

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50101131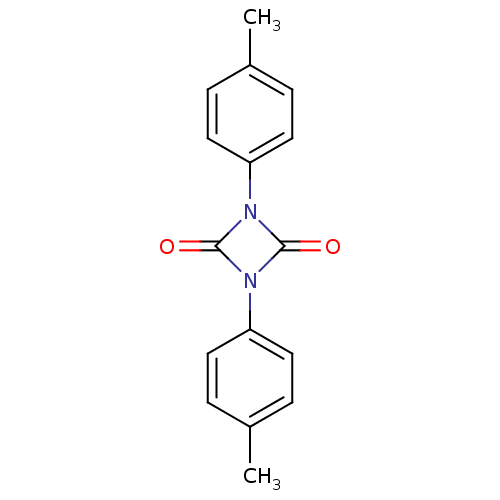 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072290 ((3S,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23700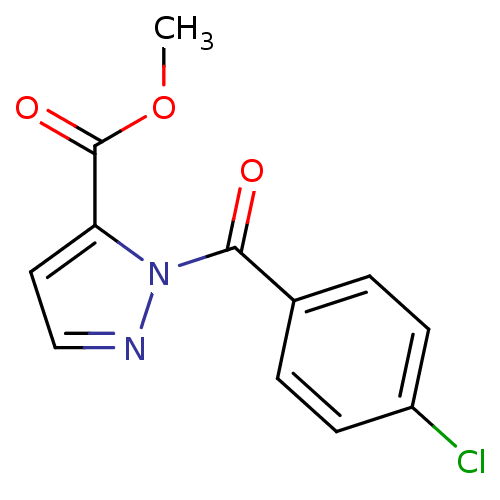 (N-Benzoylpyrazole deriv., 2 | methyl 1-[(4-chlorop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23705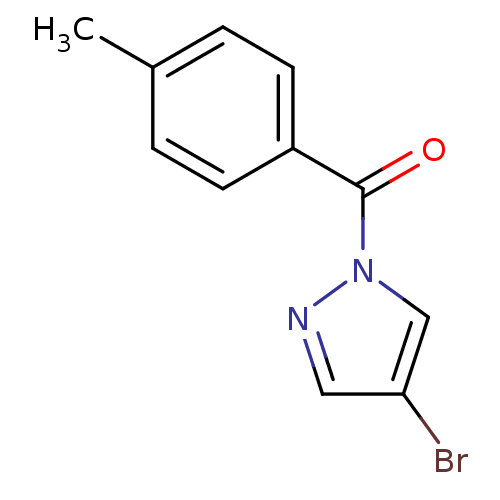 (4-bromo-1-[(4-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072282 ((3R,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23698 (4-chloro-1-[(4-fluorophenyl)carbonyl]-1H-pyrazole ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120284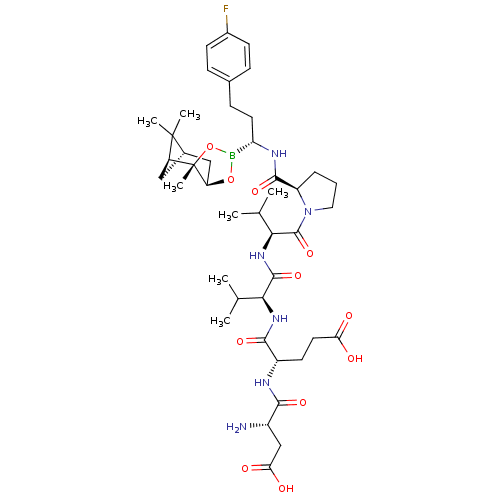 (CHEMBL107656 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23709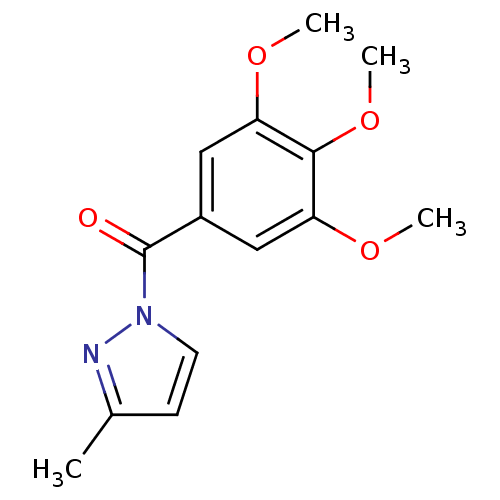 (3-methyl-1-[(3,4,5-trimethoxyphenyl)carbonyl]-1H-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23704 (1-benzoyl-N-phenyl-1H-pyrazole-3-carboxamide | N-B...