 Found 326 hits of ki for UniProtKB: P28845
Found 326 hits of ki for UniProtKB: P28845 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
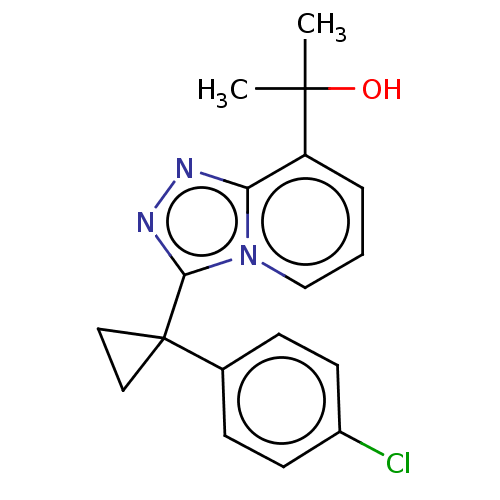
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317218
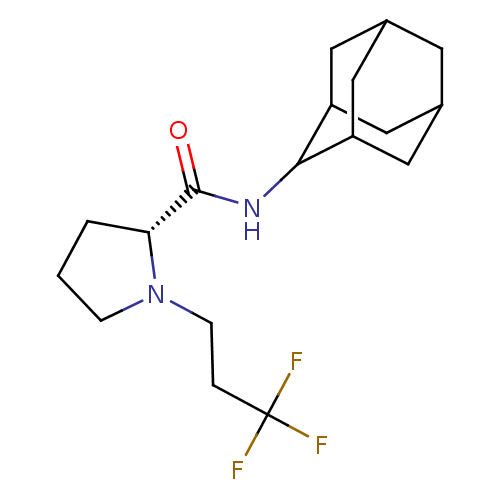
((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317224
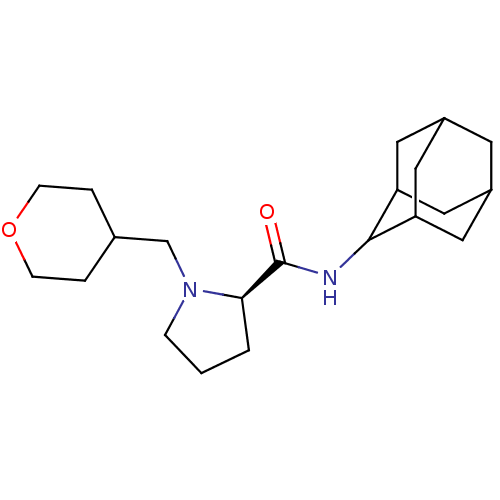
((2R)-N-(adamantan-2-yl)-1-(oxan-4-ylmethyl)pyrroli...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCOCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(16.19,2.36,;14.84,1.61,;14.82,.07,;13.47,-.68,;13.46,-2.21,;12.06,-2.57,;10.72,-2.07,;9.51,-3.36,;11.02,-2.93,;12.44,-3.5,;11.01,-1.34,;12.06,-.09,;10.7,-.58,;13.33,2.12,;12.11,1.14,;10.87,2.05,;11.33,3.52,;12.87,3.53,;13.64,4.87,;15.19,4.87,;15.96,3.52,;17.5,3.52,;18.28,4.86,;17.51,6.2,;15.96,6.21,)| Show InChI InChI=1S/C21H34N2O2/c24-21(19-2-1-5-23(19)13-14-3-6-25-7-4-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317223
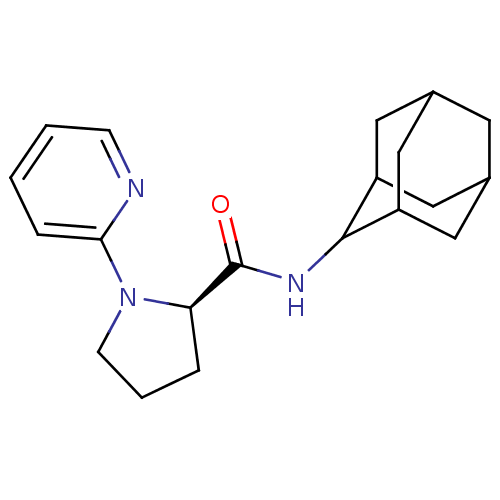
((2R)-N-(adamantan-2-yl)-1-(pyridin-2-yl)pyrrolidin...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1c1ccccn1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(6.04,2.3,;4.69,1.55,;4.66,.01,;3.32,-.73,;3.31,-2.26,;1.91,-2.62,;.57,-2.12,;-.63,-3.4,;.88,-2.98,;2.29,-3.54,;.87,-1.39,;1.92,-.15,;.56,-.63,;3.18,2.06,;1.97,1.09,;.73,1.99,;1.2,3.45,;2.72,3.47,;4.03,4.27,;4,5.82,;5.31,6.62,;6.67,5.87,;6.7,4.35,;5.39,3.54,)| Show InChI InChI=1S/C20H27N3O/c24-20(17-4-3-7-23(17)18-5-1-2-6-21-18)22-19-15-9-13-8-14(11-15)12-16(19)10-13/h1-2,5-6,13-17,19H,3-4,7-12H2,(H,22,24)/t13?,14?,15?,16?,17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317221
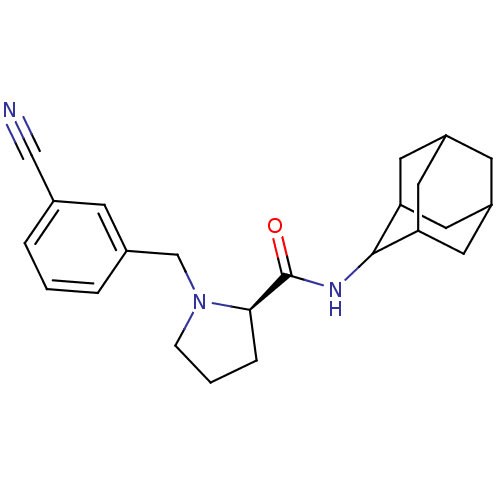
((2R)-N-(adamantan-2-yl)-1-[(3-cyanophenyl)methyl]p...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1Cc1cccc(c1)C#N |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(29.81,-42.16,;28.46,-42.91,;28.44,-44.45,;27.09,-45.2,;27.08,-46.73,;25.68,-47.08,;24.34,-46.59,;23.15,-47.87,;24.65,-47.44,;26.06,-48.01,;24.64,-45.86,;25.69,-44.62,;24.33,-45.1,;26.95,-42.4,;25.74,-43.38,;24.5,-42.47,;24.97,-41.02,;26.5,-41,;27.4,-39.75,;26.63,-38.41,;25.1,-38.41,;24.33,-37.08,;25.11,-35.74,;26.65,-35.75,;27.41,-37.09,;27.43,-34.42,;28.2,-33.09,)| Show InChI InChI=1S/C23H29N3O/c24-13-15-3-1-4-16(7-15)14-26-6-2-5-21(26)23(27)25-22-19-9-17-8-18(11-19)12-20(22)10-17/h1,3-4,7,17-22H,2,5-6,8-12,14H2,(H,25,27)/t17?,18?,19?,20?,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
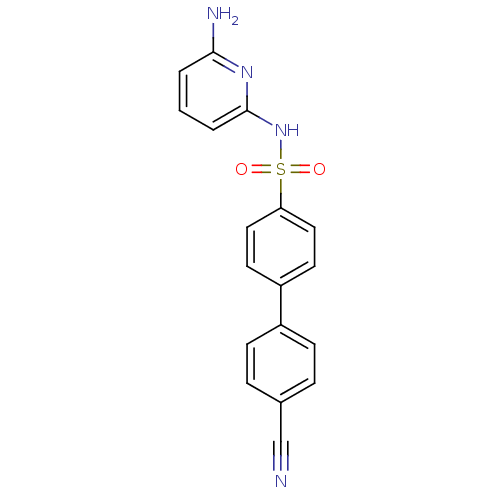
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | <-12.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317217
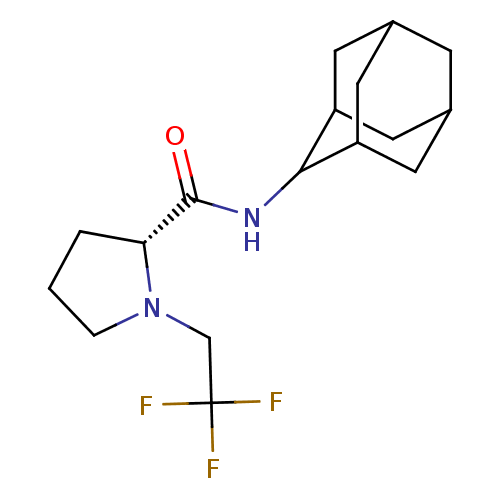
((2R)-N-(adamantan-2-yl)-1-(2,2,2-trifluoroethyl)py...)Show SMILES FC(F)(F)CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:9.10,TLB:19:18:22:15.14.13,19:14:17.18.20:22,THB:12:13:17.18.20:22,13:14:17:20.21.22,13:21:17:15.19.14,(27.97,-20.63,;27.2,-21.96,;25.66,-21.96,;26.42,-20.62,;27.97,-23.29,;27.07,-24.54,;25.53,-24.56,;25.07,-26.02,;26.31,-26.92,;27.52,-25.95,;29.03,-26.46,;30.37,-25.71,;29,-28,;27.66,-28.74,;27.65,-30.27,;26.25,-30.63,;24.91,-30.13,;23.71,-31.41,;25.22,-30.99,;26.63,-31.56,;25.21,-29.4,;26.26,-28.16,;24.9,-28.65,)| Show InChI InChI=1S/C17H25F3N2O/c18-17(19,20)9-22-3-1-2-14(22)16(23)21-15-12-5-10-4-11(7-12)8-13(15)6-10/h10-15H,1-9H2,(H,21,23)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317211
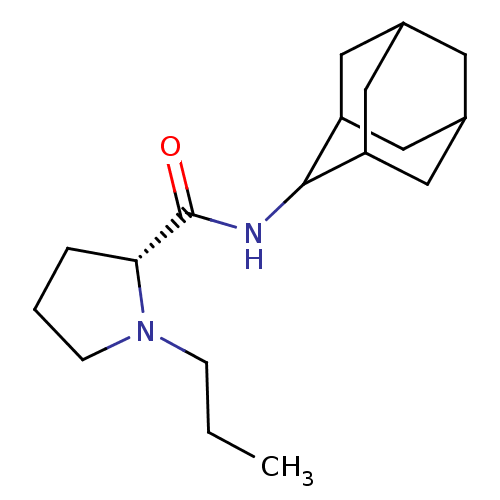
((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209
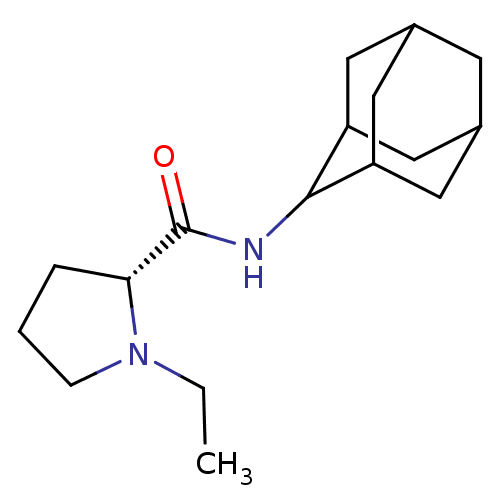
((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317212
