

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456483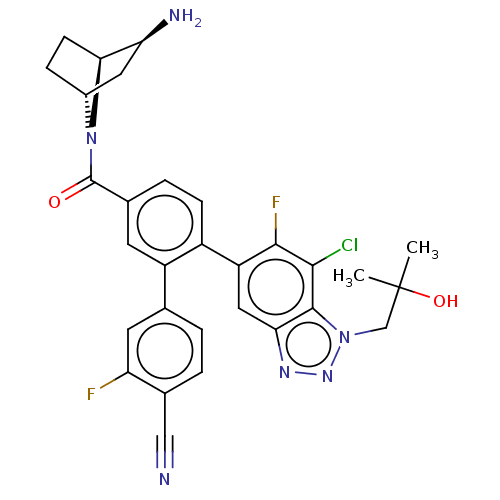 (US10723742, Example 209 | US10723742, Example 213 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581928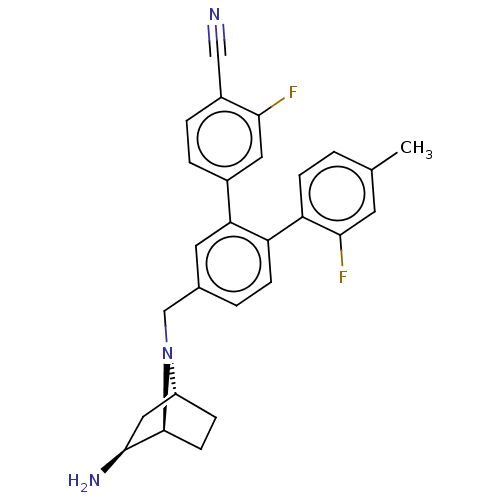 (US11510915, Example 283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456491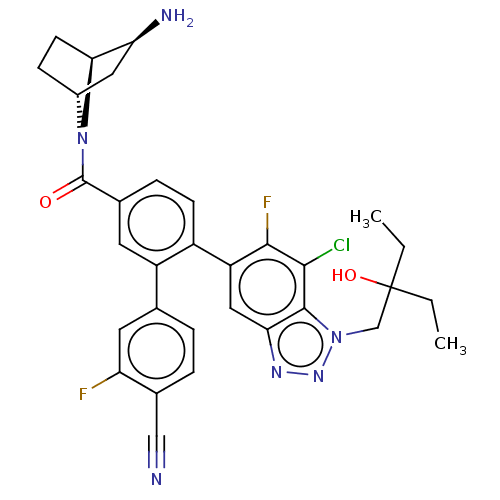 (US10723742, Example 218 | US10723742, Example 221 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581933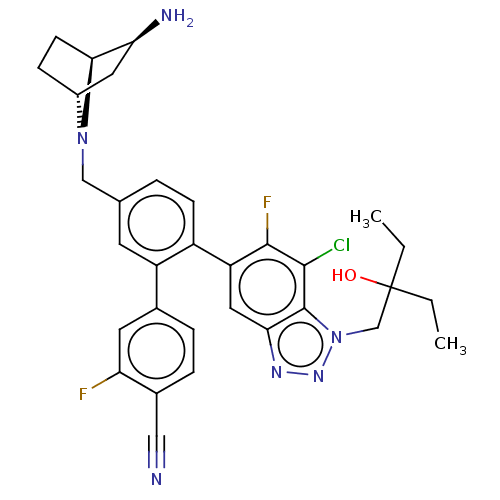 (US11510915, Example 288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581938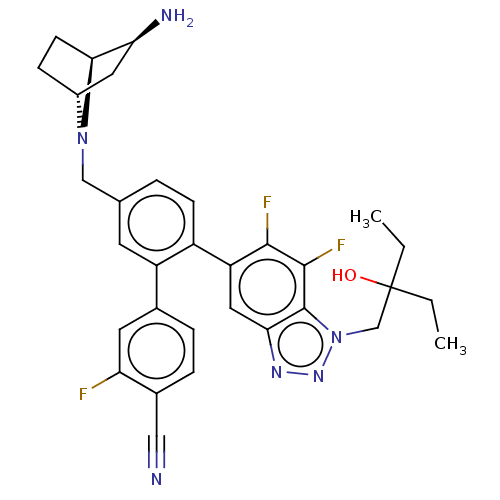 (US11510915, Example 293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456476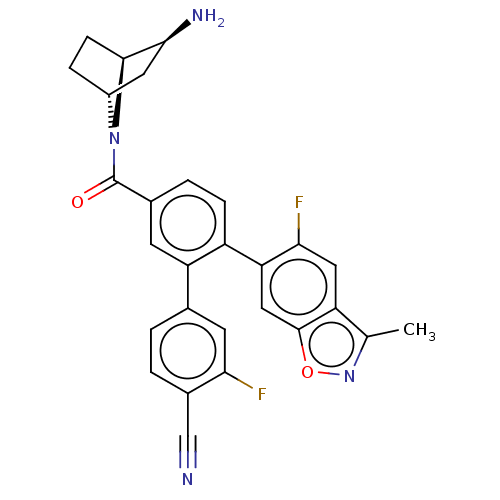 (US10723742, Example 202 | US11510915, Example 