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120311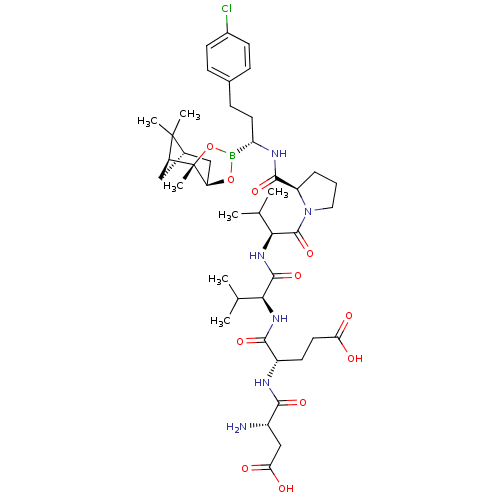 (CHEMBL320814 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072284 ((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120309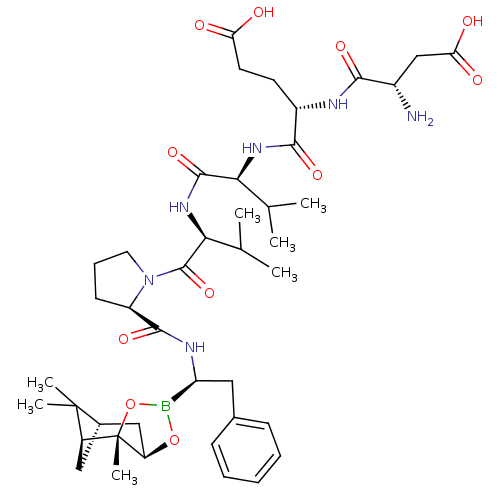 (CHEMBL108189 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120298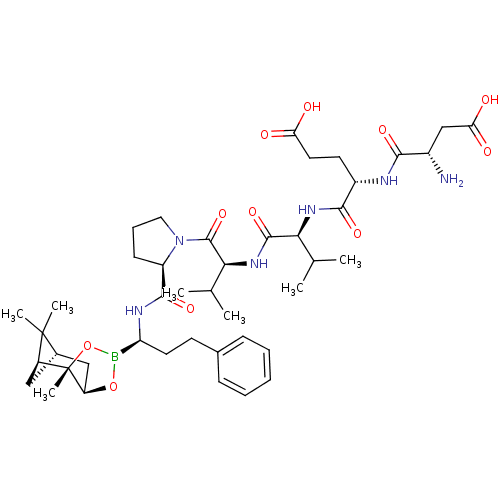 (CHEMBL109434 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072285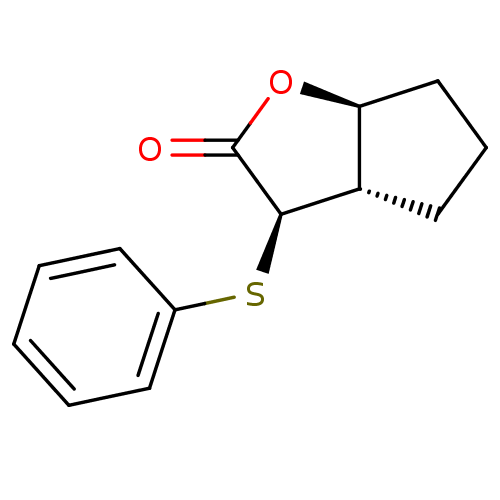 ((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072292 ((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072289 ((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072286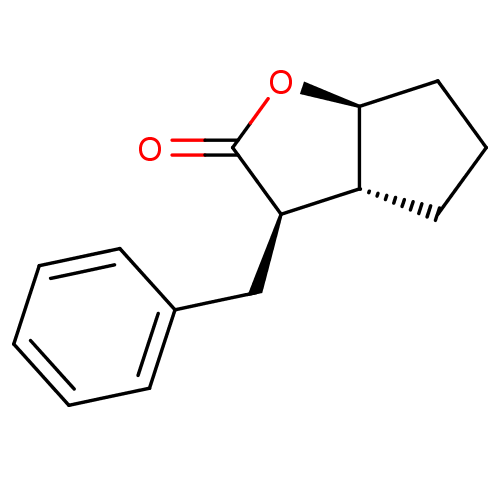 ((3R,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50072283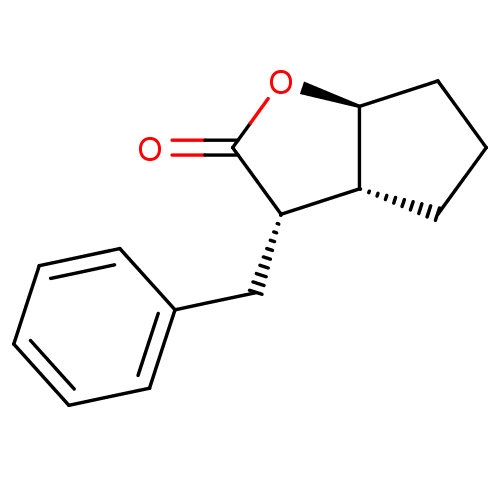 ((3S,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Chymotrypsinogen | Bioorg Med Chem Lett 8: 2955-60 (1999) Article DOI: 10.1016/S0960-894X(98)00531-9 BindingDB Entry DOI: 10.7270/Q2JQ105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23702 (3-[(2,4-dimethylphenyl)carbonyl]-1-[(4-fluoropheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23706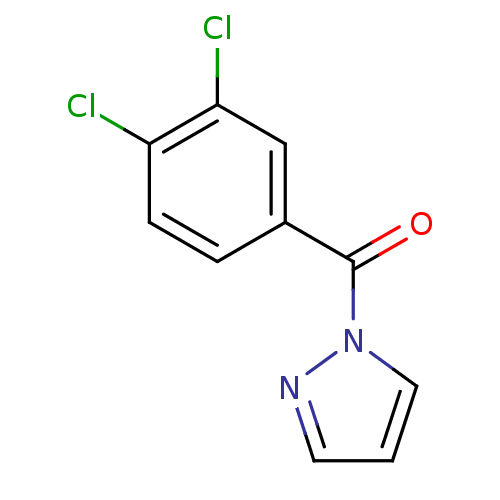 (1-[(3,4-dichlorophenyl)carbonyl]-1H-pyrazole | N-B...