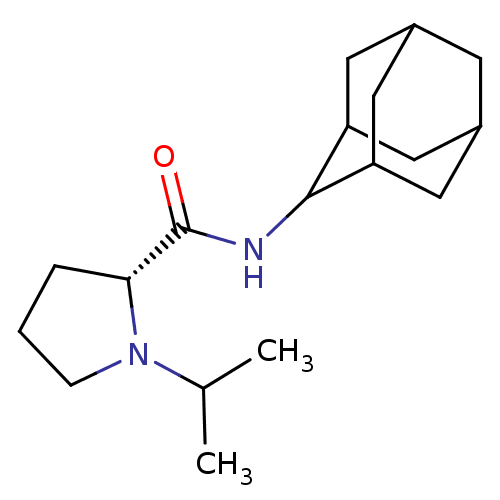
((2R)-N-(adamantan-2-yl)-1-(propan-2-yl)pyrrolidine...)Show SMILES CC(C)N1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(12.99,-6.92,;13.62,-8.33,;15.15,-8.49,;12.71,-9.58,;11.18,-9.59,;10.72,-11.05,;11.95,-11.95,;13.17,-10.98,;14.67,-11.49,;16.02,-10.74,;14.65,-13.03,;13.3,-13.78,;13.3,-15.31,;11.9,-15.66,;10.56,-15.17,;9.36,-16.45,;10.87,-16.02,;12.28,-16.59,;10.86,-14.44,;11.9,-13.19,;10.55,-13.68,)| Show InChI InChI=1S/C18H30N2O/c1-11(2)20-5-3-4-16(20)18(21)19-17-14-7-12-6-13(9-14)10-15(17)8-12/h11-17H,3-10H2,1-2H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29863

(N-(Pyridin-2-yl) arylsulfonamide, 25)Show SMILES CC(C)c1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C21H19N3O2S/c1-15(2)20-4-3-5-21(23-20)24-27(25,26)19-12-10-18(11-13-19)17-8-6-16(14-22)7-9-17/h3-13,15H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | -11.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29862
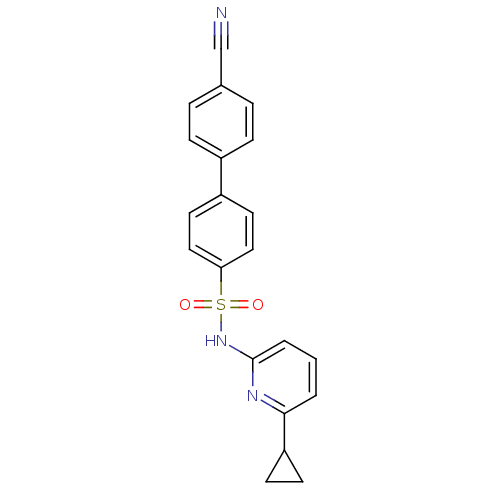
(N-(Pyridin-2-yl) arylsulfonamide, 24)Show SMILES O=S(=O)(Nc1cccc(n1)C1CC1)c1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H17N3O2S/c22-14-15-4-6-16(7-5-15)17-10-12-19(13-11-17)27(25,26)24-21-3-1-2-20(23-21)18-8-9-18/h1-7,10-13,18H,8-9H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | -11.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195289
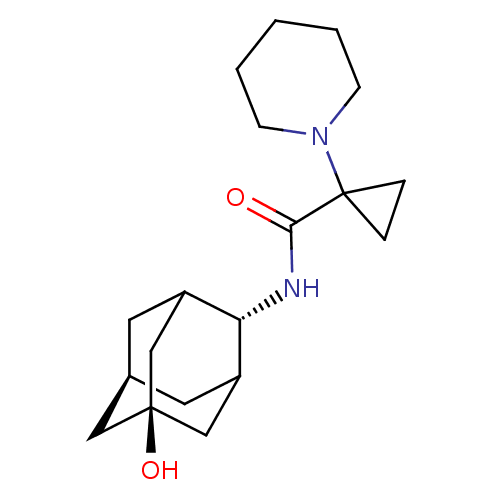
(1-piperidin-1-yl-cyclopropanecarboxylic acid (5-hy...)Show SMILES O[C@@]12C[C@H]3CC(C1)[C@H](NC(=O)C1(CC1)N1CCCCC1)C(C3)C2 |wU:7.8,wD:1.0,3.2,TLB:4:3:22:6.5.7,4:5:2.3.21:22,THB:8:7:2.3.21:22,(1.12,-7.02,;2.66,-7.15,;1.37,-8.33,;2.9,-8.03,;4.25,-8.7,;5.36,-7.5,;3.94,-7.74,;5.49,-5.98,;6.83,-5.23,;8.16,-6.02,;8.13,-7.56,;9.5,-5.27,;10.29,-3.94,;8.75,-3.92,;10.82,-6.06,;10.79,-7.6,;12.11,-8.38,;13.46,-7.64,;13.48,-6.1,;12.16,-5.3,;4.14,-5.3,;3.01,-6.44,;2.76,-5.67,)| Show InChI InChI=1S/C19H30N2O2/c22-17(19(4-5-19)21-6-2-1-3-7-21)20-16-14-8-13-9-15(16)12-18(23,10-13)11-14/h13-16,23H,1-12H2,(H,20,22)/t13-,14?,15?,16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317219
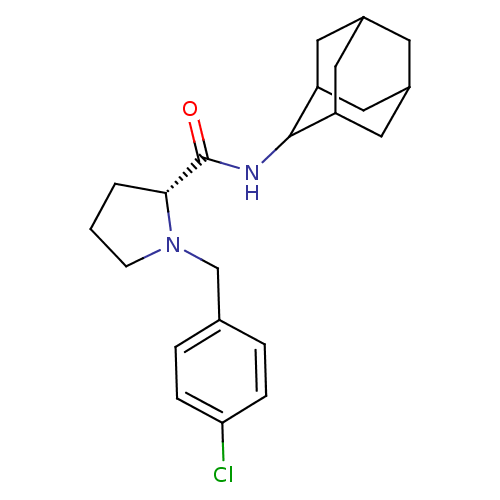
((2R)-N-(adamantan-2-yl)-1-[(4-chlorophenyl)methyl]...)Show SMILES Clc1ccc(CN2CCC[C@@H]2C(=O)NC2C3CC4CC(C3)CC2C4)cc1 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(2.87,-33.68,;3.64,-35.01,;5.18,-35.02,;5.94,-36.36,;5.16,-37.69,;5.93,-39.03,;5.02,-40.28,;3.49,-40.29,;3.03,-41.75,;4.27,-42.66,;5.48,-41.68,;6.99,-42.19,;8.34,-41.44,;6.96,-43.73,;5.62,-44.48,;5.61,-46.01,;4.21,-46.36,;2.87,-45.87,;1.67,-47.15,;3.18,-46.72,;4.59,-47.29,;3.17,-45.14,;4.22,-43.9,;2.86,-44.38,;3.63,-37.69,;2.86,-36.35,)| Show InChI InChI=1S/C22H29ClN2O/c23-19-5-3-14(4-6-19)13-25-7-1-2-20(25)22(26)24-21-17-9-15-8-16(11-17)12-18(21)10-15/h3-6,15-18,20-21H,1-2,7-13H2,(H,24,26)/t15?,16?,17?,18?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317210
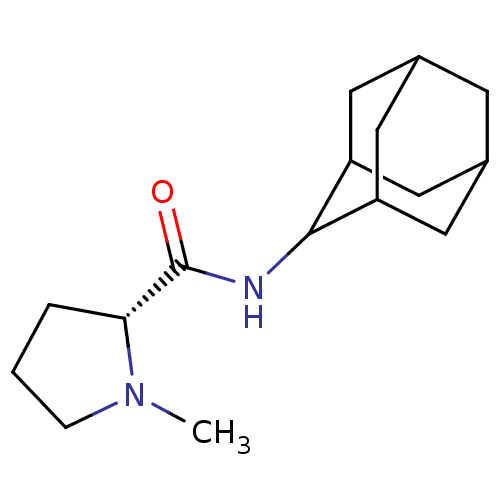
((2R)-N-(adamantan-2-yl)-1-methylpyrrolidine-2-carb...)Show SMILES CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:5.6,TLB:15:14:18:11.10.9,15:10:13.14.16:18,THB:8:9:13.14.16:18,9:10:13:16.17.18,9:17:13:11.15.10,(-4.95,-6.94,;-5.86,-8.19,;-7.4,-8.21,;-7.87,-9.67,;-6.63,-10.58,;-5.41,-9.6,;-3.89,-10.12,;-2.54,-9.36,;-3.91,-11.66,;-5.26,-12.4,;-5.27,-13.94,;-6.68,-14.3,;-8.02,-13.8,;-9.23,-15.09,;-7.71,-14.66,;-6.29,-15.23,;-7.72,-13.07,;-6.67,-11.82,;-8.03,-12.3,)| Show InChI InChI=1S/C16H26N2O/c1-18-4-2-3-14(18)16(19)17-15-12-6-10-5-11(8-12)9-13(15)7-10/h10-15H,2-9H2,1H3,(H,17,19)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317216
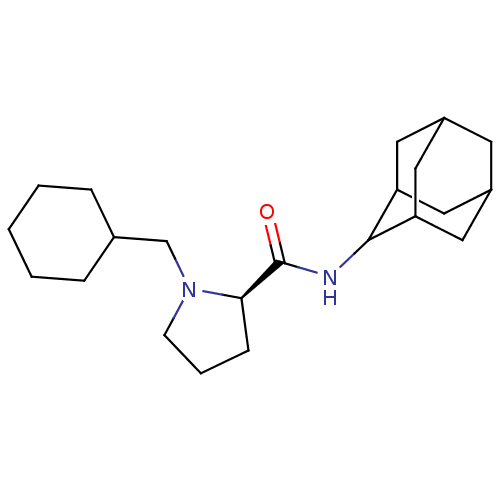
