

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137948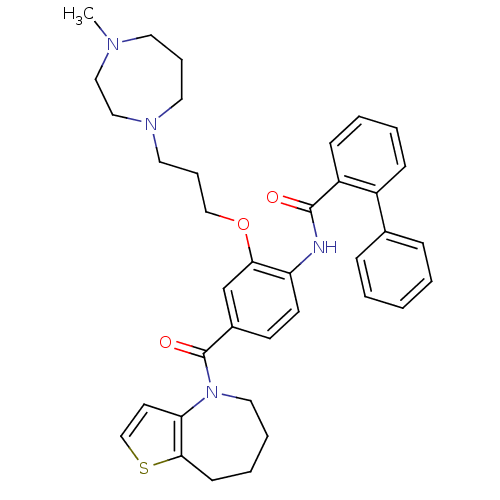 (Biphenyl-2-carboxylic acid [2-[3-(4-methyl-[1,4]di...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129464 (CHEMBL71355 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137945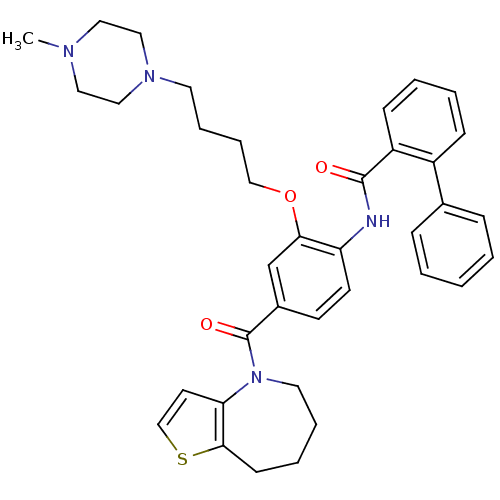 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50087552 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity for rat V1a receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137953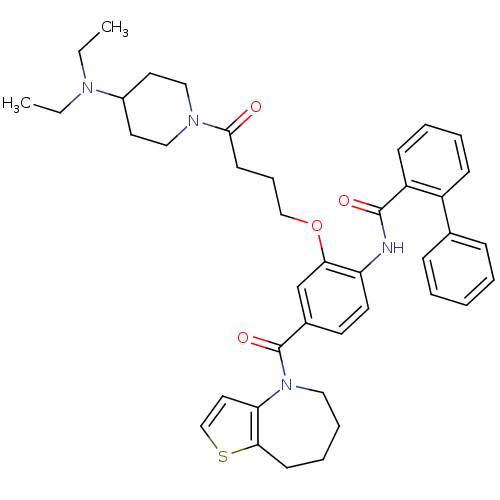 (Biphenyl-2-carboxylic acid [2-[4-(4-diethylamino-p...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078652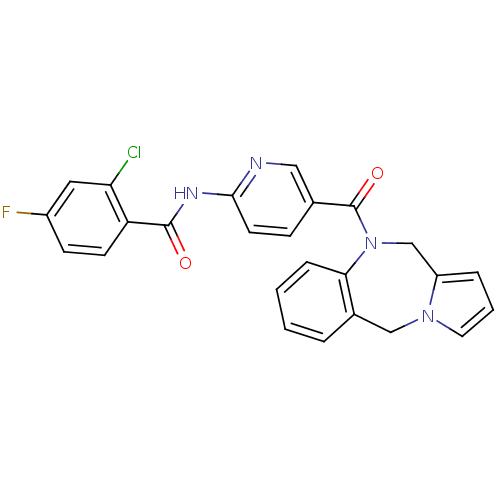 (CHEMBL301788 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137940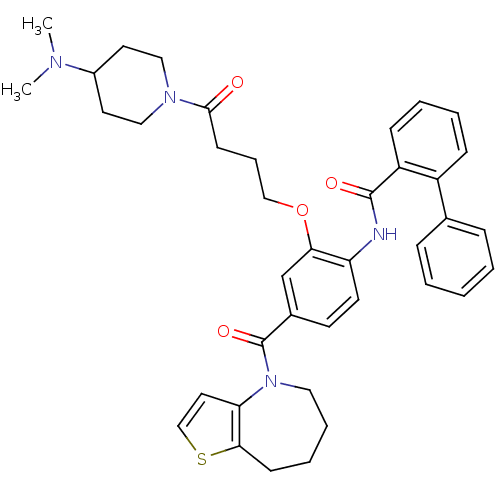 (Biphenyl-2-carboxylic acid [2-[4-(4-dimethylamino-...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078660 (CHEMBL299532 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065122 (CHEMBL306970 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129469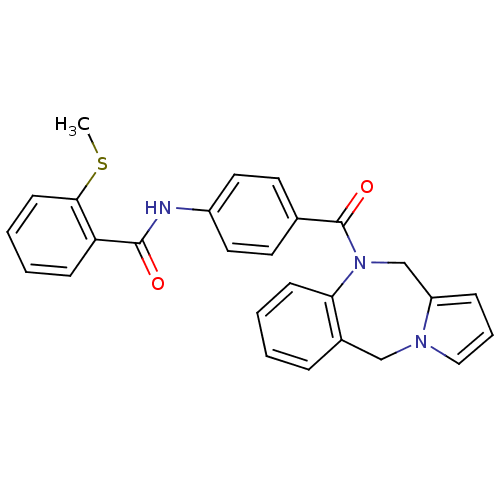 (CHEMBL304060 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078656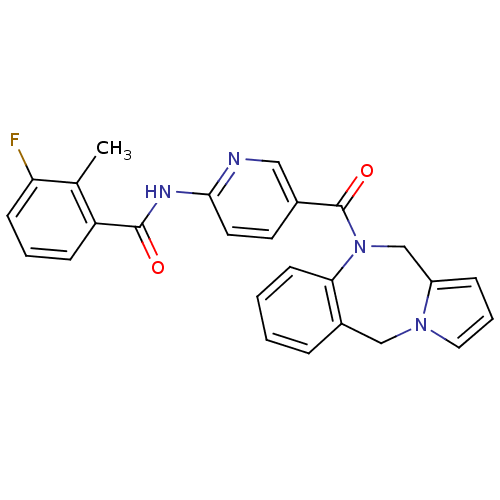 (CHEMBL46295 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50291329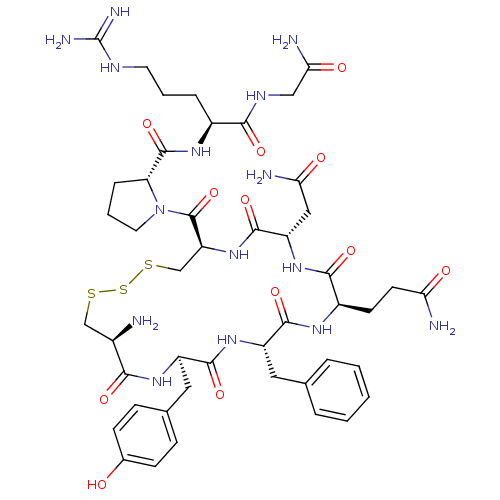 (8-arginine vasopressin trisulphide | CHEMBL267405) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the TYR(Me)2 arginine-vasopressin as radioligand at 0.3 nM in A7r5 cells | Bioorg Med Chem Lett 7: 719-724 (1997) Article DOI: 10.1016/S0960-894X(97)00050-4 BindingDB Entry DOI: 10.7270/Q2QC03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the TYR(Me)2 arginine-vasopressin as radioligand at 0.3 nM in A7r5 cells | Bioorg Med Chem Lett 7: 719-724 (1997) Article DOI: 10.1016/S0960-894X(97)00050-4 BindingDB Entry DOI: 10.7270/Q2QC03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065120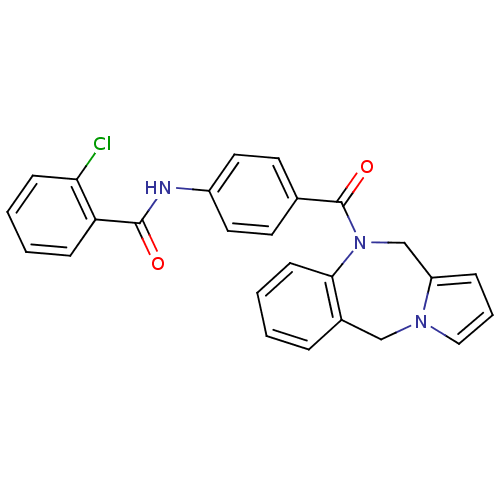 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065120 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078651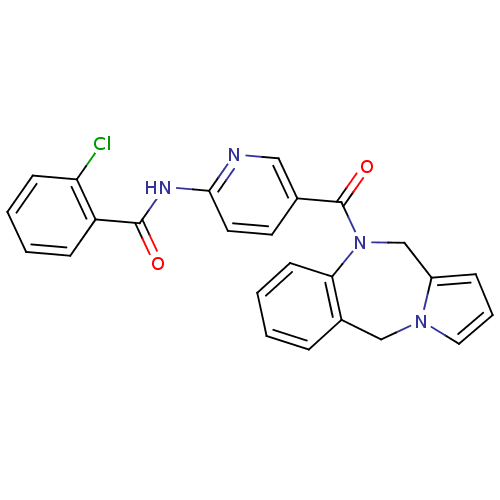 (CHEMBL300963 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50087541 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity for rat V1a receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129472 (CHEMBL70949 | N-[4-(5,11-Dihydro-dibenzo[b,e][1,4]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065124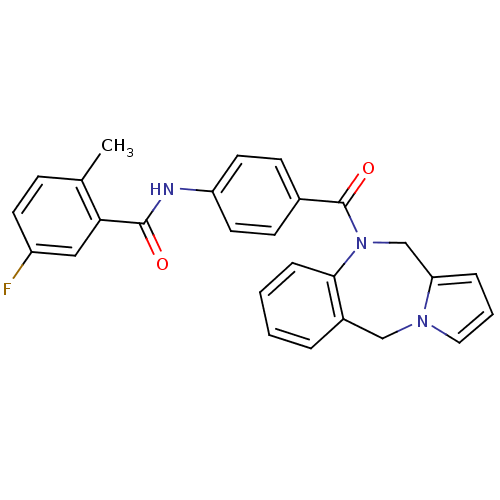 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137949 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-[1,4]di...