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50093722 ((6R,7R)-3-(4-Carboxymethyl-4H-[1,2,4]triazol-3-yls...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against alpha-chymotrypsin | Bioorg Med Chem Lett 10: 2397-401 (2001) BindingDB Entry DOI: 10.7270/Q27H1HVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23710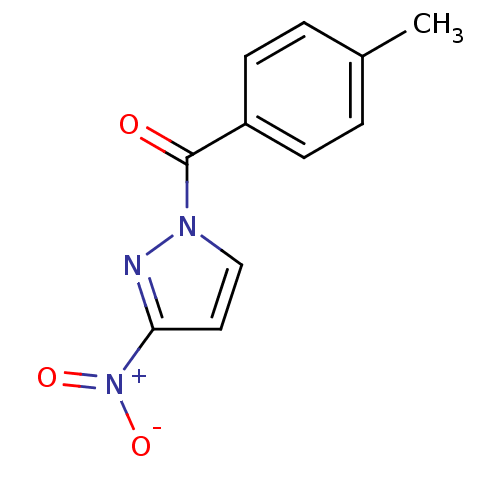 (1-[(4-methylphenyl)carbonyl]-3-nitro-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23712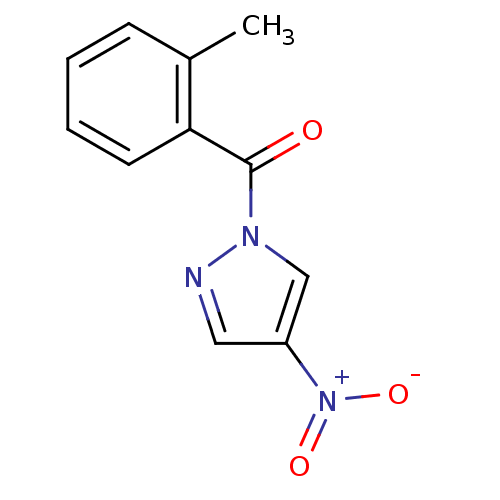 (1-[(2-methylphenyl)carbonyl]-4-nitro-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120286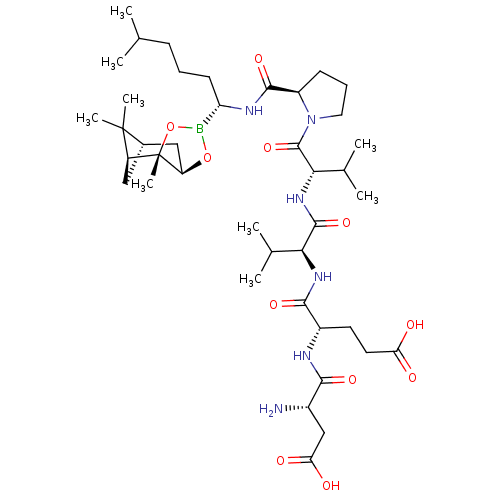 (CHEMBL322933 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120292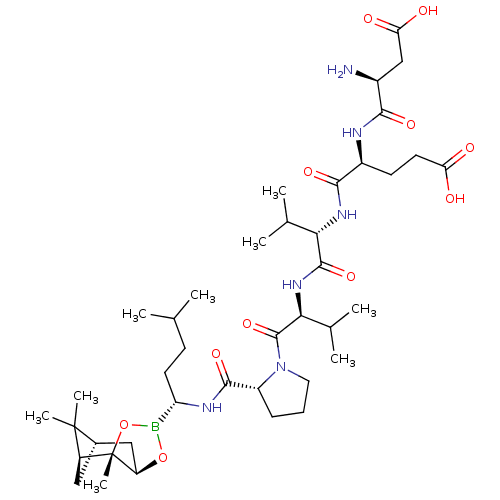 (CHEMBL322110 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50096724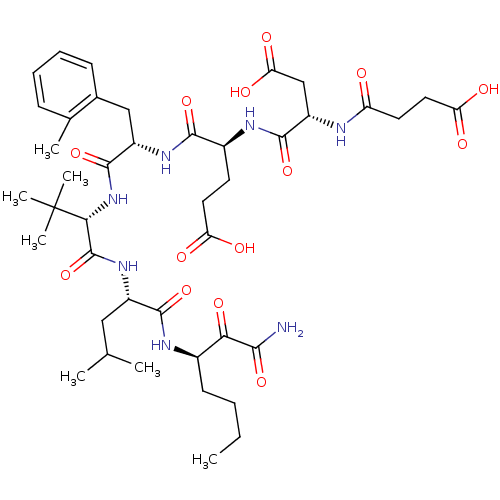 ((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn Curated by ChEMBL | Assay Description Compound was tested