((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(18.88,-25.64,;17.53,-26.39,;17.51,-27.93,;16.16,-28.68,;16.15,-30.21,;14.75,-30.56,;13.41,-30.07,;12.22,-31.35,;13.72,-30.92,;15.13,-31.49,;13.71,-29.34,;14.76,-28.1,;13.4,-28.58,;16.02,-25.88,;14.81,-26.86,;13.57,-25.95,;14.04,-24.49,;15.57,-24.48,;16.47,-23.23,;15.7,-21.89,;16.48,-20.57,;15.71,-19.24,;14.17,-19.23,;13.4,-20.56,;14.17,-21.9,)| Show InChI InChI=1S/C22H36N2O/c25-22(20-7-4-8-24(20)14-15-5-2-1-3-6-15)23-21-18-10-16-9-17(12-18)13-19(21)11-16/h15-21H,1-14H2,(H,23,25)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202094
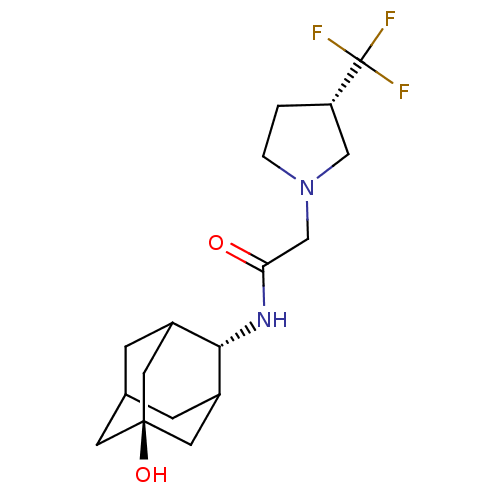
(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197404
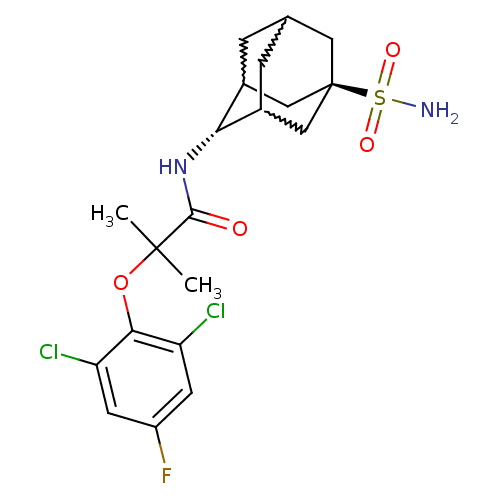
(2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...)Show SMILES CC(C)(Oc1c(Cl)cc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.19,17.28,21.21,wU:16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:17:24.19.20:22,16:21:24:25.18.17,TEB:18:19:22:25.17.16,20:21:25:24.19.18,(24.03,-36.5,;24.84,-37.8,;25.66,-39.11,;26.17,-37.02,;27.51,-37.78,;28.83,-37,;28.81,-35.46,;30.17,-37.75,;30.18,-39.3,;31.52,-40.06,;28.85,-40.08,;27.52,-39.32,;26.19,-40.1,;23.56,-38.65,;23.65,-40.19,;22.18,-37.97,;20.9,-38.82,;20.89,-40.35,;19.87,-41.62,;18.47,-41.06,;18.46,-39.47,;19.5,-38.24,;18.15,-38.72,;18.16,-40.2,;16.97,-41.48,;19.49,-40.69,;16.67,-39.79,;15.17,-39.39,;17.07,-38.31,;16.26,-41.28,)| Show InChI InChI=1S/C20H25Cl2FN2O4S/c1-19(2,29-17-14(21)5-13(23)6-15(17)22)18(26)25-16-11-3-10-4-12(16)9-20(7-10,8-11)30(24,27)28/h5-6,10-12,16H,3-4,7-9H2,1-2H3,(H,25,26)(H2,24,27,28)/t10?,11?,12?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13746
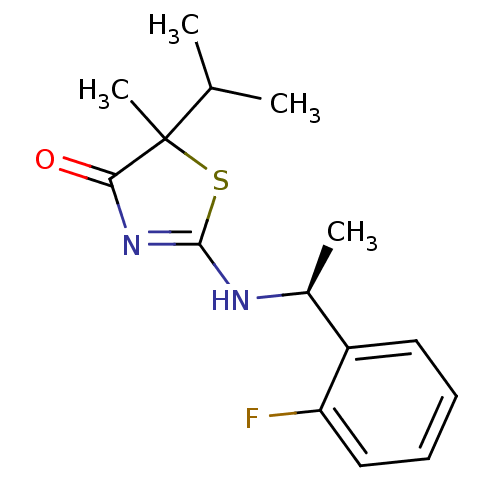
(2-((S)-1-(2-fluorophenyl)ethylamino)-5-isopropyl-5...)Show SMILES CC(C)C1(C)SC(N[C@@H](C)c2ccccc2F)=NC1=O |r,c:17| Show InChI InChI=1S/C15H19FN2OS/c1-9(2)15(4)13(19)18-14(20-15)17-10(3)11-7-5-6-8-12(11)16/h5-10H,1-4H3,(H,17,18,19)/t10-,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -11.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Amgen
| Assay Description
Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... |
J Med Chem 50: 429-32 (2007)
Article DOI: 10.1021/jm061214f
BindingDB Entry DOI: 10.7270/Q20Z71JH |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM22473
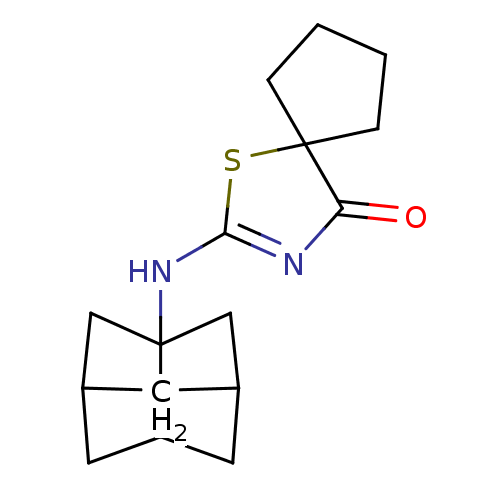
(2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...)Show SMILES O=C1N=C(NC23CC4CC2CC(C3)C4)SC11CCCC1 |t:2,TLB:12:11:5.6:8,4:5:8:11.10.13,4:5:10:7.8.13,THB:6:5:10:7.8.13,6:7:5.12:10,12:5:8:11.10.13| Show InChI InChI=1S/C16H22N2OS/c19-13-16(3-1-2-4-16)20-14(17-13)18-15-8-10-5-11(9-15)7-12(15)6-10/h10-12H,1-9H2,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -11.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB
| Assay Description
The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... |
J Med Chem 51: 2933-43 (2008)
Article DOI: 10.1021/jm701551j
BindingDB Entry DOI: 10.7270/Q2HM56Q8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317208
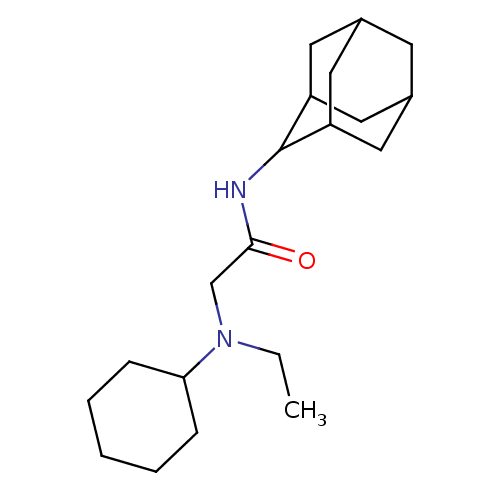
(CHEMBL1088115 | N-(adamantan-2-yl)-2-[cyclohexyl(e...)Show SMILES CCN(CC(=O)NC1C2CC3CC(C2)CC1C3)C1CCCCC1 |TLB:13:12:16:9.8.7,13:8:11.12.14:16,THB:6:7:11.12.14:16,7:8:11:14.15.16,7:15:11:9.13.8,(27.37,5.32,;27.37,3.78,;28.7,3,;28.71,1.46,;30.04,.69,;31.38,1.46,;30.04,-.85,;28.7,-1.62,;28.7,-3.14,;27.29,-3.49,;25.97,-3.01,;24.77,-4.28,;26.26,-3.86,;27.67,-4.42,;26.25,-2.27,;27.3,-1.05,;25.96,-1.52,;30.04,3.78,;31.38,3.01,;32.71,3.78,;32.71,5.31,;31.38,6.08,;30.04,5.31,)| Show InChI InChI=1S/C20H34N2O/c1-2-22(18-6-4-3-5-7-18)13-19(23)21-20-16-9-14-8-15(11-16)12-17(20)10-14/h14-18,20H,2-13H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317222
