 Found 930 hits of ic50 for UniProtKB: P12821
Found 930 hits of ic50 for UniProtKB: P12821 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272062
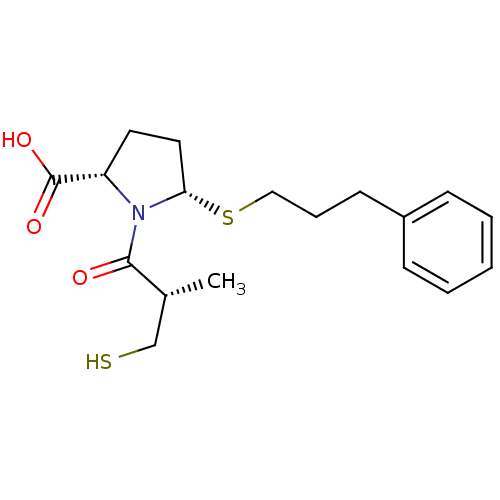
((2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(3-...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCCCc1ccccc1 |r| Show InChI InChI=1S/C18H25NO3S2/c1-13(12-23)17(20)19-15(18(21)22)9-10-16(19)24-11-5-8-14-6-3-2-4-7-14/h2-4,6-7,13,15-16,23H,5,8-12H2,1H3,(H,21,22)/t13-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879
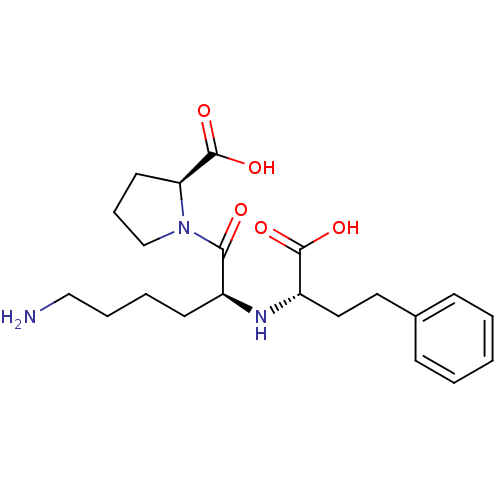
(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human serum ACE |
Bioorg Med Chem 21: 7216-21 (2013)
Article DOI: 10.1016/j.bmc.2013.08.032
BindingDB Entry DOI: 10.7270/Q21N843H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ACE using Mca-BK2 as substrate preincubated for 10 mins followed by fluorogenic substrate addition and measured after... |
ACS Med Chem Lett 10: 86-91 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00462
BindingDB Entry DOI: 10.7270/Q2D50R6V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serum |
J Med Chem 46: 3326-32 (2003)
Article DOI: 10.1021/jm021089h
BindingDB Entry DOI: 10.7270/Q2K07512 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50175518
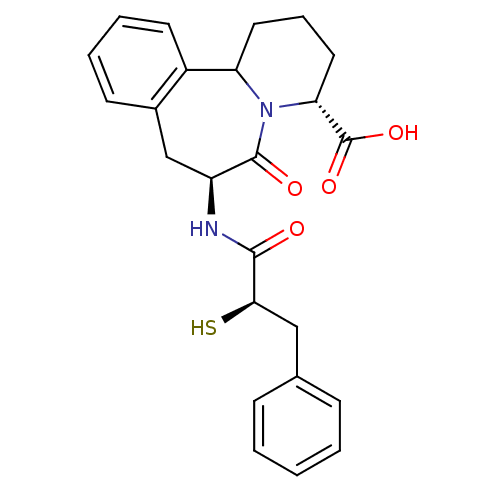
((3R,7S)-7-((R)-2-Mercapto-3-phenyl-propionylamino)...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@H](Cc1ccccc21)NC(=O)[C@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18-,19?,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin I converting enzyme |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406389
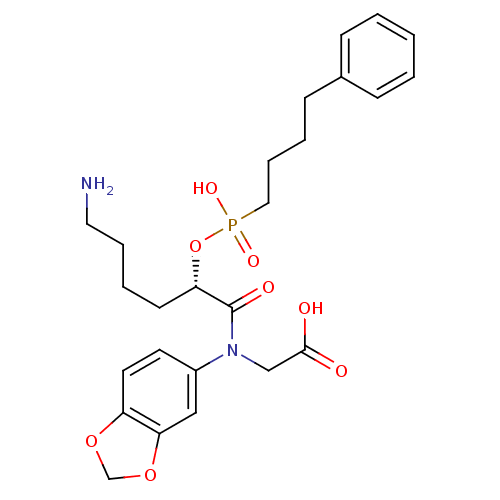
Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N(CC(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C25H33N2O8P/c26-14-6-4-11-22(35-36(31,32)15-7-5-10-19-8-2-1-3-9-19)25(30)27(17-24(28)29)20-12-13-21-23(16-20)34-18-33-21/h1-3,8-9,12-13,16,22H,4-7,10-11,14-15,17-18,26H2,(H,28,29)(H,31,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.115 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Chimica