for inhibition of Serine protease chymotrypsin | Bioorg Med Chem Lett 11: 355-7 (2001) BindingDB Entry DOI: 10.7270/Q2WQ0322 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23701 (4-chloro-1-[(3-nitrophenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120288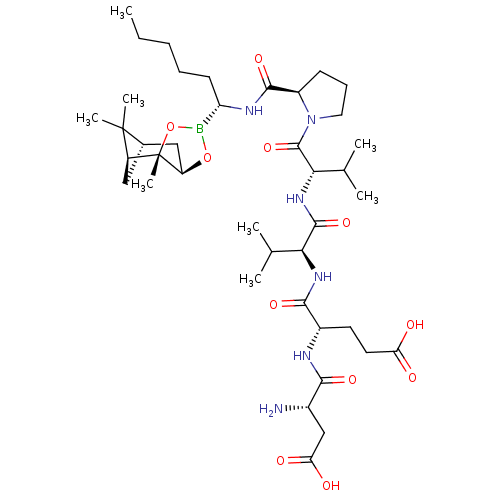 (CHEMBL432978 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120305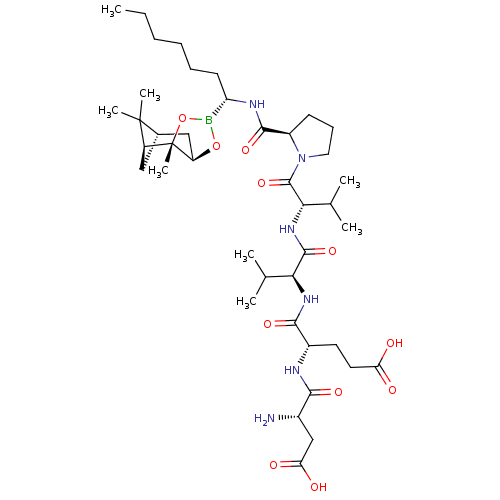 (CHEMBL111765 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50291321 (CHEMBL163002 | {1-[1-(1-Benzyl-2-oxo-ethylcarbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of alpha-Chymotrypsin, activity expressed as IC50 | Bioorg Med Chem Lett 7: 705-710 (1997) Article DOI: 10.1016/S0960-894X(97)00091-7 BindingDB Entry DOI: 10.7270/Q2ZW1KXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50167307 (1-Benzo[b]thiophen-2-ylmethyl-5-iodo-1H-indole-2,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Chymotrypsin (serine protease) | Bioorg Med Chem Lett 15: 3058-62 (2005) Article DOI: 10.1016/j.bmcl.2005.04.027 BindingDB Entry DOI: 