

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22947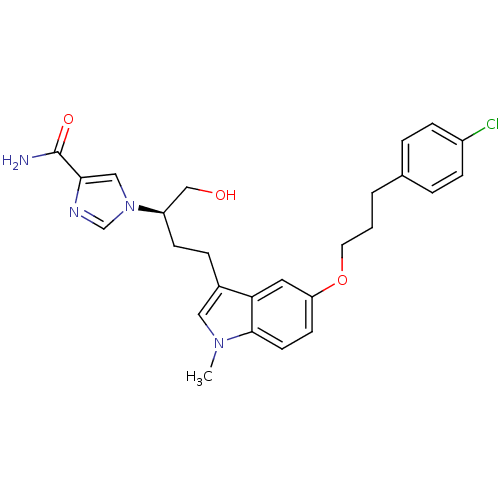 (1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22937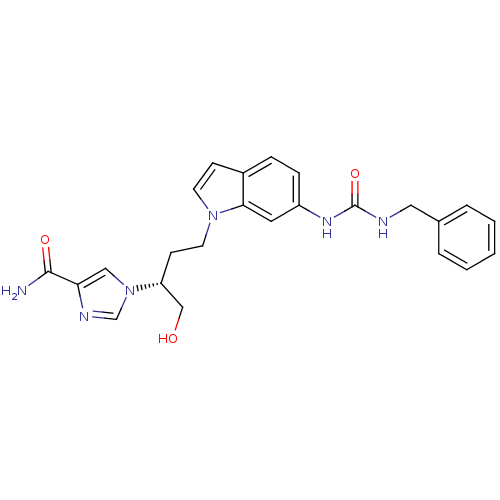 (1-[(2R)-4-{6-[(benzylcarbamoyl)amino]-1H-indol-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.5 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22920 (1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.70 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22931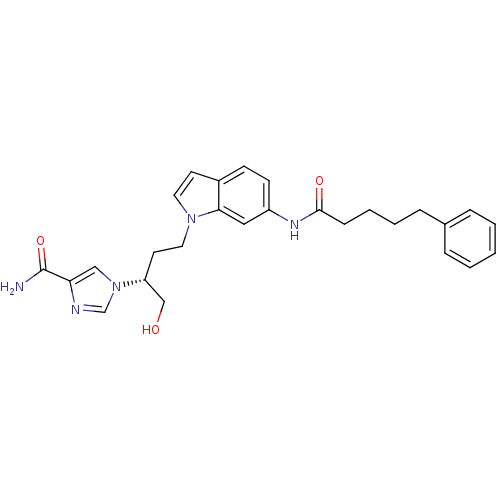 (1-[(2R)-1-hydroxy-4-[6-(5-phenylpentanamido)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22939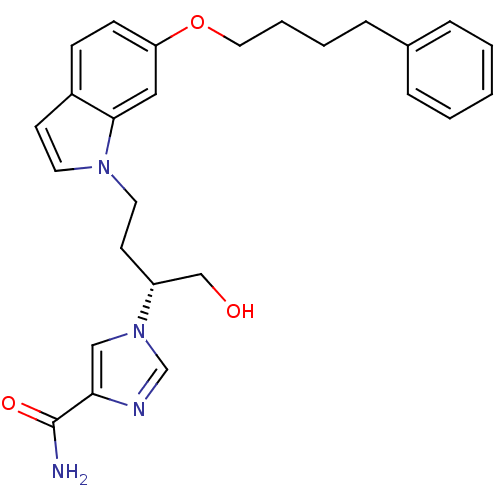 (1-[(2R)-1-hydroxy-4-[6-(4-phenylbutoxy)-1H-indol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22946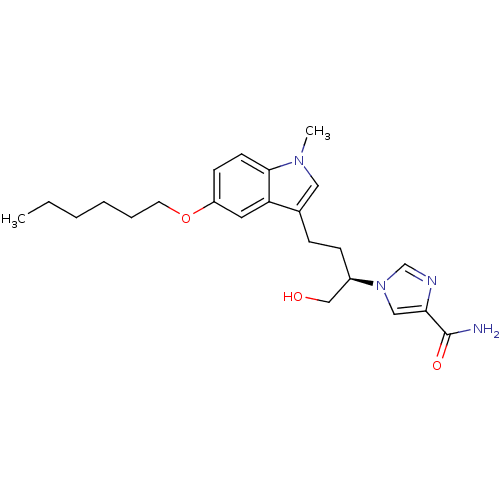 (1-[(2R)-4-[5-(hexyloxy)-1-methyl-1H-indol-3-yl]-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22942 (1-[(2R)-4-{6-[3-(4-chlorophenyl)propoxy]-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22930 (1-[(2R)-1-hydroxy-4-[6-(4-phenylbutanamido)-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22936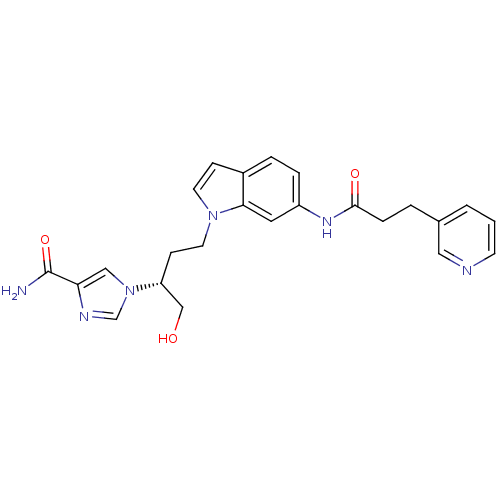 (1-[(2R)-1-hydroxy-4-{6-[3-(pyridin-3-yl)propanamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22938 (1-[(2R)-1-hydroxy-4-[6-(3-phenylpropoxy)-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22928 (1-[(2R)-4-(6-hexanamido-1H-indol-1-yl)-1-hydroxybu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22945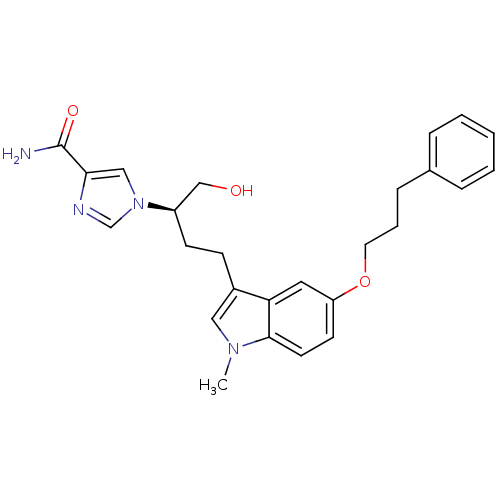 (1-[(2R)-1-hydroxy-4-[1-methyl-5-(3-phenylpropoxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22929 (1-[(2R)-1-hydroxy-4-[6-(3-phenylpropanamido)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22935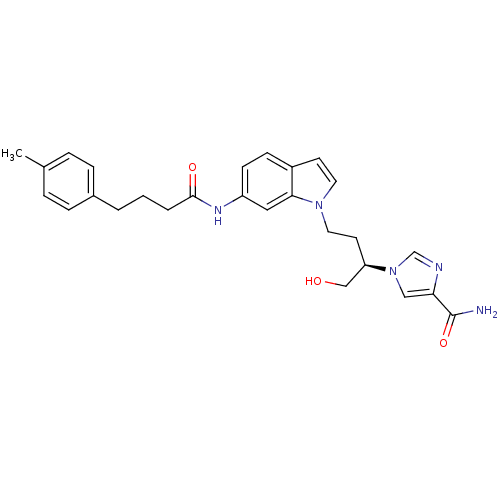 (1-[(2R)-1-hydroxy-4-{6-[4-(4-methylphenyl)butanami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22933 (1-[(2R)-1-hydroxy-4-{6-[3-(4-methylphenyl)propanam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22940 (1-[(2R)-4-[6-(hexyloxy)-1H-indol-1-yl]-1-hydroxybu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22934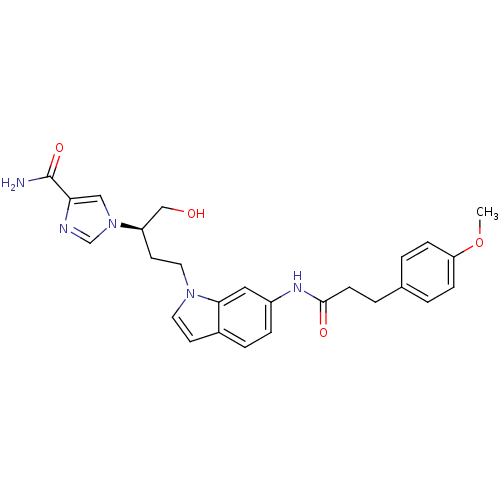 (1-[(2R)-1-hydroxy-4-{6-[3-(4-methoxyphenyl)propana...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22932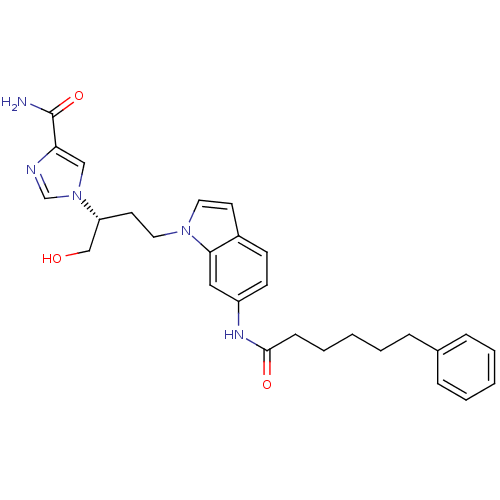 (1-[(2R)-1-hydroxy-4-[6-(6-phenylhexanamido)-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 91 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22941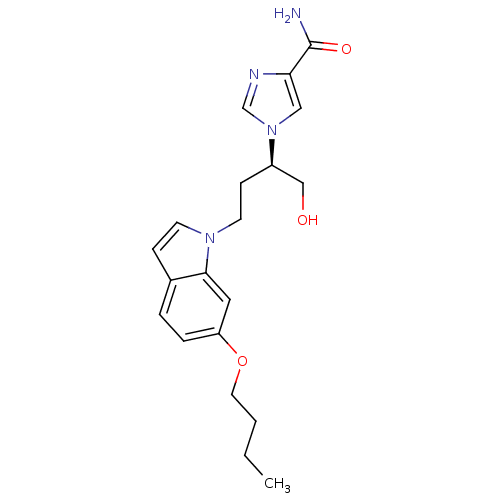 (1-[(2R)-4-(6-butoxy-1H-indol-1-yl)-1-hydroxybutan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22927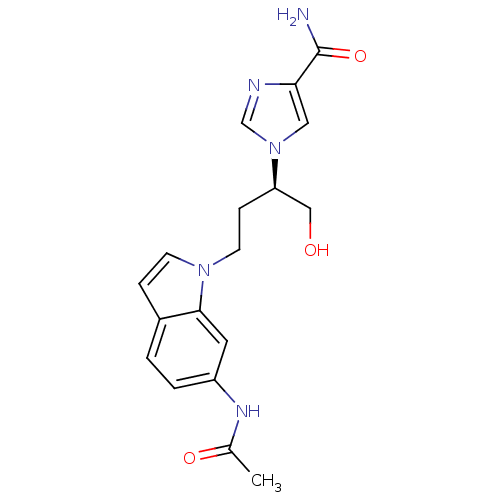 (1-[(2R)-4-(6-acetamido-1H-indol-1-yl)-1-hydroxybut...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22943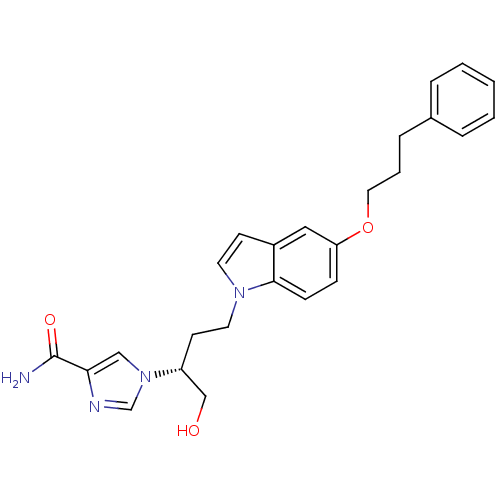 (1-[(2R)-1-hydroxy-4-[5-(3-phenylpropoxy)-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22944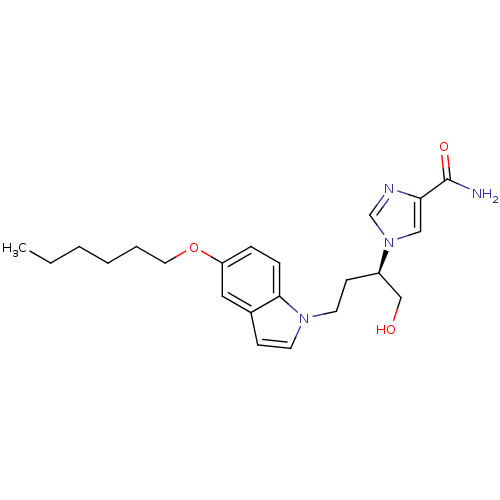 (1-[(2R)-4-[5-(hexyloxy)-1H-indol-1-yl]-1-hydroxybu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||