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137956 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137942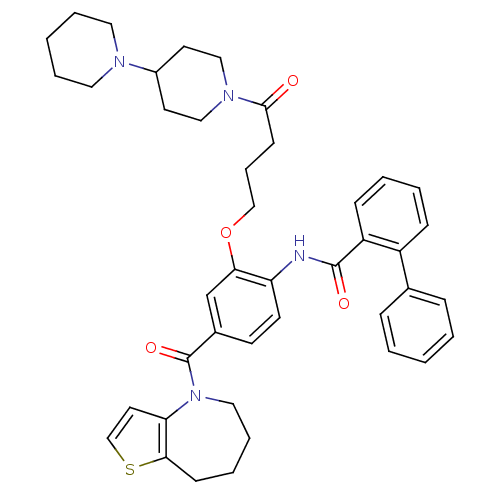 (Biphenyl-2-carboxylic acid [2-(4-[1,4']bipiperidin...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137951 (Biphenyl-2-carboxylic acid [2-{4-[(2-dimethylamino...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137946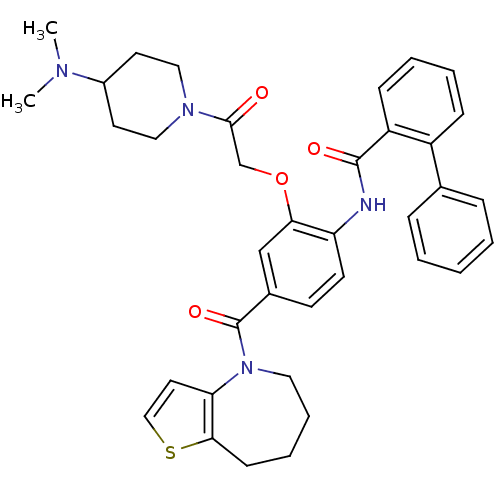 (Biphenyl-2-carboxylic acid [2-[2-(4-dimethylamino-...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078654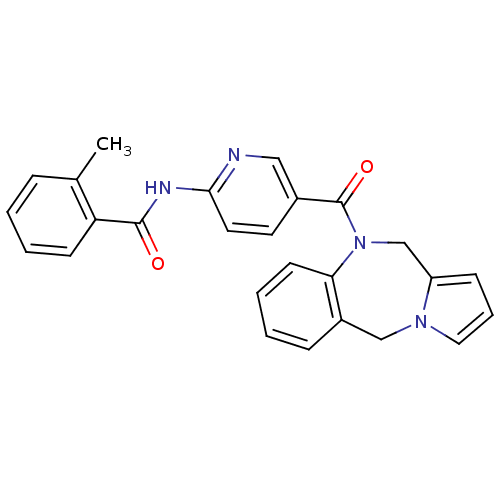 (CHEMBL297990 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of (Phe-3,4,5 [3H]-) AVP binding towards isolated rat hepatic Vasopressin V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity for rat V1a receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065130 (CHEMBL71305 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065119 (CHEMBL70981 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065130 (CHEMBL71305 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065119 (CHEMBL70981 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129463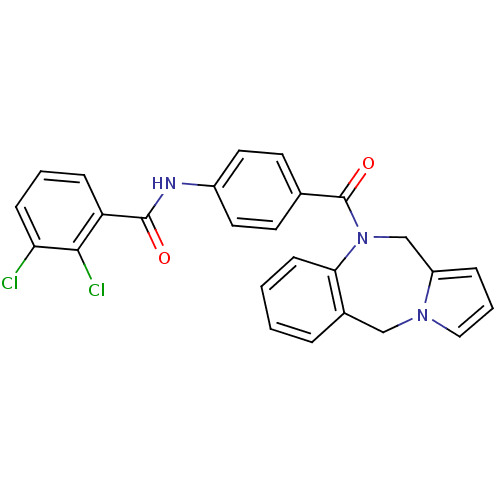 (CHEMBL71282 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078659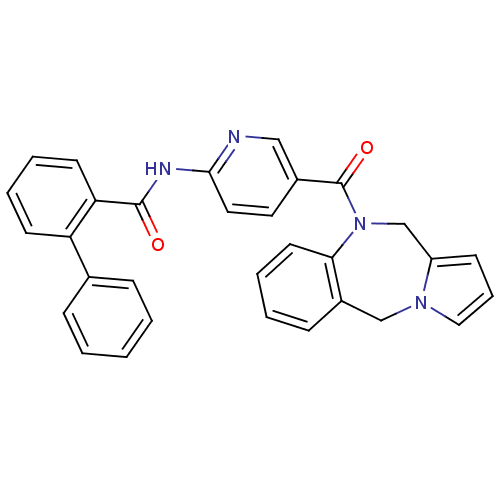 (Biphenyl-2-carboxylic acid [5-(5H,11H-benzo[e]pyrr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065117 (CHEMBL304956 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065126 (CHEMBL73286 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065126 (CHEMBL73286 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50366915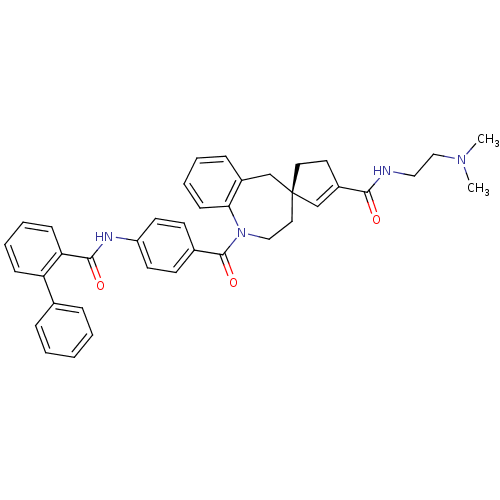 (CHEMBL1788220) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for binding affinity towards vasopressin V1a receptor in rat | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065131 (CHEMBL82376 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078663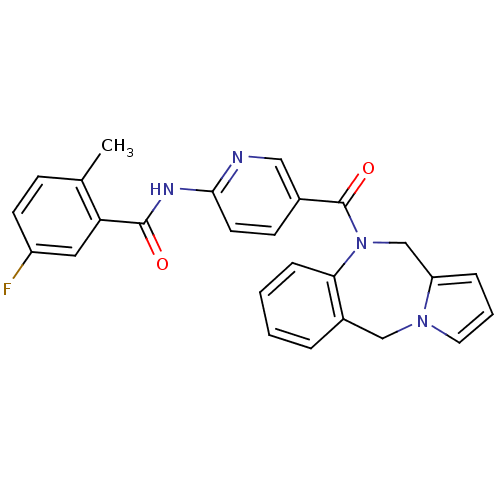 (CHEMBL300946 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065113 (CHEMBL310581 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065127 (CHEMBL71797 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065127 (CHEMBL71797 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078655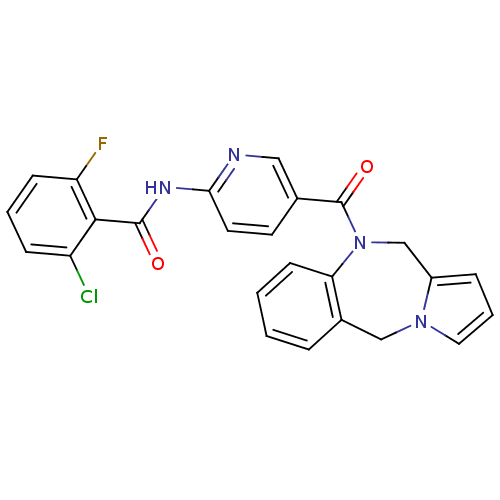 (CHEMBL49824 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129465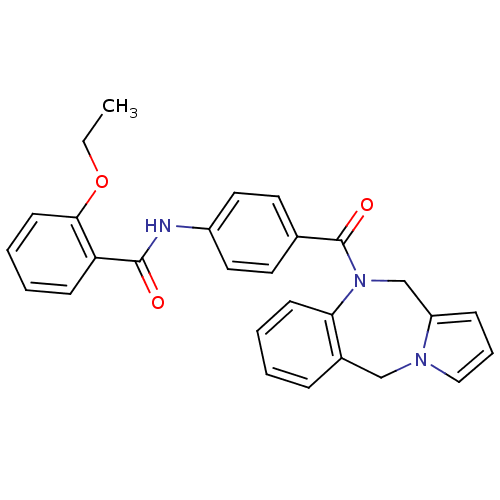 (CHEMBL309096 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065125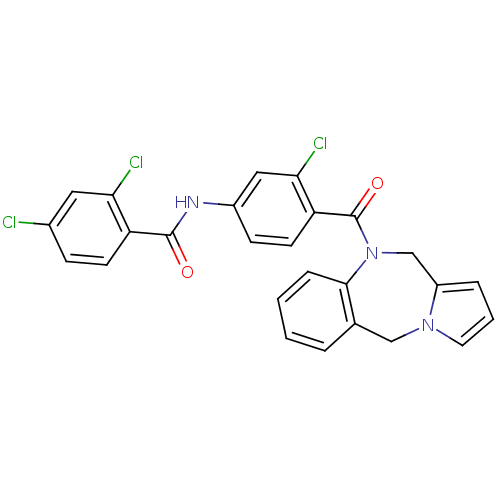 (CHEMBL311230 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50052909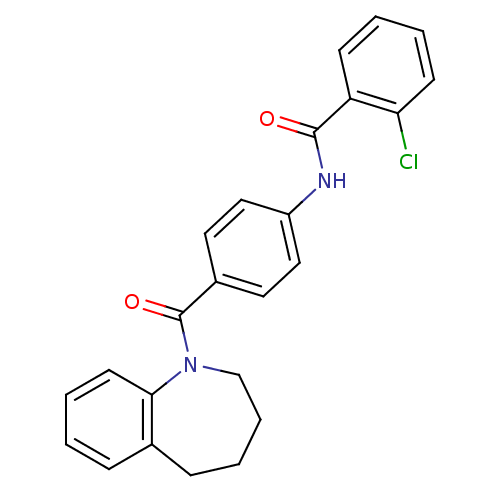 (2-Chloro-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co. Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from AVP-V1a receptor binding site in rat liver | J Med Chem 39: 3547-55 (1996) Article DOI: 10.1021/jm960133o BindingDB Entry DOI: 10.7270/Q2DZ07CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065121 (CHEMBL310416 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129476 (2-Methyl-N-[4-(6H-phenanthridine-5-carbonyl)-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50052954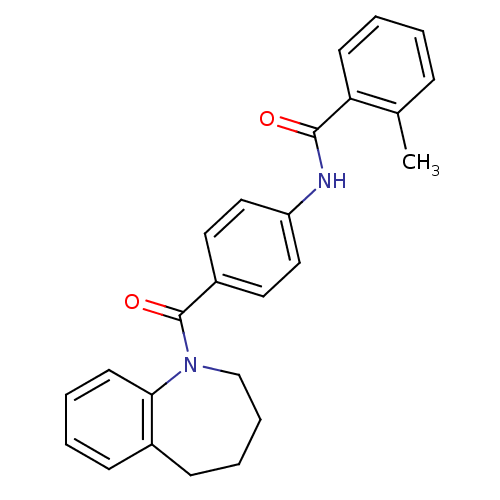 (2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Co. Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from AVP-V1a receptor binding site in rat liver | J Med Chem 39: 3547-55 (1996) Article DOI: 10.1021/jm960133o BindingDB Entry DOI: 10.7270/Q2DZ07CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 603 total ) | Next | Last >> |