202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581930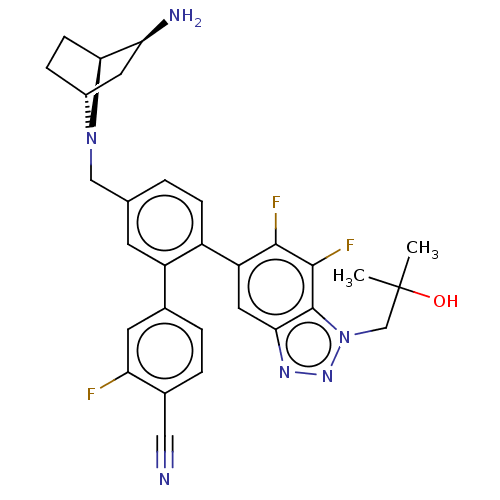 (US11510915, Example 285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581931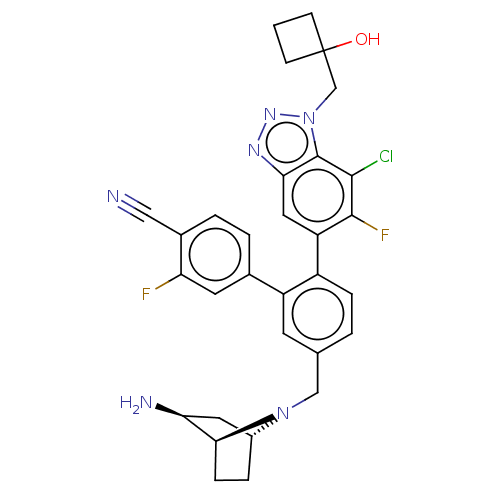 (US11510915, Example 286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456487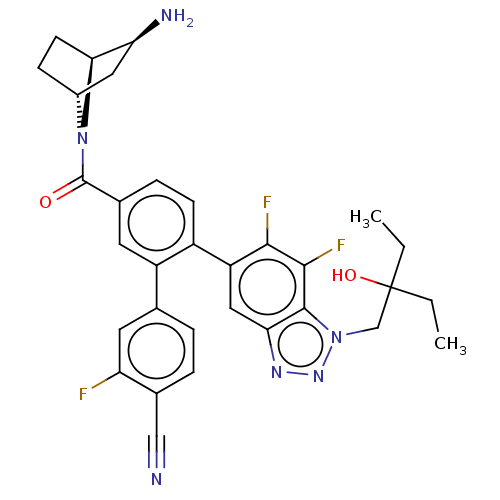 (US10723742, Example 214 | US10723742, Example 217 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456492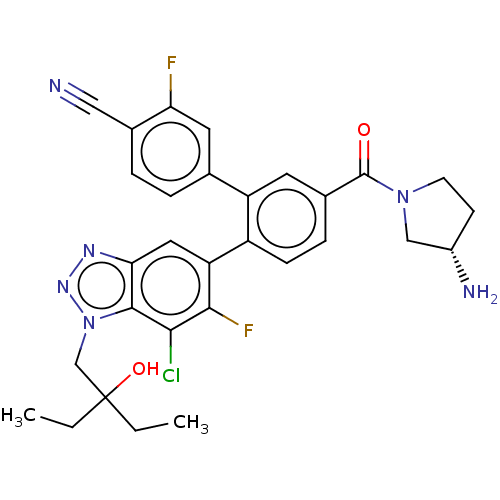 (US10723742, Example 219 | US11510915, Example 219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581932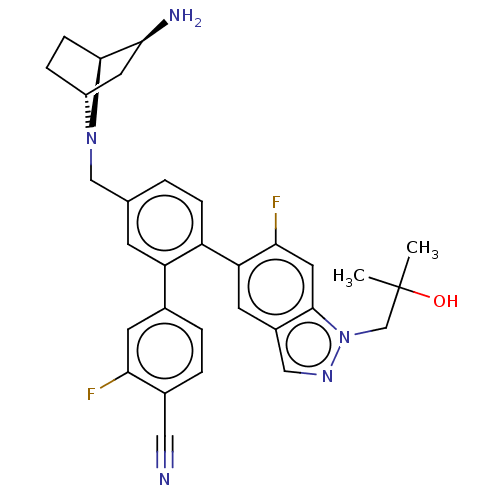 (US11510915, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456471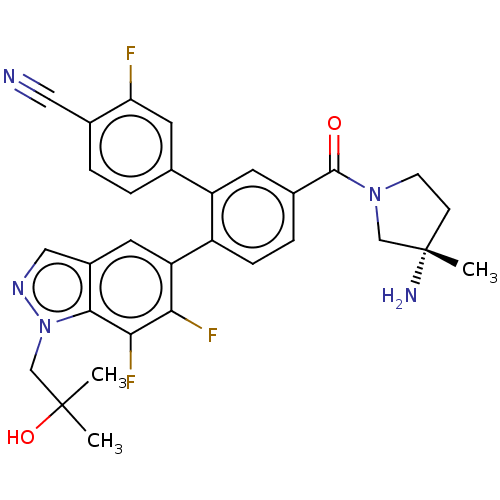 (US10723742, Example 197 | US11510915, Example 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456500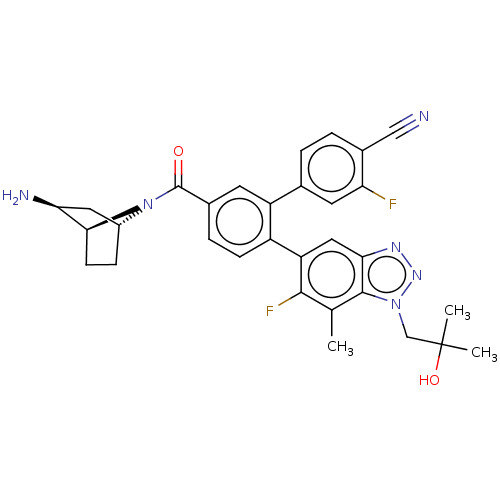 (US10723742, Example 228 | US10723742, Example 241 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456304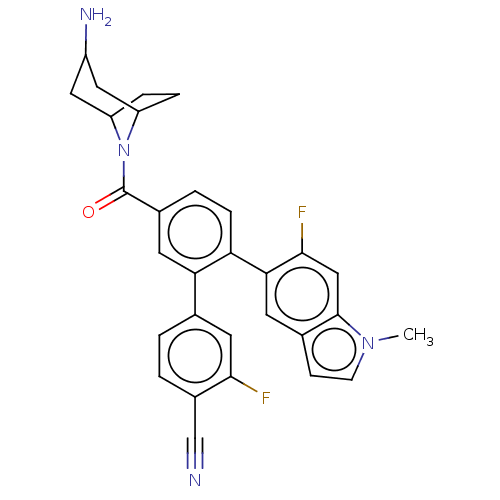 (US10723742, Example 123 | US10723742, Example 23 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456493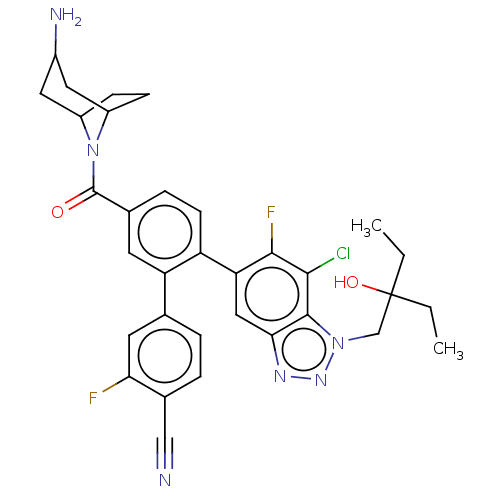 (US10723742, Example 220 | US11510915, Example 220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456528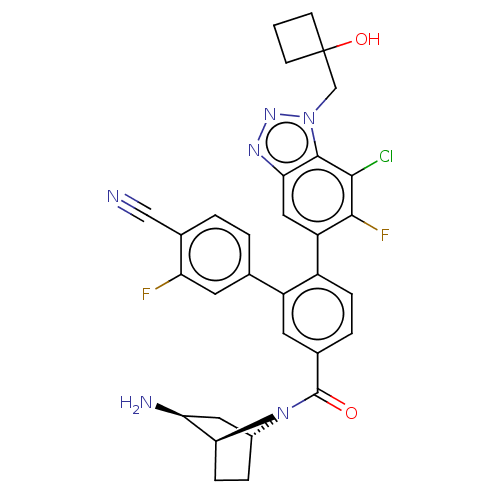 (US10723742, Example 257 | US10723742, Example 269 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456525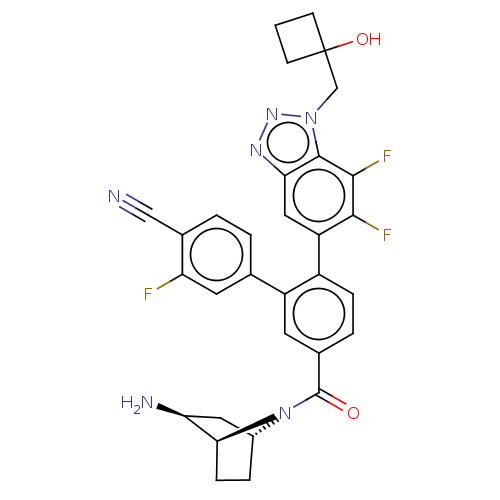 (US10723742, Example 254 | US10723742, Example 268 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456529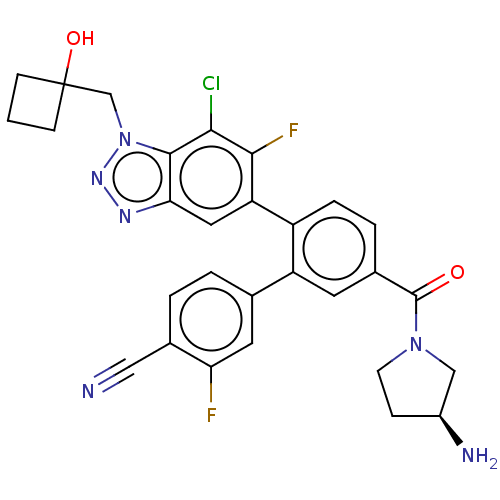 (US10723742, Example 258 | US11510915, Example 258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456449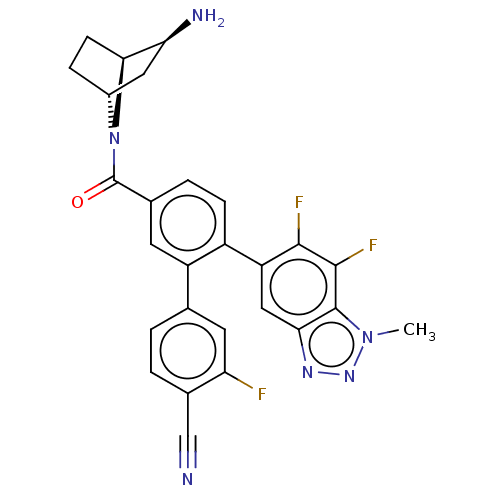 (US10723742, Example 175 | US10723742, Example 185 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456470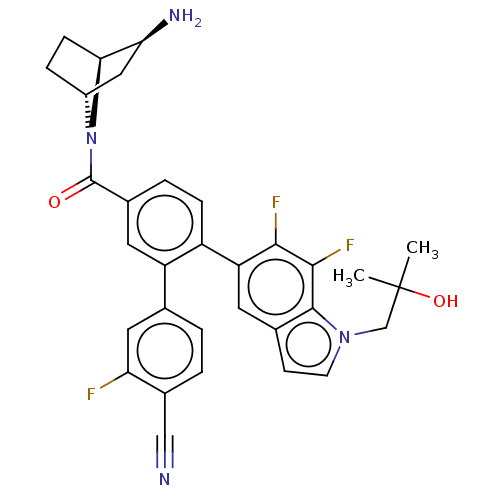 (US10723742, Example 196 | US11510915, Example 196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456485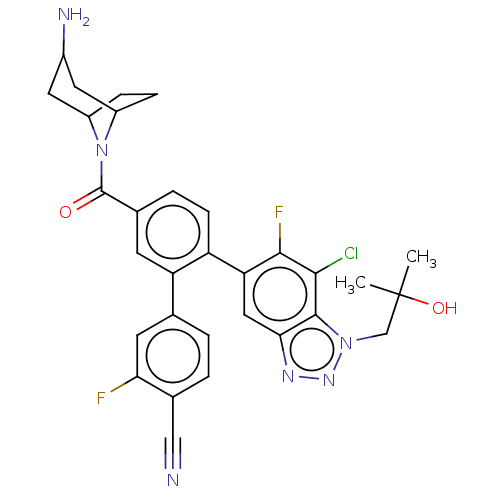 (US10723742, Example 211 | US11510915, Example 211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581904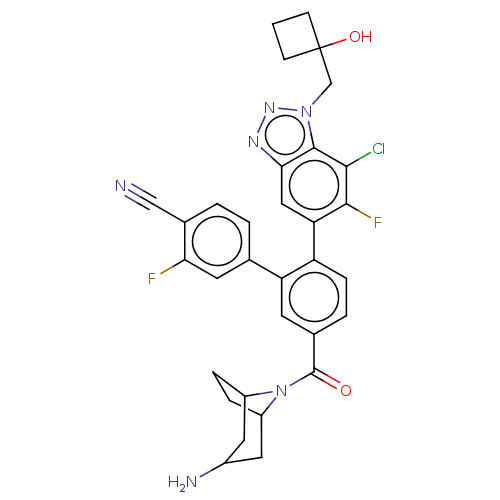 (US11510915, Example 259) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456531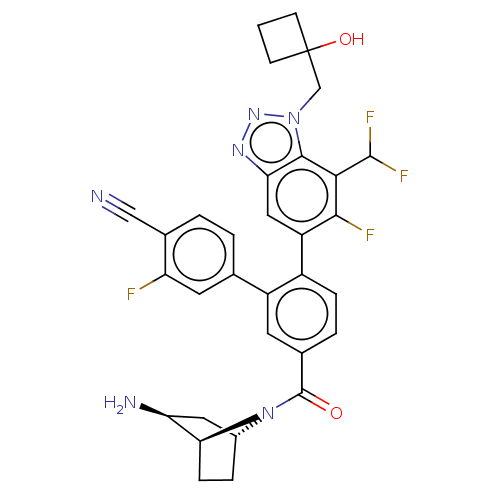 (US10723742, Example 260 | US10723742, Example 263 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581939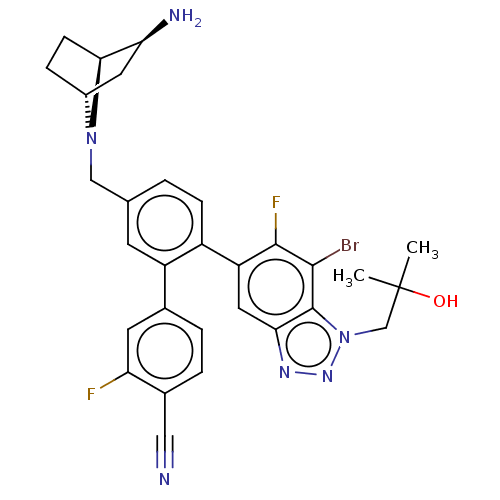 (US11510915, Example 294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456464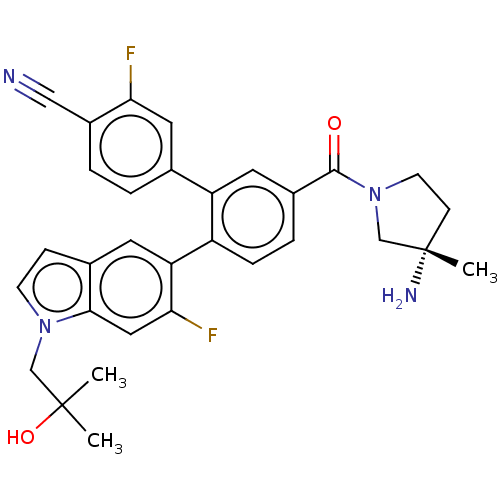 (US10723742, Example 190 | US11510915, Example 190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456484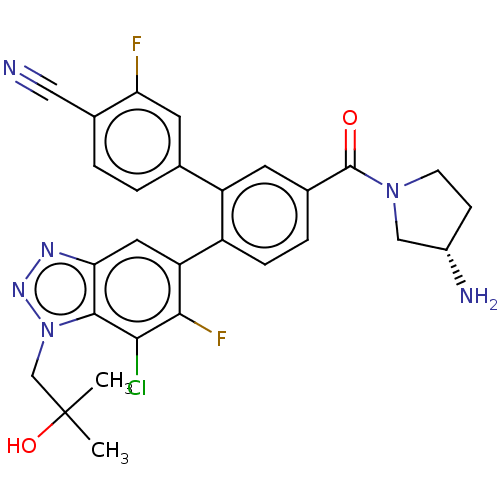 (US10723742, Example 210 | US11510915, Example 210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456483 (US10723742, Example 209 | US10723742, Example 213 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456541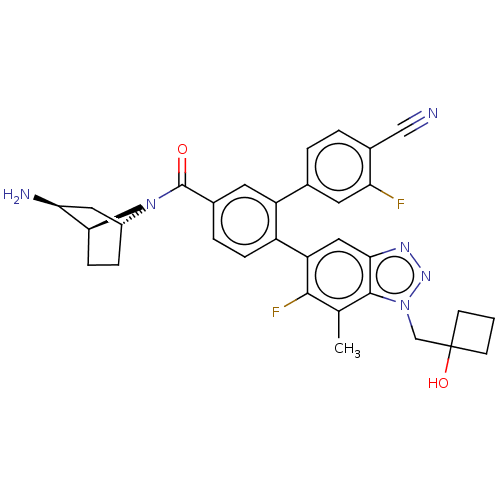 (US10723742, Example 270 | US10723742, Example 276 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581940 (US11510915, Example 295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456517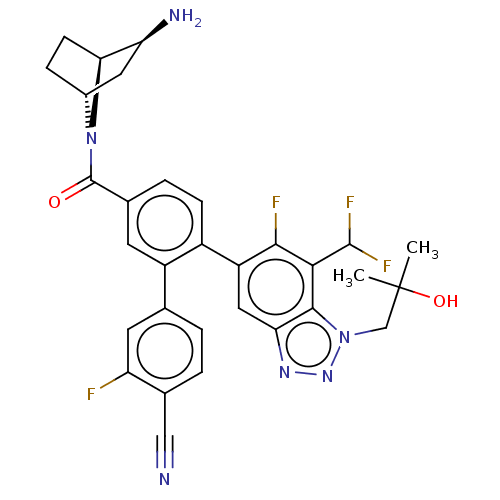 (US10723742, Example 246 | US10723742, Example 252 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581686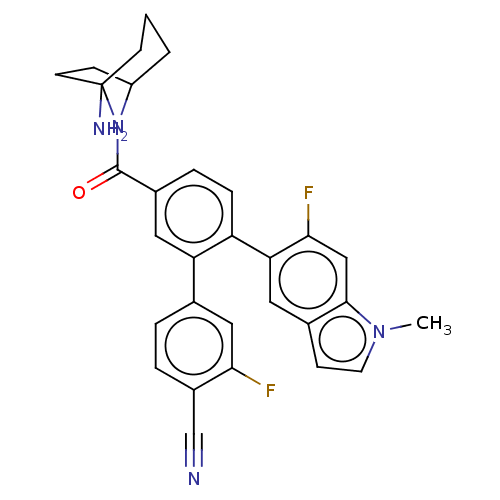 (US11510915, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456472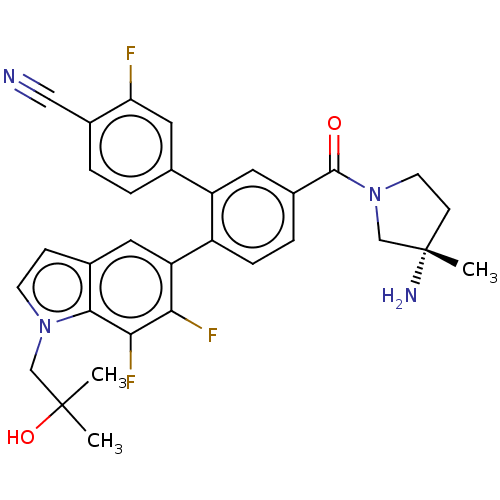 (US10723742, Example 198 | US11510915, Example 198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581934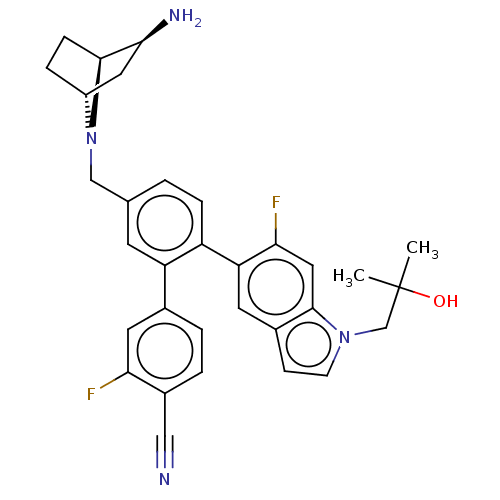 (US11510915, Example 289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456544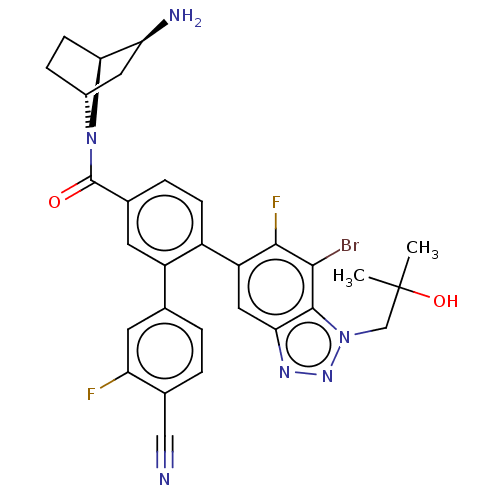 (US10723742, Example 273 | US10723742, Example 277 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456469 (US10723742, Example 195 | US11510915, Example 195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456491 (US10723742, Example 218 | US10723742, Example 221 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456480 (US10723742, Example 206 | US10723742, Example 225 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456508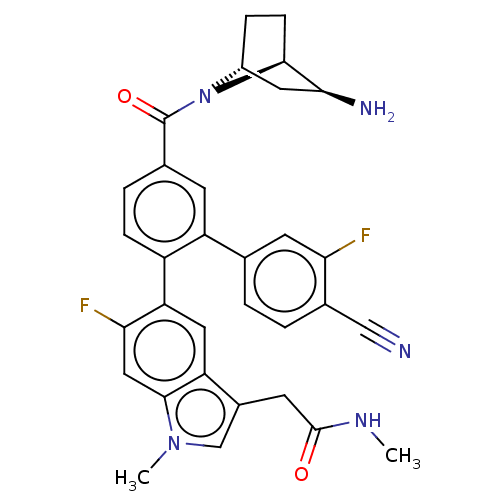 (US10723742, Example 237 | US10723742, Example 243 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456382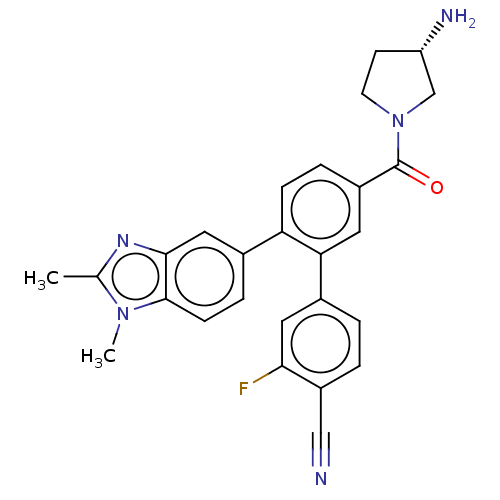 (US10723742, Example 103 | US11510915, Example 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456413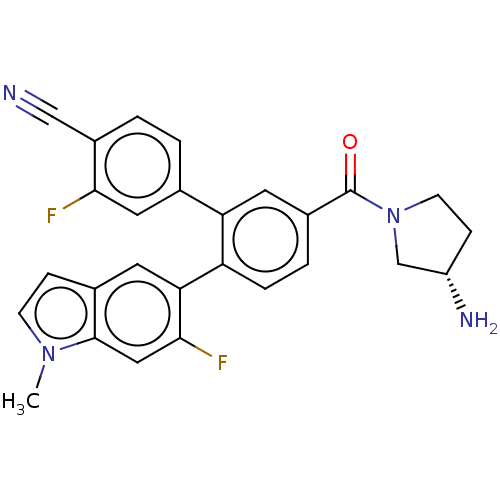 (US10723742, Example 134 | US11510915, Example 134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456487 (US10723742, Example 214 | US10723742, Example 217 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456546 (US10723742, Example 275 | US11510915, Example 275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456465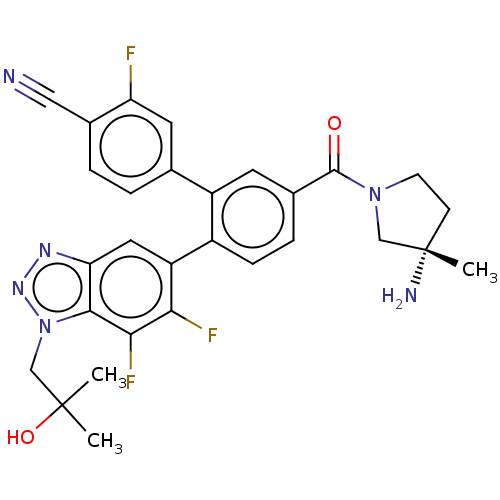 (US10723742, Example 191 | US11510915, Example 191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456451 (US10723742, Example 177 | US10723742, Example 192 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456518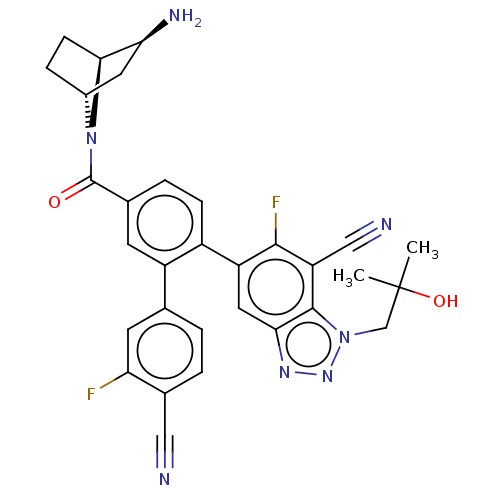 (US10723742, Example 247 | US11510915, Example 247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456526 (US10723742, Example 255 | US11510915, Example 255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456542 (US10723742, Example 271 | US11510915, Example 271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM581935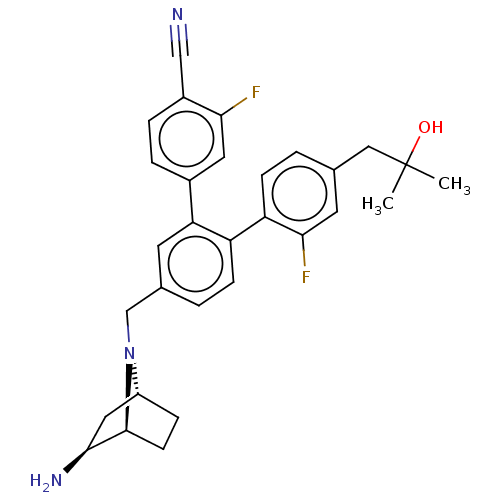 (US11510915, Example 290) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456477 (US10723742, Example 203 | US11510915, Example 203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456527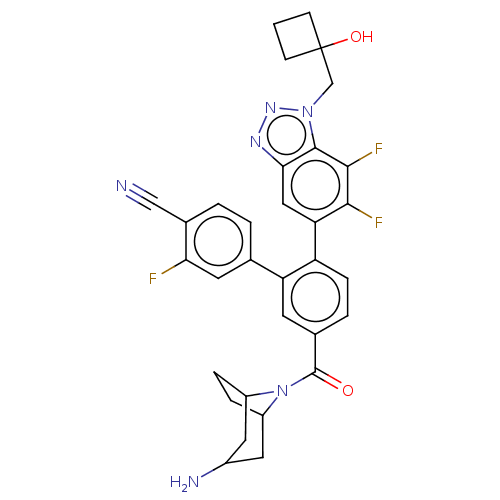 (US10723742, Example 256 | US11510915, Example 256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC6513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 278 total ) | Next | Last >> |