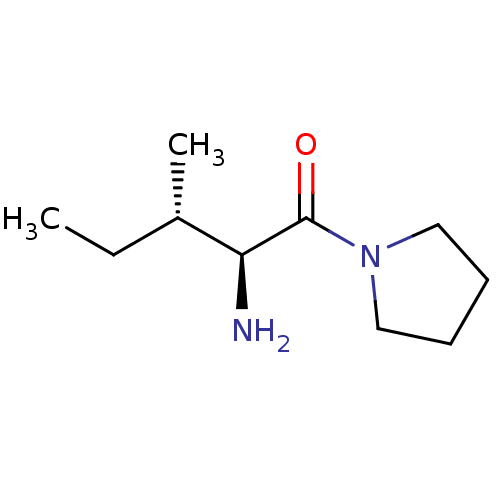 Purchase Purchase | Wt: 168.2 BDBM50118944  Purchase Purchase | Wt: 142.1 BDBM50118951 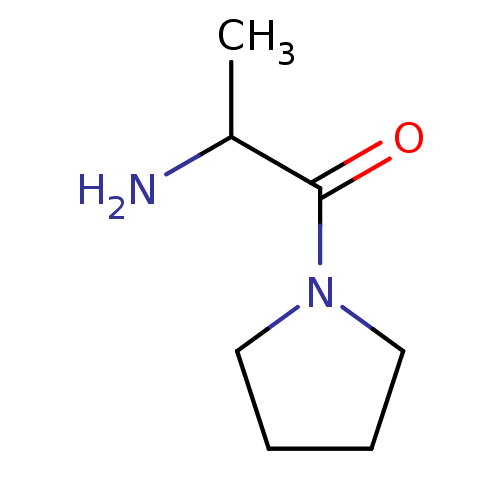 Purchase Purchase |
| Displayed 1 to 15 (of 39 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12188 (2-(pyrrolidin-2-ylcarbonyl)-1,2,3,4-tetrahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12192 ((2S)-2-amino-2-cyclohexyl-1-(2,3-dihydro-1H-isoind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12188 (2-(pyrrolidin-2-ylcarbonyl)-1,2,3,4-tetrahydroisoq...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12192 ((2S)-2-amino-2-cyclohexyl-1-(2,3-dihydro-1H-isoind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories | Assay Description The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... | Bioorg Med Chem Lett 17: 49-52 (2007) Article DOI: 10.1016/j.bmcl.2006.09.099 BindingDB Entry DOI: 10.7270/Q2F47MC2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM16285 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd. | Assay Description Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... | J Med Chem 50: 2297-300 (2007) Article DOI: 10.1021/jm070104l BindingDB Entry DOI: 10.7270/Q2TM78C5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM16285 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd. | Assay Description Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... | J Med Chem 50: 2297-300 (2007) Article DOI: 10.1021/jm070104l BindingDB Entry DOI: 10.7270/Q2TM78C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | n/a |
China Pharmaceutical University | Assay Description The reaction contained 10 uM of Ala-Pro-AMC, 50 pM of enzyme DDP-4, different concentrations of test compounds, and assay buffer (100 mM HEPES, pH 7.... | Chem Biol Drug Des 84: 364-77 (2014) Article DOI: 10.1111/cbdd.12327 BindingDB Entry DOI: 10.7270/Q2D21W96 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University | Assay Description The HypoGen module in DS2.5 was employed to produce pharmaphores with the training set compounds. | Chem Biol Drug Des 84: 364-77 (2014) Article DOI: 10.1111/cbdd.12327 BindingDB Entry DOI: 10.7270/Q2D21W96 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 2.0 | n/a |
Trustees of Tufts College US Patent | Assay Description The inhibitor solution is prepared by dissolving 3-5 mg of inhibitor in pH 2 solution (0.01 N HCl), such that the concentration of the solution is eq... | US Patent US8933056 (2015) BindingDB Entry DOI: 10.7270/Q2ST7NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Trustees of Tufts College US Patent | Assay Description The inhibitor solution is prepared by dissolving 3-5 mg of inhibitor in pH 2 solution (0.01 N HCl), such that the concentration of the solution is eq... | US Patent US8933056 (2015) BindingDB Entry DOI: 10.7270/Q2ST7NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute | Assay Description DPP-IV inhibitory activity was determined by measuring the p-nitroaniline (pNA) released from the chromogenic substrate hydrolysis (H-Gly-Pro-pNA). T... | Chem Biol Drug Des 85: 439-46 (2015) Article DOI: 10.1111/cbdd.12426 BindingDB Entry DOI: 10.7270/Q2542M9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 [K6R,T557I] (Homo sapiens (Human)) | BDBM16285 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University | Assay Description The DPP-4 Drug Discovery Kit (Enzo Life Sciences International, Inc.) was used for the assay of inhibition of DPP-4 activity. The assay is based on t... | Chem Biol Drug Des 86: 849-56 (2015) Article DOI: 10.1111/cbdd.12560 BindingDB Entry DOI: 10.7270/Q2FJ2FJG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 [K6R,T557I] (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University | Assay Description A 200-ÁL reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... | Chem Biol Drug Des 86: 1292-303 (2015) Article DOI: 10.1111/cbdd.12593 BindingDB Entry DOI: 