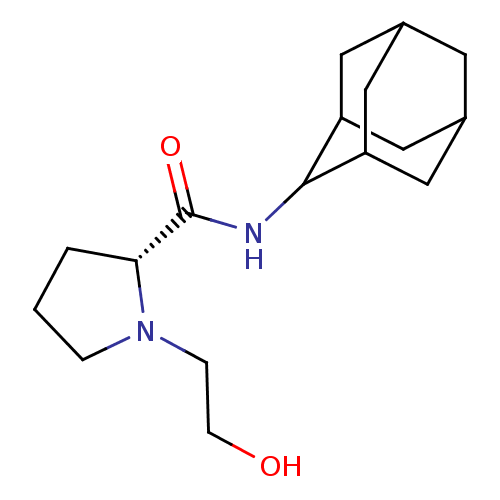
((2R)-N-(adamantan-2-yl)-1-(2-hydroxyethyl)pyrrolid...)Show SMILES OCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(-3.11,5.86,;-3.88,4.52,;-5.43,4.52,;-6.21,3.18,;-7.75,3.17,;-8.21,1.7,;-6.97,.79,;-5.75,1.77,;-4.23,1.26,;-2.88,2.01,;-4.26,-.28,;-5.6,-1.03,;-5.61,-2.57,;-7.02,-2.92,;-8.36,-2.43,;-9.57,-3.71,;-8.06,-3.29,;-6.63,-3.86,;-8.06,-1.69,;-7.01,-.44,;-8.38,-.93,)| Show InChI InChI=1S/C17H28N2O2/c20-5-4-19-3-1-2-15(19)17(21)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16,20H,1-10H2,(H,18,21)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202085
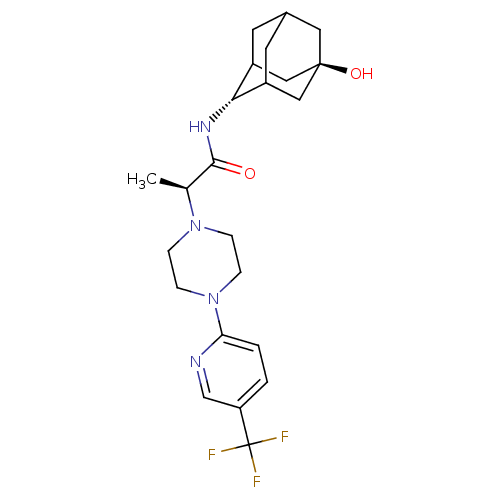
((R)-N-[(E)-5-hydroxy-2-adamantyl]-2-{4-[5-(trifluo...)Show SMILES C[C@H](N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,1.0,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(19.54,-43.75,;19.58,-45.29,;20.93,-46.02,;20.92,-47.57,;22.25,-48.34,;23.59,-47.58,;23.59,-46.04,;22.26,-45.26,;24.89,-48.41,;24.81,-49.94,;26.11,-50.77,;27.48,-50.06,;27.54,-48.51,;26.24,-47.69,;28.78,-50.88,;30.09,-51.68,;27.97,-52.19,;29.6,-49.58,;18.27,-46.09,;18.31,-47.63,;16.91,-45.36,;15.6,-46.16,;15.54,-47.69,;14.48,-48.93,;13.09,-48.31,;13.14,-46.73,;14.23,-45.53,;12.86,-45.96,;12.82,-47.45,;11.5,-46.64,;11.58,-48.68,;14.13,-47.98,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14-,15?,16?,17?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202085

((R)-N-[(E)-5-hydroxy-2-adamantyl]-2-{4-[5-(trifluo...)Show SMILES C[C@H](N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,1.0,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(19.54,-43.75,;19.58,-45.29,;20.93,-46.02,;20.92,-47.57,;22.25,-48.34,;23.59,-47.58,;23.59,-46.04,;22.26,-45.26,;24.89,-48.41,;24.81,-49.94,;26.11,-50.77,;27.48,-50.06,;27.54,-48.51,;26.24,-47.69,;28.78,-50.88,;30.09,-51.68,;27.97,-52.19,;29.6,-49.58,;18.27,-46.09,;18.31,-47.63,;16.91,-45.36,;15.6,-46.16,;15.54,-47.69,;14.48,-48.93,;13.09,-48.31,;13.14,-46.73,;14.23,-45.53,;12.86,-45.96,;12.82,-47.45,;11.5,-46.64,;11.58,-48.68,;14.13,-47.98,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14-,15?,16?,17?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202087
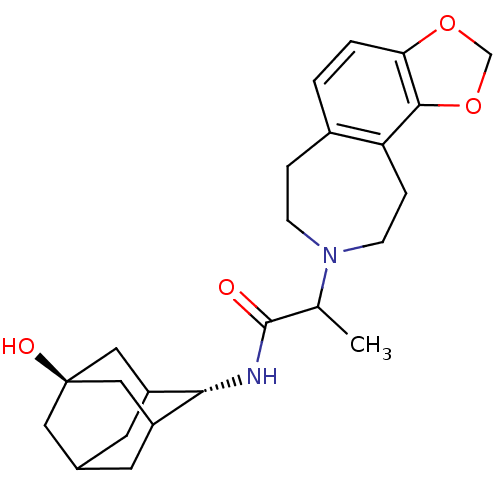
(CHEMBL374728 | N-[(1R,3S)-5-hydroxy-2-adamantyl]-2...)Show SMILES CC(N1CCc2ccc3OCOc3c2CC1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:19.21,wD:26.30,TLB:19:20:28:23.24.25,18:19:28.22.23:25,THB:21:22:25:29.20.19,21:20:28.22.23:25,19:24:28:29.21.20,(.95,-44.73,;.96,-46.27,;2.31,-47.02,;2.21,-48.56,;3.35,-49.6,;4.88,-49.34,;5.66,-50.67,;7.21,-50.65,;7.96,-49.29,;9.45,-48.94,;9.58,-47.41,;8.17,-46.82,;7.17,-47.97,;5.63,-48,;5.05,-46.58,;3.56,-46.15,;-.36,-47.05,;-.34,-48.59,;-1.7,-46.3,;-2.98,-47.15,;-2.99,-48.68,;-3.99,-49.97,;-5.4,-49.41,;-5.41,-47.83,;-4.38,-46.58,;-5.73,-47.07,;-5.71,-48.55,;-7.26,-47.91,;-6.9,-49.84,;-4.38,-49.04,)| Show InChI InChI=1S/C24H32N2O4/c1-14(23(27)25-21-17-8-15-9-18(21)12-24(28,10-15)11-17)26-6-4-16-2-3-20-22(30-13-29-20)19(16)5-7-26/h2-3,14-15,17-18,21,28H,4-13H2,1H3,(H,25,27)/t14?,15?,17?,18?,21-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50216902

(2-(adamantan-1-ylamino)-5,5-diethyl-oxazol-4-one |...)Show SMILES CCC1(CC)OC(NC23CC4CC(CC(C4)C2)C3)=NC1=O |c:20,TLB:7:8:11:15.13.14,THB:16:14:11:17.8.9,16:8:11:15.13.14,13:12:9:15.14.16,13:14:11.12.17:9| Show InChI InChI=1S/C17H26N2O2/c1-3-17(4-2)14(20)18-15(21-17)19-16-8-11-5-12(9-16)7-13(6-11)10-16/h11-13H,3-10H2,1-2H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Binding affinity to human 11beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 17: 4837-40 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.054
