

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334463 (1,3,5-tri-{5-[1-(3-picolinium)]-pent-1-ynyl}benzen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/alpha-6/beta-3 (Rattus norvegicus-Rattus norvegicus (Rat)) | BDBM50445330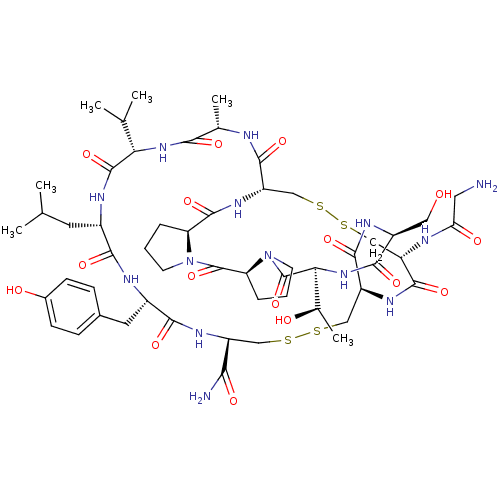 (CHEMBL3104243) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Inhibition of rat alpha6/alpha3beta2beta3 nAChR expressed in Xenopus oocytes assessed as inhibition of ACh-induced current by voltage clamp electroph... | J Med Chem 56: 9655-63 (2014) Article DOI: 10.1021/jm401254c BindingDB Entry DOI: 10.7270/Q22Z171M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334472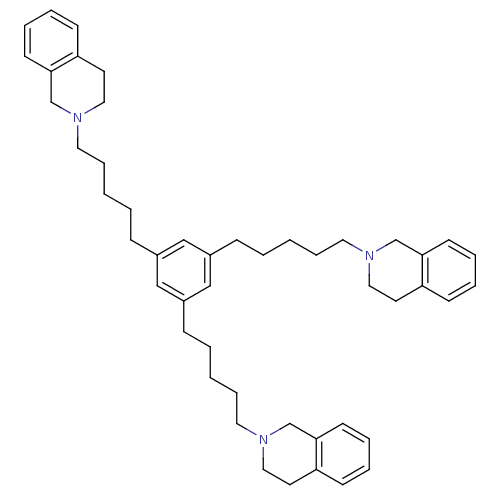 (1,3,5-tris(5-(3,4-dihydroisoquinolin-2(1H)-yl)pent...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334464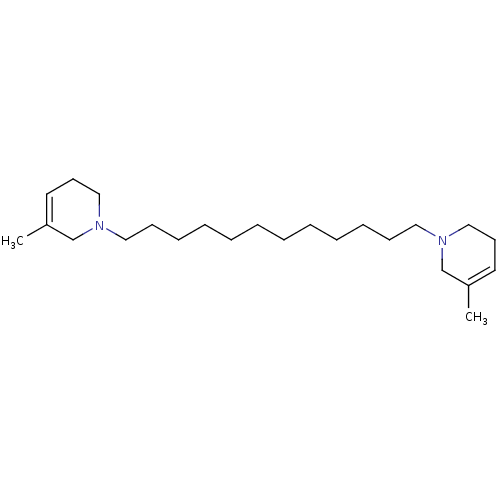 (1,12-bis(3-methyl-5,6-dihydropyridin-1(2H)-yl)dode...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50119774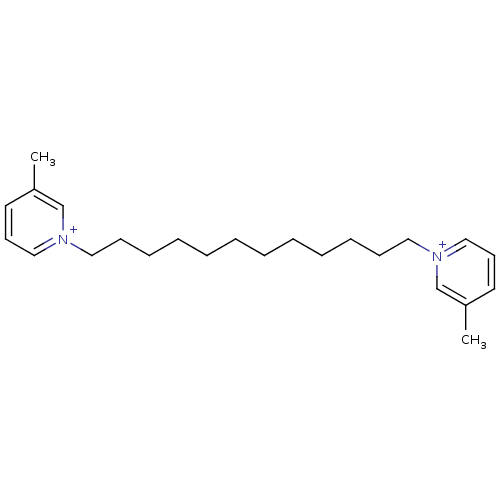 (1,1'-(dodecane-1,12-diyl)bis(3-methylpyridinium)br...