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorimetry |
Bioorg Med Chem Lett 19: 4715-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.064
BindingDB Entry DOI: 10.7270/Q2GH9JWT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406391

Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@H](C[C@H]1C(O)=O)c1ccccc1 Show InChI InChI=1S/C27H37N2O6P/c28-17-9-7-16-25(35-36(33,34)18-10-8-13-21-11-3-1-4-12-21)26(30)29-20-23(19-24(29)27(31)32)22-14-5-2-6-15-22/h1-6,11-12,14-15,23-25H,7-10,13,16-20,28H2,(H,31,32)(H,33,34)/t23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272061
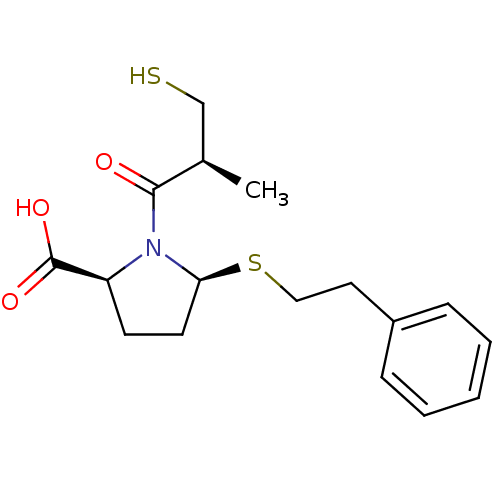
((2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(ph...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCCc1ccccc1 |r| Show InChI InChI=1S/C17H23NO3S2/c1-12(11-22)16(19)18-14(17(20)21)7-8-15(18)23-10-9-13-5-3-2-4-6-13/h2-6,12,14-15,22H,7-11H2,1H3,(H,20,21)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406942
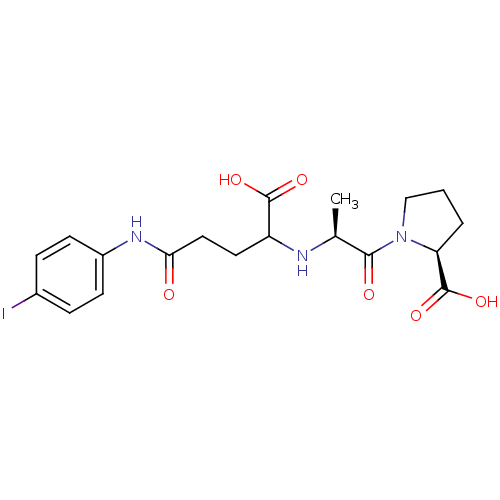
(CHEMBL80665)Show SMILES C[C@H](NC(CCC(=O)Nc1ccc(I)cc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H24IN3O6/c1-11(17(25)23-10-2-3-15(23)19(28)29)21-14(18(26)27)8-9-16(24)22-13-6-4-12(20)5-7-13/h4-7,11,14-15,21H,2-3,8-10H2,1H3,(H,22,24)(H,26,27)(H,28,29)/t11-,14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.229 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406908

(CHEMBL78629)Show SMILES OC(=O)[C@@H](CCC(=O)N1[C@@H](Cc2ccccc12)C(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H31N3O8/c35-27(34-25-14-8-7-13-22(25)18-26(34)30(39)40)16-15-23(29(37)38)32-28(36)24(17-20-9-3-1-4-10-20)33-31(41)42-19-21-11-5-2-6-12-21/h1-14,23-24,26H,15-19H2,(H,32,36)(H,33,41)(H,37,38)(H,39,40)/t23-,24?,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406375