BindingDB Entry DOI: 10.7270/Q2MP52ZC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50216921

(2-(1-adamantylamino)-1-oxa-3-azaspiro[4.4]non-2-en...)Show SMILES O=C1N=C(NC23CC4CC(CC(C4)C2)C3)OC11CCCC1 |t:2,TLB:4:5:8:12.10.11,THB:13:11:8:14.5.6,13:5:8:12.10.11,10:9:6:12.11.13,10:11:8.9.14:6| Show InChI InChI=1S/C17H24N2O2/c20-14-17(3-1-2-4-17)21-15(18-14)19-16-8-11-5-12(9-16)7-13(6-11)10-16/h11-13H,1-10H2,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Binding affinity to human 11beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 17: 4837-40 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.054
BindingDB Entry DOI: 10.7270/Q2MP52ZC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197399
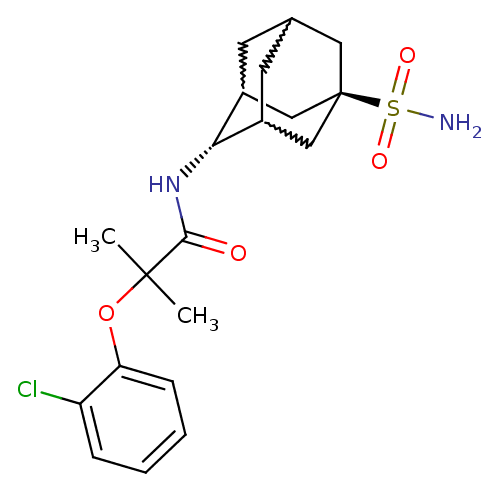
(2-(2-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(20.57,1.97,;21.38,.67,;22.2,-.64,;22.71,1.45,;24.05,.69,;24.05,-.85,;25.39,-1.61,;26.72,-.83,;26.71,.72,;25.37,1.47,;25.35,3.01,;20.1,-.18,;20.19,-1.72,;18.72,.5,;17.44,-.35,;17.42,-1.88,;16.41,-3.16,;15.01,-2.59,;15,-1,;16.04,.23,;14.69,-.25,;14.7,-1.73,;13.5,-3.01,;16.03,-2.22,;13.21,-1.32,;11.71,-.92,;13.61,.16,;12.8,-2.81,)| Show InChI InChI=1S/C20H27ClN2O4S/c1-19(2,27-16-6-4-3-5-15(16)21)18(24)23-17-13-7-12-8-14(17)11-20(9-12,10-13)28(22,25)26/h3-6,12-14,17H,7-11H2,1-2H3,(H,23,24)(H2,22,25,26)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29860
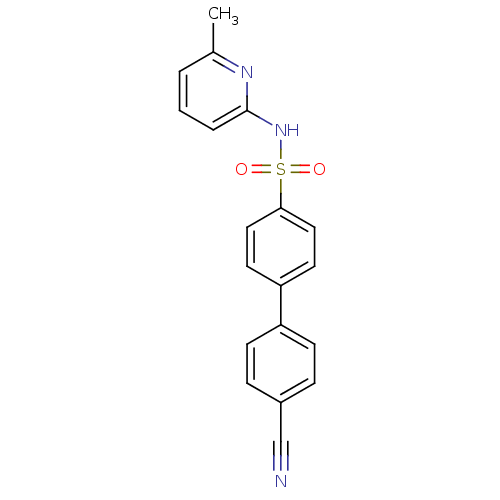
(N-(Pyridin-2-yl) arylsulfonamide, 22)Show SMILES Cc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C19H15N3O2S/c1-14-3-2-4-19(21-14)22-25(23,24)18-11-9-17(10-12-18)16-7-5-15(13-20)6-8-16/h2-12H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -11.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM13749
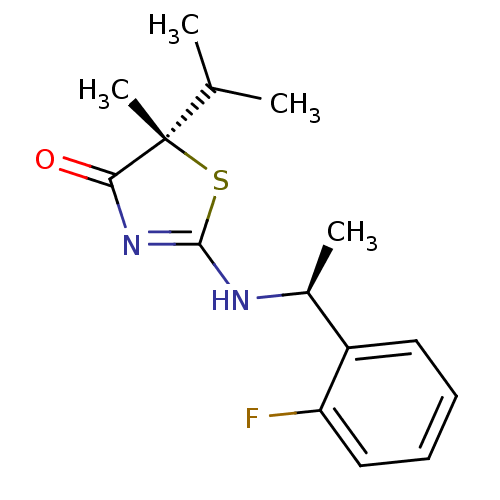
((5S)-2-{[(1S)-1-(2-fluorophenyl)ethyl]amino}-5-met...)Show SMILES CC(C)[C@]1(C)SC(N[C@@H](C)c2ccccc2F)=NC1=O |r,c:17| Show InChI InChI=1S/C15H19FN2OS/c1-9(2)15(4)13(19)18-14(20-15)17-10(3)11-7-5-6-8-12(11)16/h5-10H,1-4H3,(H,17,18,19)/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | -11.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Amgen
| Assay Description
Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... |
J Med Chem 50: 429-32 (2007)
Article DOI: 10.1021/jm061214f
BindingDB Entry DOI: 10.7270/Q20Z71JH |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195504
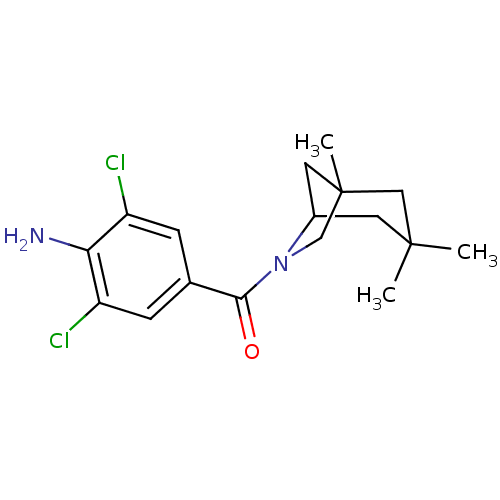
((4-amino-3,5-dichlorophenyl)(1,3,3-trimethyl-6-aza...)Show SMILES CC12CC(CC(C)(C)C1)N(C2)C(=O)c1cc(Cl)c(N)c(Cl)c1 Show InChI InChI=1S/C17H22Cl2N2O/c1-16(2)6-11-7-17(3,8-16)9-21(11)15(22)10-4-12(18)14(20)13(19)5-10/h4-5,11H,6-9,20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli by SPA |
Bioorg Med Chem Lett 16: 6241-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.035
BindingDB Entry DOI: 10.7270/Q2B857R4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195506
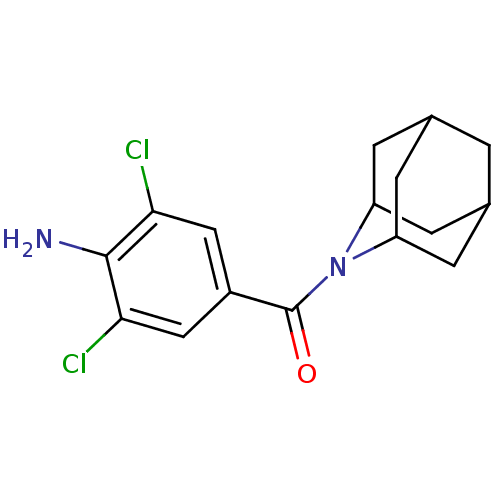
((4-amino-3,5-dichloro-phenyl)-(2-aza-tricyclo[3.3....)Show SMILES Nc1c(Cl)cc(cc1Cl)C(=O)N1C2CC3CC(C2)CC1C3 |TLB:20:19:15.14.13:17,THB:9:11:15.14.13:17,20:14:17:11.18.19,15:14:11:16.17.18,15:16:11:14.13.20| Show InChI InChI=1S/C16H18Cl2N2O/c17-13-6-10(7-14(18)15(13)19)16(21)20-11-2-8-1-9(4-11)5-12(20)3-8/h6-9,11-12H,1-5,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli by SPA |
Bioorg Med Chem Lett 16: 6241-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.035
BindingDB Entry DOI: 10.7270/Q2B857R4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197408