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at nAChRalpha6beta2 in Sprague-Dawley rat striatal slices assessed as inhibition of nicotine-evoked [3H]dopamine uptake by liquid... | Bioorg Med Chem 17: 4477-85 (2009) Article DOI: 10.1016/j.bmc.2009.05.010 BindingDB Entry DOI: 10.7270/Q2XG9R5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50119774 (1,1'-(dodecane-1,12-diyl)bis(3-methylpyridinium)br...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334474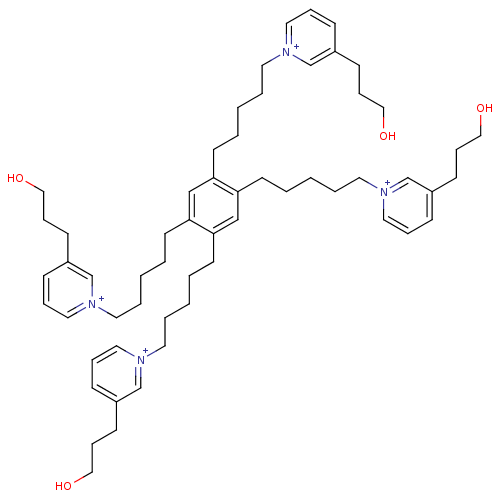 (1,1',1'',1'''-(5,5',5'',5'''-(benzene-1,2,4,5-tetr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334466 (1,3,5-tris(5-(3-methyl-5,6-dihydropyridin-1(2H)-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334470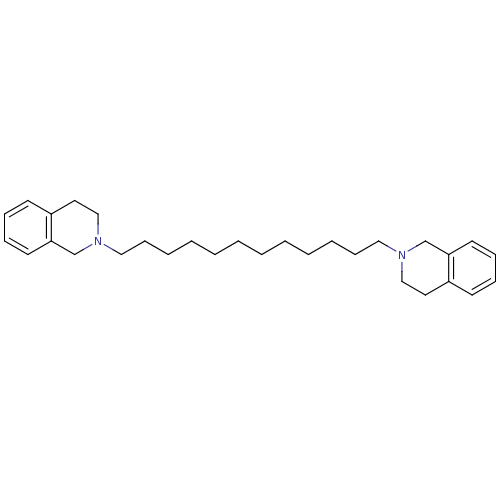 (1,12-bis(3,4-dihydroisoquinolin-2(1H)-yl)dodecane ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334471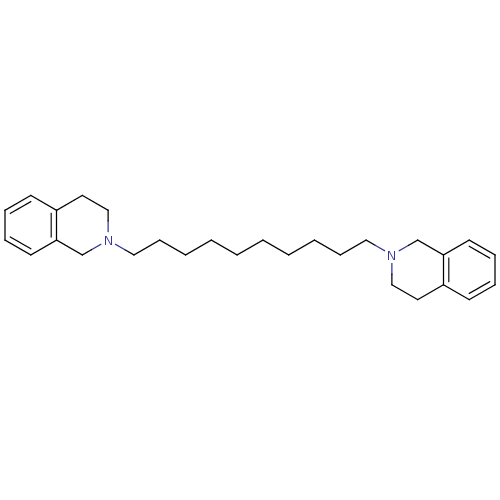 (1,10-bis(3,4-dihydroisoquinolin-2(1H)-yl)decane | ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/alpha-6/beta-3 (Rattus norvegicus-Rattus norvegicus (Rat)) | BDBM50445322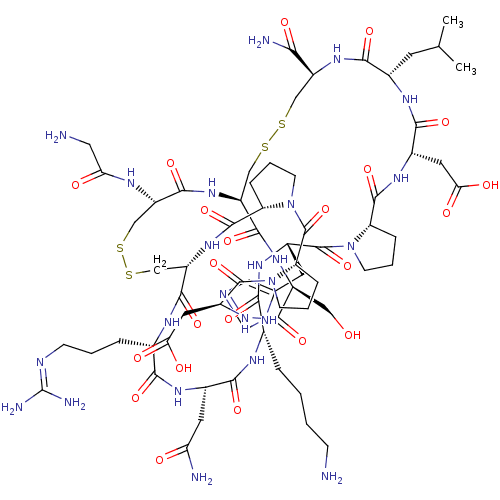 (CHEMBL3104235) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Inhibition of rat alpha6/alpha3beta2beta3 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of ACh-induced current by voltage clamp el... | J Med Chem 56: 9655-63 (2014) Article