10.7270/Q2M32THN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.80E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Universiteit Antwerp; Fox Chase Cancer Center US Patent | Assay Description Enzyme activities were determined kinetically in a final volume of 200 μl for 10 minutes at 37° C. by measuring the initial velocities of pNA ... | US Patent US9346814 (2016) BindingDB Entry DOI: 10.7270/Q2W37V6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50118943 ((2S,3S)-2-amino-3-methyl-1-(pyrrolidin-1-yl)pentan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase II. | Bioorg Med Chem Lett 12: 2825-8 (2002) BindingDB Entry DOI: 10.7270/Q279457M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of QPP (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of FAP (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of PEP (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of DPP8 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of DPP9 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of Cav1.2 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of Nav1.5 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of DPP4 in mouse plasma | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUV-UNIL Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | J Med Chem 57: 2197-212 (2014) Article DOI: 10.1021/jm400658e BindingDB Entry DOI: 10.7270/Q2TB18F1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUV-UNIL Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | J Med Chem 57: 2197-212 (2014) Article DOI: 10.1021/jm400658e BindingDB Entry DOI: 10.7270/Q2TB18F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda Curated by ChEMBL | Assay Description Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis | Bioorg Med Chem Lett 24: 1918-22 (2014) Article DOI: 10.1016/j.bmcl.2014.03.009 BindingDB Entry DOI: 10.7270/Q2G73G7F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG by MK-499 displacement binding analysis | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Competitive reversible inhibition of DPP4 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50003020 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Competitive reversible inhibition of DPP4 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP2 purified from human seminal plasma using Lys-Ala-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP4 purified from human seminal plasma using Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP4 purified from human seminal plasma using Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant mouse FAP purified from HEK293 cell supernatant using Ala-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP2 purified from human seminal plasma using Lys-Ala-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant human PREP purified from Escherichia coli using Z-Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of recombinant human PREP purified from Escherichia coli using Z-Gly-Pro-p-nitroanilide as substrate by spectrophotometry | J Med Chem 57: 3053-74 (2014) Article DOI: 10.1021/jm500031w BindingDB Entry DOI: 10.7270/Q2P84DFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human FAP | Bioorg Med Chem Lett 17: 507-10 (2007) Article DOI: 10.1016/j.bmcl.2006.10.012 BindingDB Entry DOI: 10.7270/Q2T43SQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FAP | Bioorg Med Chem Lett 17: 3384-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.095 BindingDB Entry DOI: 10.7270/Q2NK3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FAP | Bioorg Med Chem Lett 17: 3877-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.106 BindingDB Entry DOI: 10.7270/Q20K2884 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human FAP | Bioorg Med Chem 17: 2388-99 (2009) Article DOI: 10.1016/j.bmc.2009.02.020 BindingDB Entry DOI: 10.7270/Q2416WZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| << First | Previous | Displayed 51 to 100 (of 521 total ) | Next | Last >> |