(CHEMBL35682)Show SMILES COc1ccc(cc1OC)N(CC(O)=O)C(=O)[C@H](CCCCN)OP(O)(=O)CCCCc1ccccc1 Show InChI InChI=1S/C26H37N2O8P/c1-34-22-15-14-21(18-24(22)35-2)28(19-25(29)30)26(31)23(13-6-8-16-27)36-37(32,33)17-9-7-12-20-10-4-3-5-11-20/h3-5,10-11,14-15,18,23H,6-9,12-13,16-17,19,27H2,1-2H3,(H,29,30)(H,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406380

(CHEMBL284734)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O Show InChI InChI=1S/C25H33N2O6P/c26-16-8-6-15-23(33-34(31,32)17-9-7-12-19-10-2-1-3-11-19)24(28)27-21-14-5-4-13-20(21)18-22(27)25(29)30/h1-5,10-11,13-14,22-23H,6-9,12,15-18,26H2,(H,29,30)(H,31,32)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.295 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406392
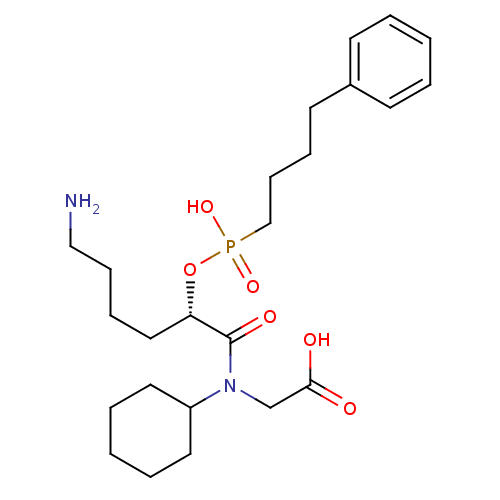
Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N(CC(O)=O)C1CCCCC1 Show InChI InChI=1S/C24H39N2O6P/c25-17-9-7-16-22(24(29)26(19-23(27)28)21-14-5-2-6-15-21)32-33(30,31)18-10-8-13-20-11-3-1-4-12-20/h1,3-4,11-12,21-22H,2,5-10,13-19,25H2,(H,27,28)(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.295 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50084629
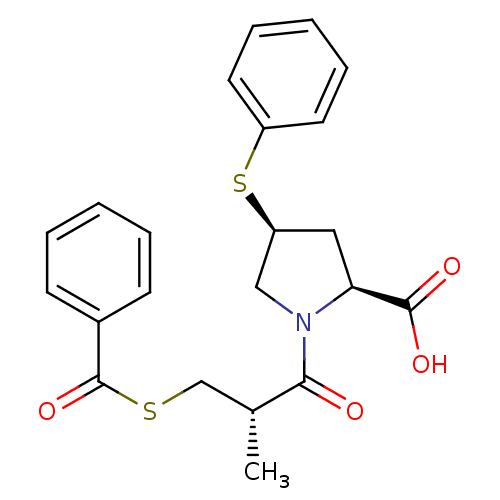
(1-(3-Benzoylsulfanyl-2-methyl-propionyl)-4-phenyls...)Show SMILES C[C@H](CSC(=O)c1ccccc1)C(=O)N1C[C@H](C[C@H]1C(O)=O)Sc1ccccc1 Show InChI InChI=1S/C22H23NO4S2/c1-15(14-28-22(27)16-8-4-2-5-9-16)20(24)23-13-18(12-19(23)21(25)26)29-17-10-6-3-7-11-17/h2-11,15,18-19H,12-14H2,1H3,(H,25,26)/t15-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme (ACE) |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406394

Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@H](C[C@H]1C(O)=O)C1CCCCC1 Show InChI InChI=1S/C27H43N2O6P/c28-17-9-7-16-25(35-36(33,34)18-10-8-13-21-11-3-1-4-12-21)26(30)29-20-23(19-24(29)27(31)32)22-14-5-2-6-15-22/h1,3-4,11-12,22-25H,2,5-10,13-20,28H2,(H,31,32)(H,33,34)/t23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125
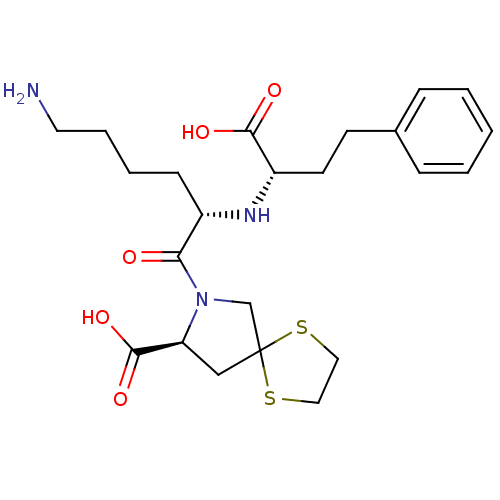