DOI: 10.1021/jm401254c BindingDB Entry DOI: 10.7270/Q22Z171M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334475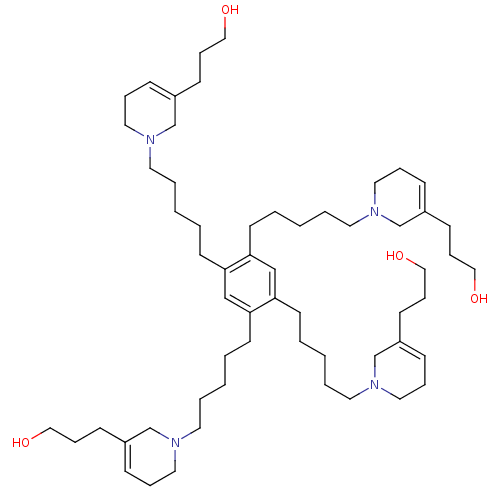 (3,3',3'',3'''-(1,1',1'',1'''-(5,5',5'',5'''-(benze...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334465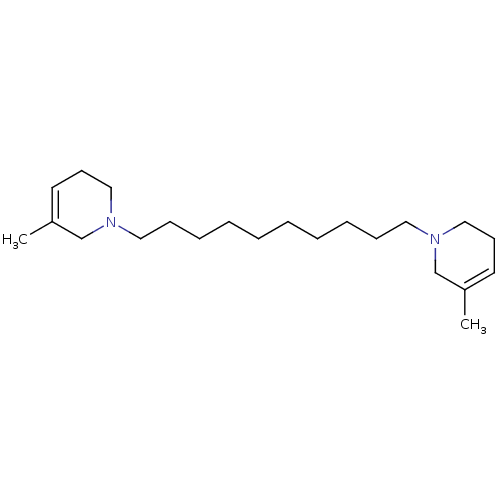 (1,10-bis(3-methyl-5,6-dihydropyridin-1(2H)-yl)deca...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50119805 (1,12-di(2-isoquinoliniumyl)dodecane; with dibromid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334469 (2,2',2'',2'''-(5,5',5'',5'''-(benzene-1,2,4,5-tetr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334467 (1,2-bis-(5-isoquinolinium-pent-1-ynyl)benzene dibr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50119780 (1,10-di(2-isoquinoliniumyl)decane; with diiodide i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/alpha-6/beta-3 (Rattus norvegicus-Rattus norvegicus (Rat)) | BDBM50445324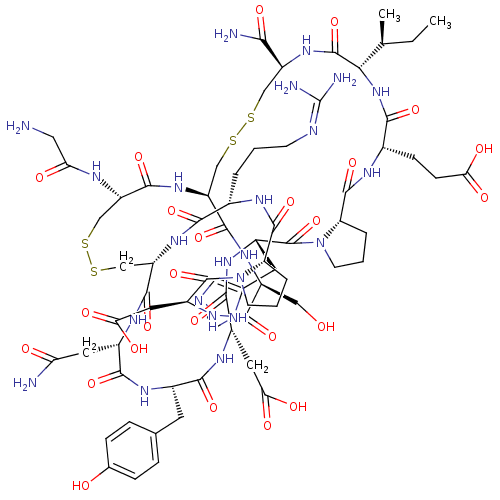 (CHEMBL3104237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Antagonist activity at Sprague-Dawley rat alpha6/alpha3beta2beta3 nAChR expressed in Xenopus oocytes assessed as inhibition of ACh-induced current by... | J Med Chem 56: 9655-63 (2014) Article DOI: 10.1021/jm401254c BindingDB Entry DOI: 10.7270/Q22Z171M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50119782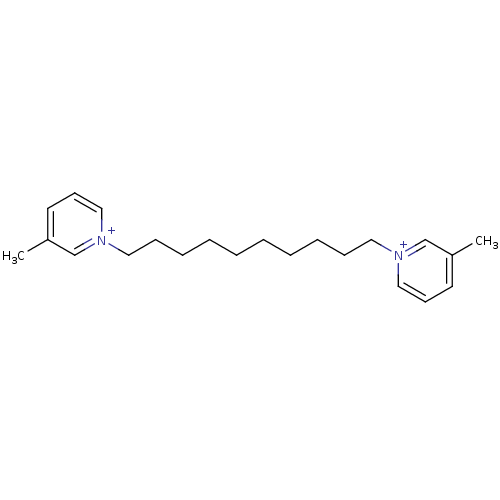 (1,1''-(decane-1,10-diyl)bis(3-methylpyridinium)iod...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-6 (Rattus norvegicus) | BDBM50334473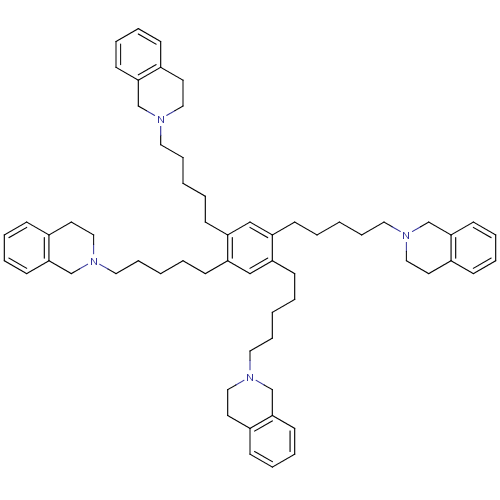 (1,2,4,5-tetrakis(5-(3,4-dihydroisoquinolin-2(1H)-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Antagonist activity at alpha6 nAChR in rat striatum assessed as inhibition of nicotine-induced [3H]dopamine release | Bioorg Med Chem Lett 21: 88-91 (2010) Article DOI: 10.1016/j.bmcl.2010.11.070 BindingDB Entry DOI: 10.7270/Q2VT1SDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/alpha-6/beta-3 (Rattus norvegicus-Rattus norvegicus (Rat)) | BDBM50445331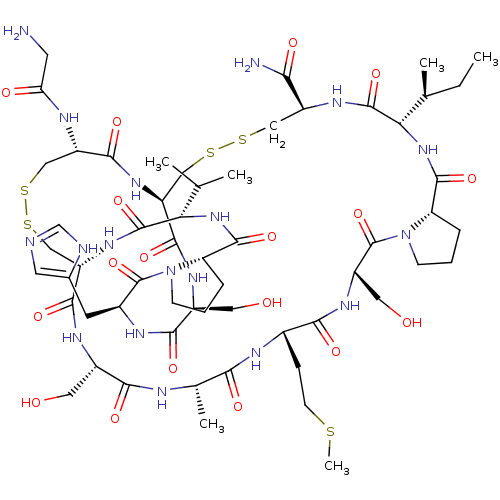 (CHEMBL3104245) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Inhibition of rat alpha6/alpha3beta2beta3 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of ACh-induced current after 2 to 4 days b... | J Med Chem 56: 9655-63 (2014) Article DOI: 10.1021/jm401254c BindingDB Entry DOI: 10.7270/Q22Z171M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||