10.7270/Q2H131JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50135494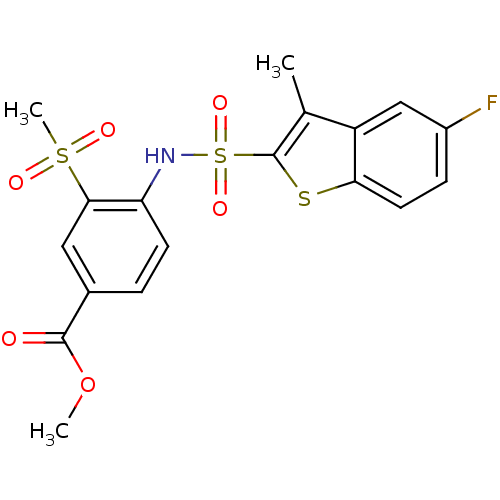 (4-(5-Fluoro-3-methyl-benzo[b]thiophene-2-sulfonyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toa Eiyo Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against bovin chymotrypsin | Bioorg Med Chem Lett 13: 4085-8 (2003) BindingDB Entry DOI: 10.7270/Q2QN665P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50135495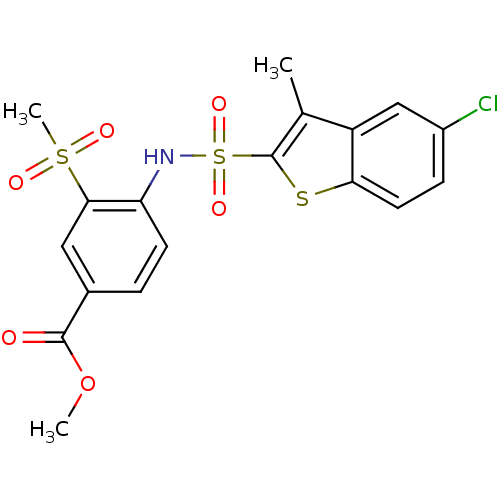 (4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toa Eiyo Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against bovin chymotrypsin | Bioorg Med Chem Lett 13: 4085-8 (2003) BindingDB Entry DOI: 10.7270/Q2QN665P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50135493 (5-Fluoro-3-methyl-benzo[b]thiophene-2-sulfonic aci...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toa Eiyo Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against bovin chymotrypsin | Bioorg Med Chem Lett 13: 4085-8 (2003) BindingDB Entry DOI: 10.7270/Q2QN665P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23707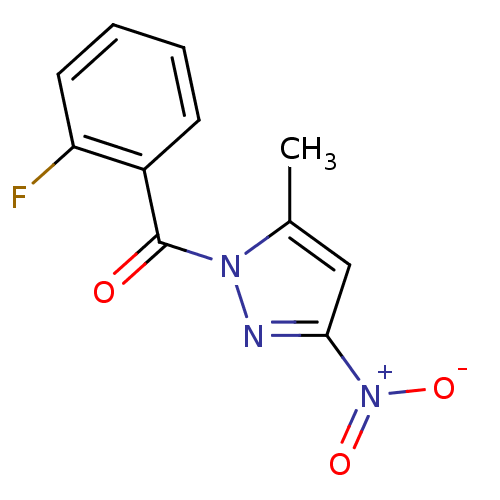 (1-[(2-fluorophenyl)carbonyl]-5-methyl-3-nitro-1H-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23717 (4-chloro-1-[(2-chlorophenyl)carbonyl]-1H-pyrazole ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23720 (1-[(4-tert-butylphenyl)carbonyl]-4-chloro-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23708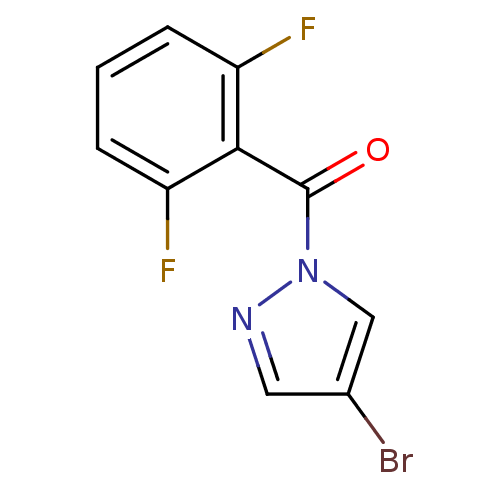 (4-bromo-1-[(2,6-difluorophenyl)carbonyl]-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120289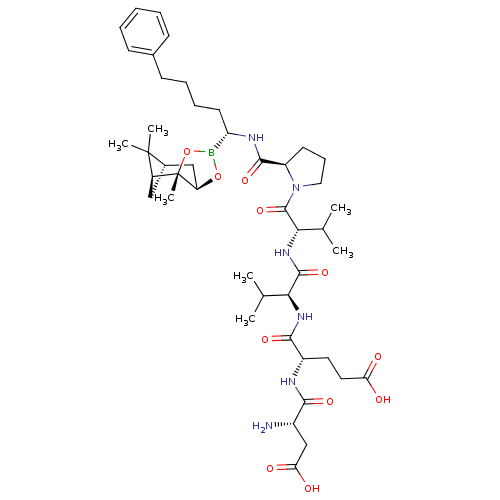 (CHEMBL419567 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50111590 (2-(4-Chloro-phenyl)-6-[4-(4-chloro-phenylsulfanyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against chymotrypsinogen | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120307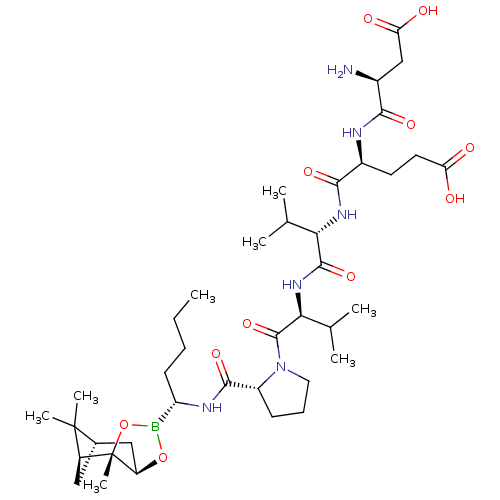 (CHEMBL263941 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23715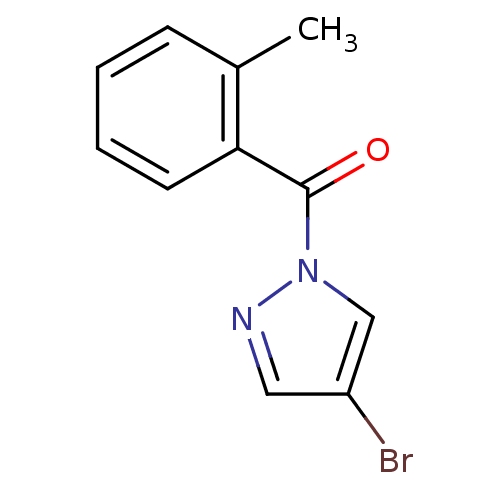 (4-bromo-1-[(2-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50111607 (3,4-Dichloro-N-[5-(5-chloro-benzothiazol-2-ylsulfa...