(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Compound tested in vitro for inhibition of Angiotensin I converting enzyme |
J Med Chem 32: 1600-6 (1989)
BindingDB Entry DOI: 10.7270/Q2M61KVW |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406369
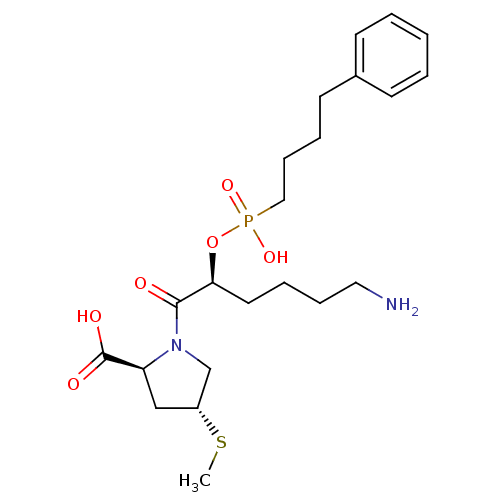
(CHEMBL289022)Show SMILES CS[C@@H]1C[C@H](N(C1)C(=O)[C@H](CCCCN)OP(O)(=O)CCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C22H35N2O6PS/c1-32-18-15-19(22(26)27)24(16-18)21(25)20(12-5-7-13-23)30-31(28,29)14-8-6-11-17-9-3-2-4-10-17/h2-4,9-10,18-20H,5-8,11-16,23H2,1H3,(H,26,27)(H,28,29)/t18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406370

(CHEMBL34832)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@H](C[C@H]1C(O)=O)Sc1ccccc1 Show InChI InChI=1S/C27H37N2O6PS/c28-17-9-7-16-25(35-36(33,34)18-10-8-13-21-11-3-1-4-12-21)26(30)29-20-23(19-24(29)27(31)32)37-22-14-5-2-6-15-22/h1-6,11-12,14-15,23-25H,7-10,13,16-20,28H2,(H,31,32)(H,33,34)/t23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50121930
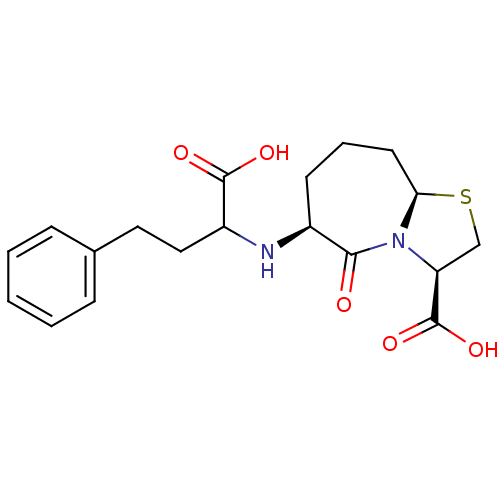
(6-(1-Carboxy-3-phenyl-propylamino)-5-oxo-octahydro...)Show SMILES OC(=O)C(CCc1ccccc1)N[C@H]1CCC[C@H]2SC[C@H](N2C1=O)C(O)=O Show InChI InChI=1S/C19H24N2O5S/c22-17-13(7-4-8-16-21(17)15(11-27-16)19(25)26)20-14(18(23)24)10-9-12-5-2-1-3-6-12/h1-3,5-6,13-16,20H,4,7-11H2,(H,23,24)(H,25,26)/t13-,14?,15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom |
J Med Chem 45: 5609-16 (2002)
BindingDB Entry DOI: 10.7270/Q270825W |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50121930

(6-(1-Carboxy-3-phenyl-propylamino)-5-oxo-octahydro...)Show SMILES OC(=O)C(CCc1ccccc1)N[C@H]1CCC[C@H]2SC[C@H](N2C1=O)C(O)=O Show InChI InChI=1S/C19H24N2O5S/c22-17-13(7-4-8-16-21(17)15(11-27-16)19(25)26)20-14(18(23)24)10-9-12-5-2-1-3-6-12/h1-3,5-6,13-16,20H,4,7-11H2,(H,23,24)(H,25,26)/t13-,14?,15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406384
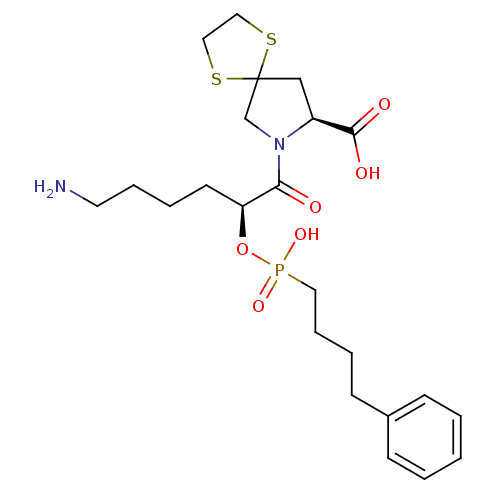
(CHEMBL34650)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H35N2O6PS2/c24-12-6-4-11-20(31-32(29,30)13-7-5-10-18-8-2-1-3-9-18)21(26)25-17-23(33-14-15-34-23)16-19(25)22(27)28/h1-3,8-9,19-20H,4-7,10-17,24H2,(H,27,28)(H,29,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50520683
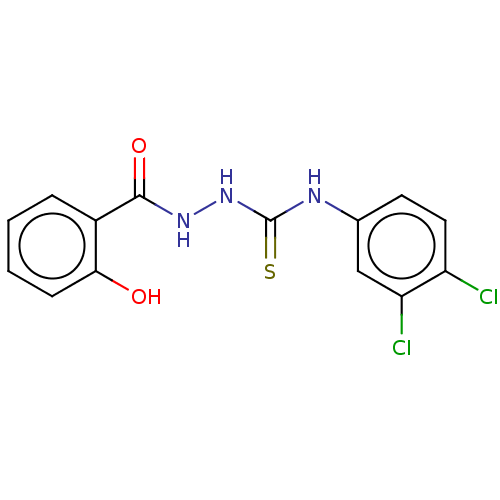
(CHEMBL4476621)Show InChI InChI=1S/C14H11Cl2N3O2S/c15-10-6-5-8(7-11(10)16)17-14(22)19-18-13(21)9-3-1-2-4-12(9)20/h1-7,20H,(H,18,21)(H2,17,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain (UCLouvain)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE using Abz-FRK(Dnp)-P-OH as substrate after 30 mins by fluorimetric analysis |
Eur J Med Chem 159: 324-338 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.067
BindingDB Entry DOI: 10.7270/Q2VM4GND |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406378

(CHEMBL431707)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@@H](C[C@H]1C(O)=O)Sc1ccccc1 Show InChI InChI=1S/C27H37N2O6PS/c28-17-9-7-16-25(35-36(33,34)18-10-8-13-21-11-3-1-4-12-21)26(30)29-20-23(19-24(29)27(31)32)37-22-14-5-2-6-15-22/h1-6,11-12,14-15,23-25H,7-10,13,16-20,28H2,(H,31,32)(H,33,34)/t23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406942

(CHEMBL80665)Show SMILES C[C@H](NC(CCC(=O)Nc1ccc(I)cc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C19H24IN3O6/c1-11(17(25)23-10-2-3-15(23)19(28)29)21-14(18(26)27)8-9-16(24)22-13-6-4-12(20)5-7-13/h4-7,11,14-15,21H,2-3,8-10H2,1H3,(H,22,24)(H,26,27)(H,28,29)/t11-,14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin I converting enzyme in silico |
J Med Chem 40: 3161-72 (1997)
Article DOI: 10.1021/jm970211n
BindingDB Entry DOI: 10.7270/Q2MG7QPB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017122
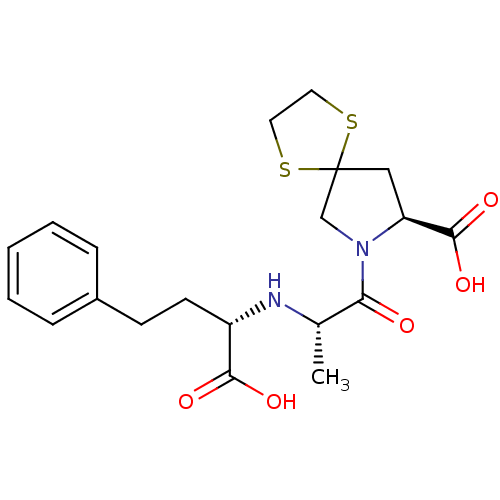
(7-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C20H26N2O5S2/c1-13(21-15(18(24)25)8-7-14-5-3-2-4-6-14)17(23)22-12-20(28-9-10-29-20)11-16(22)19(26)27/h2-6,13,15-16,21H,7-12H2,1H3,(H,24,25)(H,26,27)/t13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Compound tested in vitro for inhibition of Angiotensin I converting enzyme |
J Med Chem 32: 1600-6 (1989)
BindingDB Entry DOI: 10.7270/Q2M61KVW |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406372
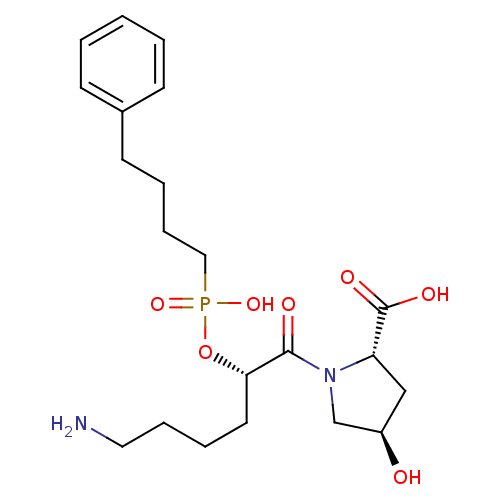
(CHEMBL33025)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@H](O)C[C@H]1C(O)=O Show InChI InChI=1S/C21H33N2O7P/c22-12-6-4-11-19(20(25)23-15-17(24)14-18(23)21(26)27)30-31(28,29)13-7-5-10-16-8-2-1-3-9-16/h1-3,8-9,17-19,24H,4-7,10-15,22H2,(H,26,27)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406373
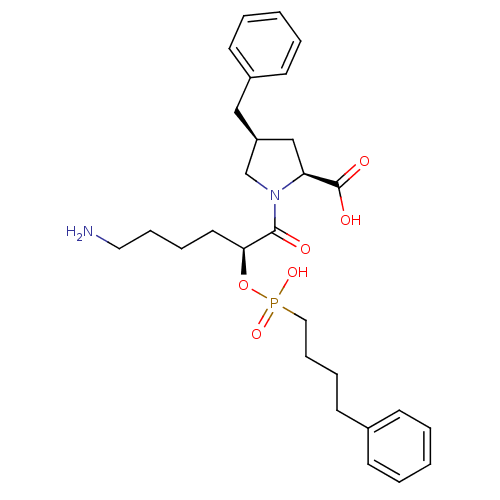
(CHEMBL284843)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@@H](Cc2ccccc2)C[C@H]1C(O)=O Show InChI InChI=1S/C28H39N2O6P/c29-17-9-7-16-26(36-37(34,35)18-10-8-13-22-11-3-1-4-12-22)27(31)30-21-24(20-25(30)28(32)33)19-23-14-5-2-6-15-23/h1-6,11-12,14-15,24-26H,7-10,13,16-21,29H2,(H,32,33)(H,34,35)/t24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272145

((2S,5S)-1-((S)-3-Mercapto-2-methyl-propionyl)-5-(3...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1cccc(C)c1 |r| Show InChI InChI=1S/C17H23NO3S2/c1-11-4-3-5-13(8-11)10-23-15-7-6-14(17(20)21)18(15)16(19)12(2)9-22/h3-5,8,12,14-15,22H,6-7,9-10H2,1-2H3,(H,20,21)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50369775
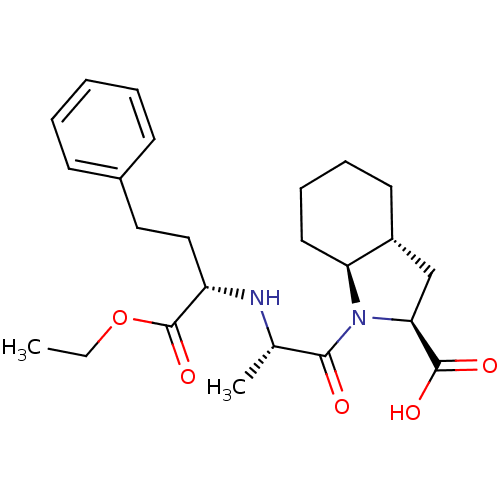
(Mavik | RU-44570 | TRANDOLAPRIL)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1[C@H]2CCCC[C@@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C24H34N2O5/c1-3-31-24(30)19(14-13-17-9-5-4-6-10-17)25-16(2)22(27)26-20-12-8-7-11-18(20)15-21(26)23(28)29/h4-6,9-10,16,18-21,25H,3,7-8,11-15H2,1-2H3,(H,28,29)/t16-,18+,19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme (ACE) |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human fully glycosylated ACE C-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... |
J Med Chem 61: 10141-10154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01309
BindingDB Entry DOI: 10.7270/Q2862K4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50146428
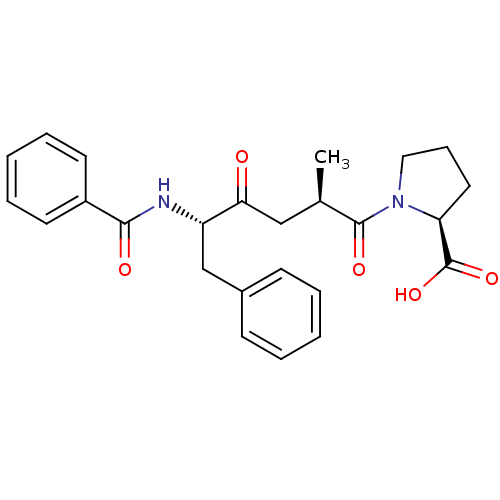
((S)-1-((1R,5S)-5-Benzoylamino-2-methyl-4-oxo-6-phe...)Show SMILES C[C@H](CC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C25H28N2O5/c1-17(24(30)27-14-8-13-21(27)25(31)32)15-22(28)20(16-18-9-4-2-5-10-18)26-23(29)19-11-6-3-7-12-19/h2-7,9-12,17,20-21H,8,13-16H2,1H3,(H,26,29)(H,31,32)/t17-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406911
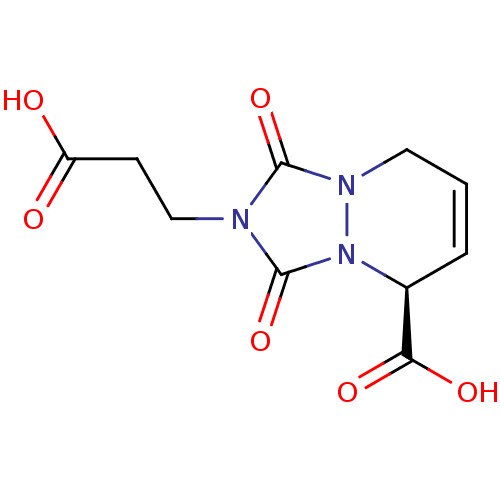
(CHEMBL310750)Show SMILES OC(=O)CCn1c(=O)n2CC=C[C@@H](C(O)=O)n2c1=O |c:10| Show InChI InChI=1S/C10H11N3O6/c14-7(15)3-5-11-9(18)12-4-1-2-6(8(16)17)13(12)10(11)19/h1-2,6H,3-5H2,(H,14,15)(H,16,17)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50130714
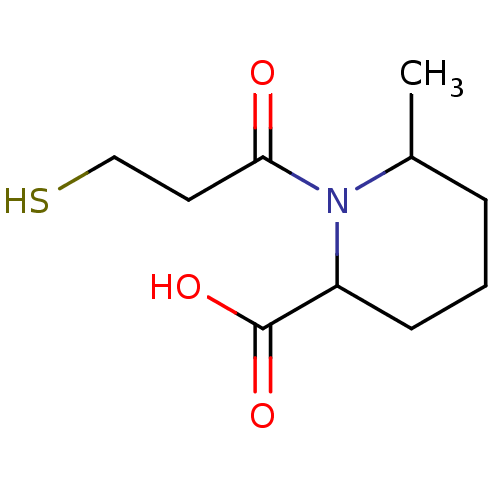
(1-(3-Mercapto-propionyl)-6-methyl-piperidine-2-car...)Show InChI InChI=1S/C10H17NO3S/c1-7-3-2-4-8(10(13)14)11(7)9(12)5-6-15/h7-8,15H,2-6H2,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidney |
J Med Chem 46: 3326-32 (2003)
Article DOI: 10.1021/jm021089h
BindingDB Entry DOI: 10.7270/Q2K07512 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50084622
((2S,4S)-4-cyclohexyl-1-[2-[(2-methyl-1-propanoylox...)Show SMILES CCC(=O)OC(OP(=O)(CCCc1ccccc1)CC(=O)N1C[C@@H](C[C@H]1C(O)=O)C1CCCCC1)C(C)C Show InChI InChI=1S/C29H44NO7P/c1-4-27(32)36-29(21(2)3)37-38(35,17-11-14-22-12-7-5-8-13-22)20-26(31)30-19-24(18-25(30)28(33)34)23-15-9-6-10-16-23/h5,7-8,12-13,21,23-25,29H,4,6,9-11,14-20H2,1-3H3,(H,33,34)/t24-,25+,29?,38?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme (ACE) |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406387
Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N(CC(O)=O)c1ccccc1 Show InChI InChI=1S/C24H33N2O6P/c25-17-9-7-16-22(24(29)26(19-23(27)28)21-14-5-2-6-15-21)32-33(30,31)18-10-8-13-20-11-3-1-4-12-20/h1-6,11-12,14-15,22H,7-10,13,16-19,25H2,(H,27,28)(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406909
(CHEMBL80779)Show SMILES C[C@H](NC(CCc1cccc2ccccc12)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C22H26N2O5/c1-14(20(25)24-13-5-10-19(24)22(28)29)23-18(21(26)27)12-11-16-8-4-7-15-6-2-3-9-17(15)16/h2-4,6-9,14,18-19,23H,5,10-13H2,1H3,(H,26,27)(H,28,29)/t14-,18?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme in Hog plasma |
J Med Chem 33: 1606-15 (1990)
BindingDB Entry DOI: 10.7270/Q2CF9QPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50017129
((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom |
J Med Chem 45: 5609-16 (2002)
BindingDB Entry DOI: 10.7270/Q270825W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom |
J Med Chem 45: 5609-16 (2002)
BindingDB Entry DOI: 10.7270/Q270825W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 36: 2051-8 (1993)
BindingDB Entry DOI: 10.7270/Q2NP2524 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
J Med Chem 36: 2051-8 (1993)
BindingDB Entry DOI: 10.7270/Q2NP2524 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Angiotensin I converting enzyme |
J Med Chem 29: 251-60 (1986)
BindingDB Entry DOI: 10.7270/Q2J67HH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021129
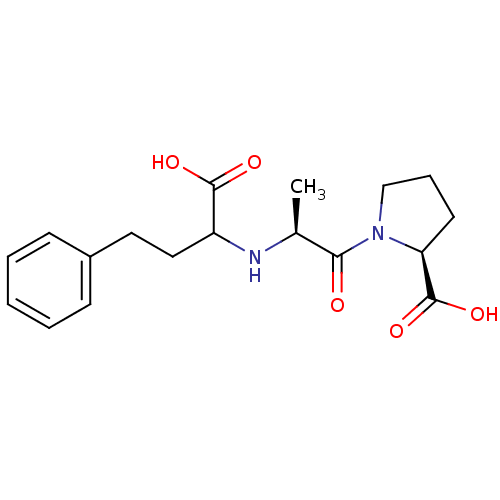
(1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-p...)Show SMILES C[C@H](NC(CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme (ACE) |
J Med Chem 36: 2390-403 (1993)
BindingDB Entry DOI: 10.7270/Q2Z320V9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027142
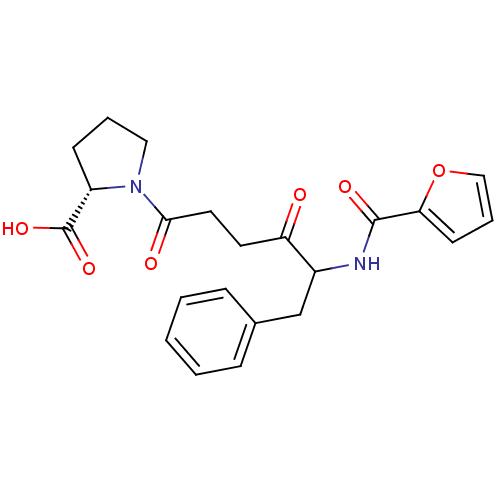
(1-{5-[(Furan-2-carbonyl)-amino]-4-oxo-6-phenyl-hex...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccco1 Show InChI InChI=1S/C22H24N2O6/c25-18(10-11-20(26)24-12-4-8-17(24)22(28)29)16(14-15-6-2-1-3-7-15)23-21(27)19-9-5-13-30-19/h1-3,5-7,9,13,16-17H,4,8,10-12,14H2,(H,23,27)(H,28,29)/t16?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data