(2-(3-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...)Show SMILES CC(C)(Oc1cccc(Cl)c1)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(.77,-7.03,;1.59,-8.34,;2.4,-9.65,;2.92,-7.56,;4.26,-8.32,;4.26,-9.86,;5.6,-10.62,;6.93,-9.84,;6.91,-8.29,;8.24,-7.5,;5.57,-7.54,;.3,-9.19,;.4,-10.73,;-1.07,-8.5,;-2.36,-9.36,;-2.37,-10.88,;-3.38,-12.16,;-4.79,-11.6,;-4.79,-10.01,;-3.75,-8.78,;-5.1,-9.26,;-5.09,-10.74,;-6.29,-12.02,;-3.77,-11.23,;-6.59,-10.33,;-8.08,-9.92,;-6.18,-8.84,;-7,-11.81,)| Show InChI InChI=1S/C20H27ClN2O4S/c1-19(2,27-16-5-3-4-15(21)8-16)18(24)23-17-13-6-12-7-14(17)11-20(9-12,10-13)28(22,25)26/h3-5,8,12-14,17H,6-7,9-11H2,1-2H3,(H,23,24)(H2,22,25,26)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29866

(N-(Pyridin-2-yl) arylsulfonamide, 28)Show SMILES CCNc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C20H18N4O2S/c1-2-22-19-4-3-5-20(23-19)24-27(25,26)18-12-10-17(11-13-18)16-8-6-15(14-21)7-9-16/h3-13H,2H2,1H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | -11.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202093
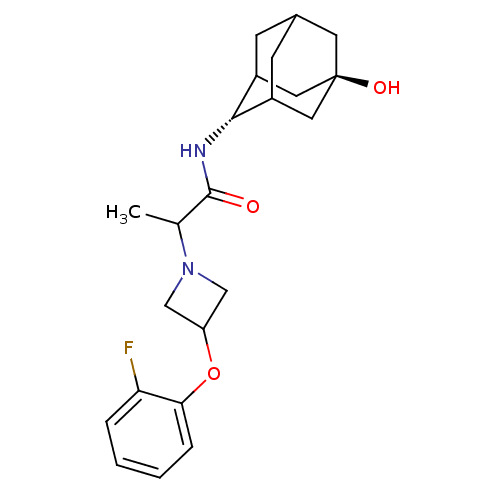
(2-[3-(2-fluoro-phenoxy)-azetidin-1-yl]-N-(5-hydrox...)Show SMILES CC(N1CC(C1)Oc1ccccc1F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:17.18,wD:24.27,TLB:17:18:26:21.22.23,16:17:26.20.21:23,THB:19:20:23:27.18.17,19:18:26.20.21:23,17:22:26:27.19.18,(24.76,-36.3,;24.78,-37.84,;26.12,-38.59,;26.53,-40.07,;28.01,-39.66,;27.6,-38.17,;29.34,-40.41,;30.67,-39.62,;32.01,-40.38,;33.33,-39.6,;33.31,-38.06,;31.96,-37.3,;30.64,-38.09,;29.3,-37.34,;23.45,-38.62,;23.47,-40.16,;22.11,-37.87,;20.83,-38.72,;20.83,-40.25,;19.82,-41.54,;18.42,-40.98,;18.4,-39.39,;19.43,-38.15,;18.09,-38.64,;18.11,-40.12,;16.55,-39.48,;16.92,-41.41,;19.44,-40.61,)| Show InChI InChI=1S/C22H29FN2O3/c1-13(25-11-17(12-25)28-19-5-3-2-4-18(19)23)21(26)24-20-15-6-14-7-16(20)10-22(27,8-14)9-15/h2-5,13-17,20,27H,6-12H2,1H3,(H,24,26)/t13?,14?,15?,16?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197402
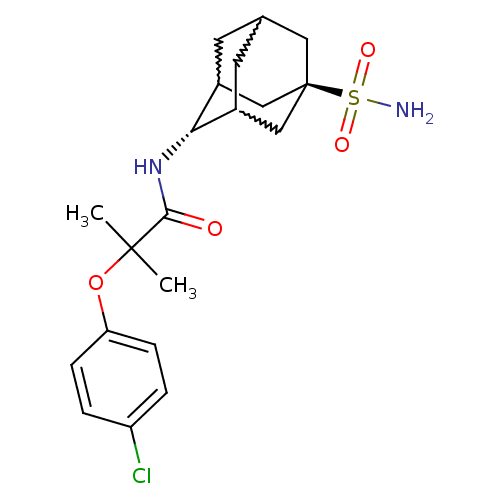
(2-(4-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...)Show SMILES CC(C)(Oc1ccc(Cl)cc1)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(13.59,-42.74,;14.41,-44.05,;15.22,-45.36,;15.73,-43.27,;17.07,-44.03,;17.08,-45.56,;18.42,-46.32,;19.75,-45.54,;21.09,-46.3,;19.73,-44,;18.39,-43.24,;13.12,-44.9,;13.22,-46.44,;11.74,-44.21,;10.46,-45.06,;10.45,-46.59,;9.43,-47.87,;8.03,-47.3,;8.02,-45.71,;9.06,-44.48,;7.72,-44.96,;7.72,-46.45,;6.53,-47.72,;9.05,-46.94,;6.23,-46.04,;4.74,-45.63,;6.64,-44.55,;5.82,-47.52,)| Show InChI InChI=1S/C20H27ClN2O4S/c1-19(2,27-16-5-3-15(21)4-6-16)18(24)23-17-13-7-12-8-14(17)11-20(9-12,10-13)28(22,25)26/h3-6,12-14,17H,7-11H2,1-2H3,(H,23,24)(H2,22,25,26)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202102
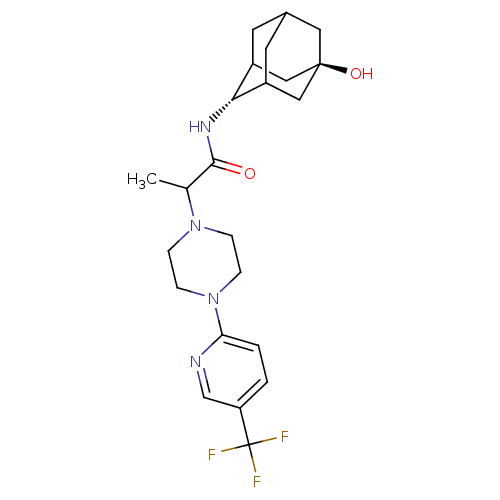
(CHEMBL375156 | N-(5-Hydroxy-adamantan-2-yl)-2-[4-(...)Show SMILES CC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(-1.15,-33.95,;-1.14,-35.49,;.19,-36.26,;.19,-37.8,;1.52,-38.56,;2.85,-37.8,;2.85,-36.25,;1.52,-35.48,;4.19,-38.57,;4.18,-40.11,;5.51,-40.88,;6.84,-40.11,;6.84,-38.56,;5.51,-37.8,;8.18,-40.88,;9.46,-41.72,;8.99,-39.57,;7.38,-42.19,;-2.48,-36.27,;-2.47,-37.81,;-3.81,-35.5,;-5.14,-36.28,;-5.24,-37.8,;-6.33,-39.02,;-7.7,-38.37,;-7.61,-36.79,;-6.5,-35.62,;-7.88,-36.01,;-7.95,-37.5,;-9.42,-37,;-9.22,-38.7,;-6.66,-38.06,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14?,15?,16?,17?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202105
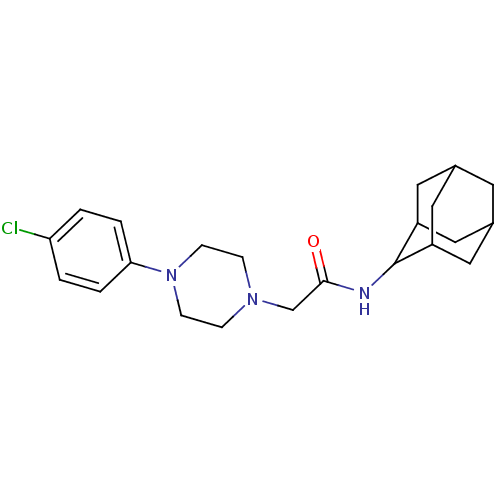
(CHEMBL218093 | N-adamantan-2-yl-2-[4-(4-chloro-phe...)Show SMILES Clc1ccc(cc1)N1CCN(CC(=O)NC2C3CC4CC(C3)CC2C4)CC1 |TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:14:15:19.20.22:24,15:16:19:22.23.24,15:23:19:17.21.16,(23.9,-3.58,;22.58,-2.79,;21.23,-3.53,;19.91,-2.74,;19.94,-1.2,;21.28,-.45,;22.6,-1.24,;18.62,-.4,;17.3,-1.2,;15.96,-.46,;15.93,1.09,;14.56,1.8,;13.26,.96,;13.34,-.58,;11.89,1.66,;10.6,.83,;10.57,-.7,;9.17,-1.03,;7.84,-.53,;6.63,-1.79,;8.14,-1.39,;9.54,-1.97,;8.15,.2,;9.21,1.42,;7.85,.96,;17.24,1.89,;18.59,1.14,)| Show InChI InChI=1S/C22H30ClN3O/c23-19-1-3-20(4-2-19)26-7-5-25(6-8-26)14-21(27)24-22-17-10-15-9-16(12-17)13-18(22)11-15/h1-4,15-18,22H,5-14H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197418
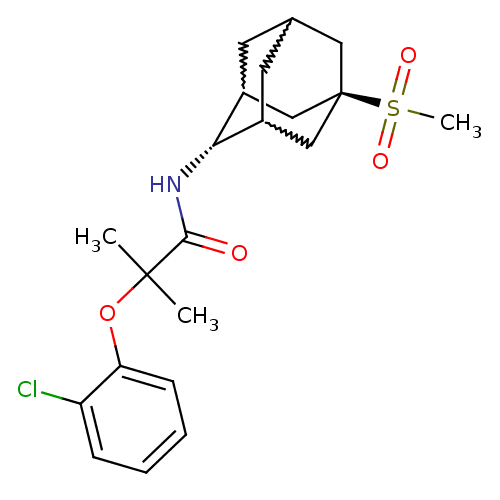
(2-(2-chloro-phenoxy)-N-(5-methanesulfonyl-adamanta...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(C)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(2.61,-15.76,;3.42,-17.07,;4.24,-18.37,;4.75,-16.28,;6.09,-17.05,;6.1,-18.59,;7.44,-19.35,;8.77,-18.57,;8.76,-17.02,;7.41,-16.26,;7.39,-14.72,;2.14,-17.92,;2.23,-19.46,;.76,-17.23,;-.53,-18.08,;-.54,-19.61,;-1.56,-20.89,;-2.97,-20.33,;-2.97,-18.74,;-1.93,-17.5,;-3.28,-17.98,;-3.27,-19.47,;-4.47,-20.75,;-1.94,-19.96,;-4.77,-19.06,;-6.26,-18.66,;-4.36,-17.57,;-5.17,-20.55,)| Show InChI InChI=1S/C21H28ClNO4S/c1-20(2,27-17-7-5-4-6-16(17)22)19(24)23-18-14-8-13-9-15(18)12-21(10-13,11-14)28(3,25)26/h4-7,13-15,18H,8-12H2,1-3H3,(H,23,24)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195298
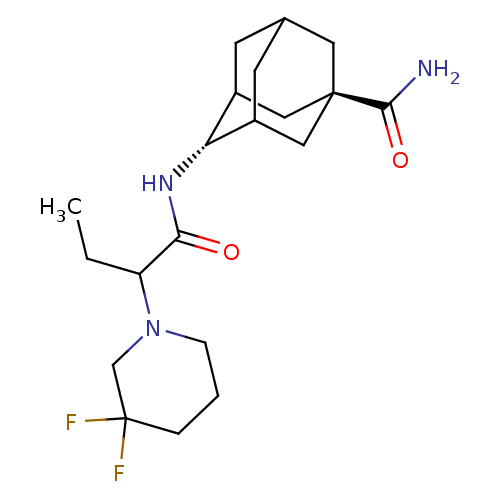
(4-[2-(3,3-difluoro-piperidin-1-yl)-butyrylamino]-a...)Show SMILES CCC(N1CCCC(F)(F)C1)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:17:20:23.15.14,16:15:22.17.18:20,14:19:22:23.16.15,(2.47,-8.55,;1.18,-9.41,;1.28,-10.95,;2.66,-11.64,;2.75,-13.17,;4.13,-13.85,;5.41,-13,;5.31,-11.46,;6.8,-11.86,;4.91,-9.99,;3.94,-10.77,;0,-11.8,;.1,-13.34,;-1.38,-11.12,;-2.66,-11.97,;-2.67,-13.5,;-3.69,-14.78,;-5.09,-14.21,;-5.1,-12.62,;-4.06,-11.39,;-5.4,-11.87,;-5.4,-13.35,;-6.59,-14.63,;-4.07,-13.84,;-6.94,-13.35,;-7.71,-14.68,;-7.7,-12.01,)| Show InChI InChI=1S/C20H31F2N3O2/c1-2-15(25-5-3-4-20(21,22)11-25)17(26)24-16-13-6-12-7-14(16)10-19(8-12,9-13)18(23)27/h12-16H,2-11H2,1H3,(H2,23,27)(H,24,26)/t12?,13?,14?,15?,16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50224221
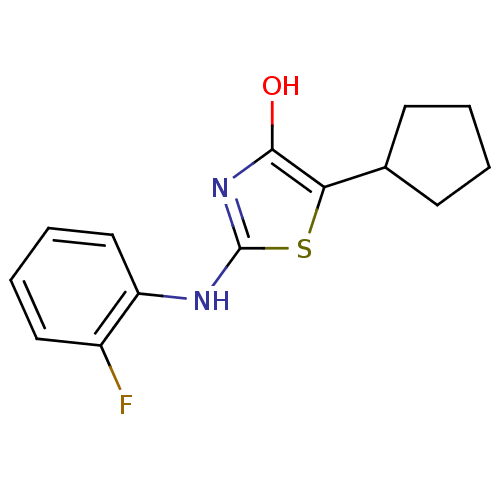
(5-cyclopentyl-2-(2-fluorophenylamino)thiazol-4(5H)...)Show InChI InChI=1S/C14H15FN2OS/c15-10-7-3-4-8-11(10)16-14-17-13(18)12(19-14)9-5-1-2-6-9/h3-4,7-9,18H,1-2,5-6H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by SPA |
Bioorg Med Chem Lett 17: 6056-61 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.070
BindingDB Entry DOI: 10.7270/Q2X34X6D |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29858
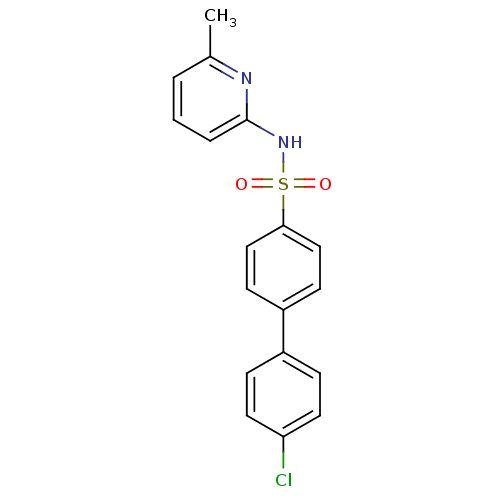
(N-(Pyridin-2-yl) arylsulfonamide, 20)Show SMILES Cc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(Cl)cc2)n1 Show InChI InChI=1S/C18H15ClN2O2S/c1-13-3-2-4-18(20-13)21-24(22,23)17-11-7-15(8-12-17)14-5-9-16(19)10-6-14/h2-12H,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | -11.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202104
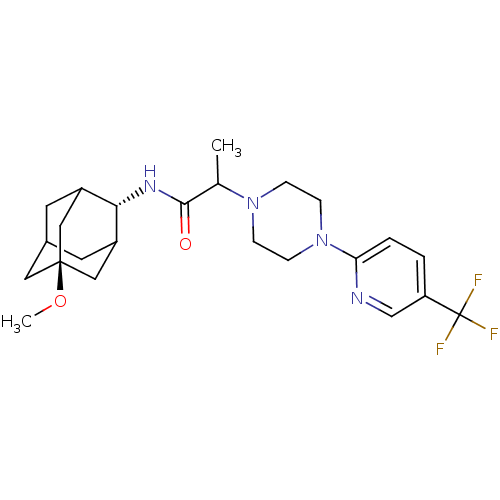
((E)-N-(5-methoxy-adamantan-2-yl)-2-[4-(5-trifluoro...)Show SMILES CO[C@@]12CC3CC(C1)[C@H](NC(=O)C(C)N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:8.9,wD:2.1,TLB:5:4:32:7.6.8,5:6:3.4.31:32,THB:8:6:3:31.30.32,8:30:3:7.5.6,9:8:3.4.31:32,(-11.76,-8.87,;-10.48,-7.77,;-8.89,-8.33,;-10.15,-9.55,;-8.63,-9.2,;-7.25,-9.82,;-6.18,-8.59,;-7.59,-8.88,;-6.1,-7.07,;-4.78,-6.27,;-3.43,-7.02,;-3.41,-8.56,;-2.11,-6.23,;-2.14,-4.69,;-.77,-6.98,;-.78,-8.53,;.54,-9.3,;1.88,-8.54,;1.89,-6.99,;.55,-6.21,;3.18,-9.36,;3.11,-10.9,;4.41,-11.72,;5.78,-11.01,;5.84,-9.47,;4.54,-8.65,;7.08,-11.84,;8.38,-12.64,;6.26,-13.14,;7.9,-10.54,;-7.47,-6.43,;-8.56,-7.61,;-8.84,-6.84,)| Show InChI InChI=1S/C24H33F3N4O2/c1-15(22(32)29-21-17-9-16-10-18(21)13-23(11-16,12-17)33-2)30-5-7-31(8-6-30)20-4-3-19(14-28-20)24(25,26)27/h3-4,14-18,21H,5-13H2,1-2H3,(H,29,32)/t15?,16?,17?,18?,21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
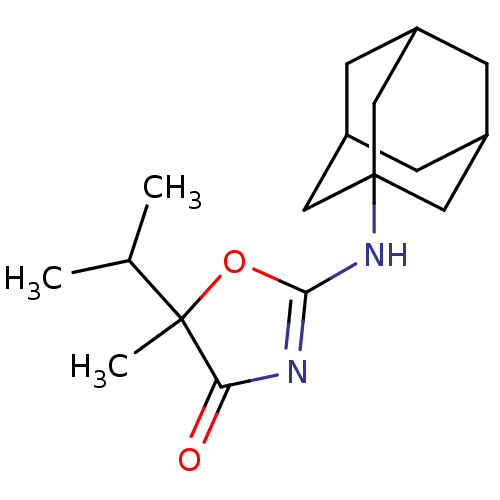
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50216904
(2-(adamantan-1-ylamino)-5-isopropyl-5-methyl-oxazo...)Show SMILES CC(C)C1(C)OC(NC23CC4CC(CC(C4)C2)C3)=NC1=O |c:20,TLB:7:8:11:15.13.14,THB:16:14:11:17.8.9,16:8:11:15.13.14,13:12:9:15.14.16,13:14:11.12.17:9| Show InChI InChI=1S/C17H26N2O2/c1-10(2)16(3)14(20)18-15(21-16)19-17-7-11-4-12(8-17)6-13(5-11)9-17/h10-13H,4-9H2,1-3H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Binding affinity to human 11beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 17: 4837-40 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.054
BindingDB Entry DOI: 10.7270/Q2MP52ZC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50216910

(2-(adamantan-2-ylamino)-5-isopropyl-5-methyl-oxazo...)Show SMILES CC(C)C1(C)OC(NC2C3CC4CC(C3)CC2C4)=NC1=O |c:20,TLB:14:13:17:10.9.8,14:9:12.13.15:17,THB:8:9:12:15.16.17,8:16:12:10.14.9,7:8:12.13.15:17,(-1.08,-29.52,;.46,-29.4,;1.33,-30.67,;1.12,-28.01,;2.66,-28.14,;-.4,-27.79,;-.62,-26.32,;-1.96,-25.56,;-3.22,-26.43,;-3.23,-27.96,;-4.63,-28.3,;-5.96,-27.82,;-7.16,-29.09,;-5.66,-28.67,;-4.25,-29.24,;-5.67,-27.08,;-4.62,-25.85,;-5.97,-26.33,;.72,-25.55,;1.82,-26.64,;3.34,-26.39,)| Show InChI InChI=1S/C17H26N2O2/c1-9(2)17(3)15(20)19-16(21-17)18-14-12-5-10-4-11(7-12)8-13(14)6-10/h9-14H,4-8H2,1-3H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB
Curated by ChEMBL
| Assay Description
Binding affinity to human 11beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 17: 4837-40 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.054
BindingDB Entry DOI: 10.7270/Q2MP52ZC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382388

(CHEMBL2023973)Show SMILES CC(=C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H20N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h9-11H,1,4-7H2,2-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50195302
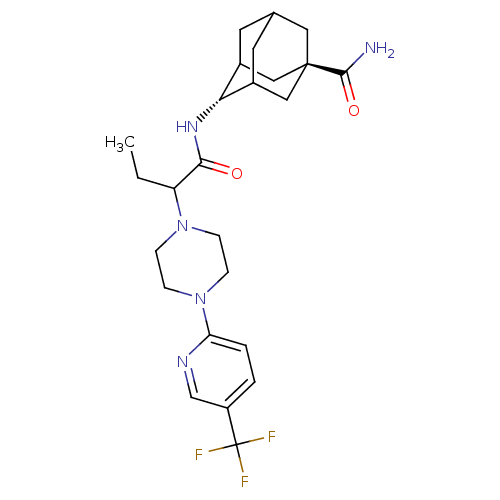
(4-{2-[4-(5-trifluoromethyl-pyridin-2-yl)-piperazin...)Show SMILES CCC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |wU:22.23,wD:29.36,TLB:22:23:30:26.27.28,21:22:30.25.26:28,THB:24:25:28:31.23.22,24:23:30.25.26:28,22:27:30:31.24.23,(22.63,-27.62,;21.34,-28.48,;21.44,-30.01,;22.82,-30.7,;22.91,-32.23,;24.28,-32.91,;25.57,-32.07,;25.48,-30.53,;24.1,-29.83,;26.95,-32.75,;27.03,-34.29,;28.41,-34.98,;29.7,-34.13,;29.6,-32.59,;28.22,-31.9,;31.08,-34.82,;32.4,-35.58,;30.29,-36.14,;31.83,-33.47,;20.16,-30.87,;20.25,-32.4,;18.78,-30.18,;17.5,-31.03,;17.49,-32.56,;16.47,-33.84,;15.07,-33.27,;15.06,-31.68,;16.1,-30.45,;14.75,-30.93,;14.76,-32.41,;13.57,-33.69,;16.09,-32.9,;13.22,-32.41,;12.45,-33.74,;12.46,-31.07,)| Show InChI InChI=1S/C25H34F3N5O2/c1-2-19(32-5-7-33(8-6-32)20-4-3-18(14-30-20)25(26,27)28)22(34)31-21-16-9-15-10-17(21)13-24(11-15,12-16)23(29)35/h3-4,14-17,19,21H,2,5-13H2,1H3,(H2,29,35)(H,31,34)/t15?,16?,17?,19?,21-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA |
Bioorg Med Chem Lett 16: 5958-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.129
BindingDB Entry DOI: 10.7270/Q23R0SJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data