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against chymotrypsinogen | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23714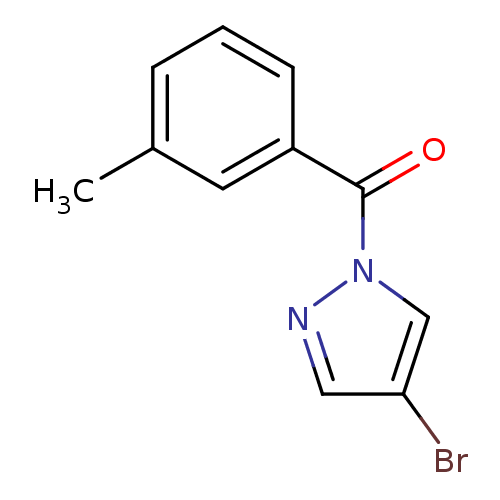 (4-bromo-1-[(3-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23703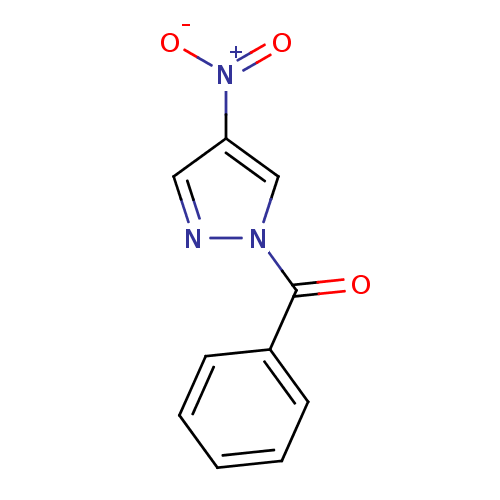 (1-benzoyl-4-nitro-1H-pyrazole | N-Benzoylpyrazole ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50111595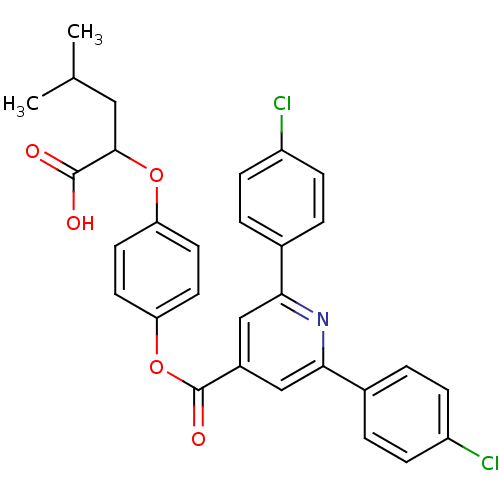 (2,6-Bis-(4-chloro-phenyl)-isonicotinic acid 4-(1-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against chymotrypsinogen | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23721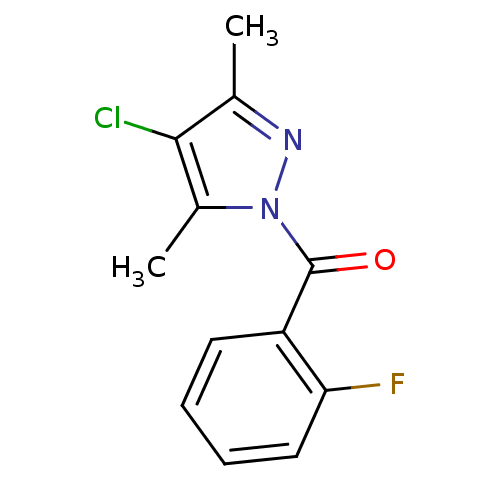 (4-chloro-1-[(2-fluorophenyl)carbonyl]-3,5-dimethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50120300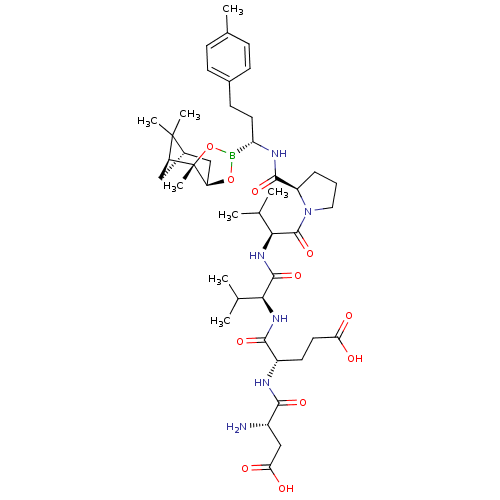 (CHEMBL431246 | Peptide Boronic Acid analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Human pancreatic Serine protease chymotrypsin | Bioorg Med Chem Lett 12: 3199-202 (2002) BindingDB Entry DOI: 10.7270/Q2R49Q38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |