

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50290967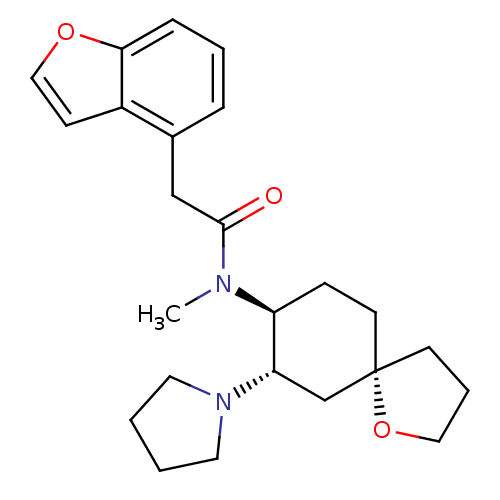 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-U-69,593 to cloned rat Opioid receptor kappa 1 expressed in CHO cell line | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50088375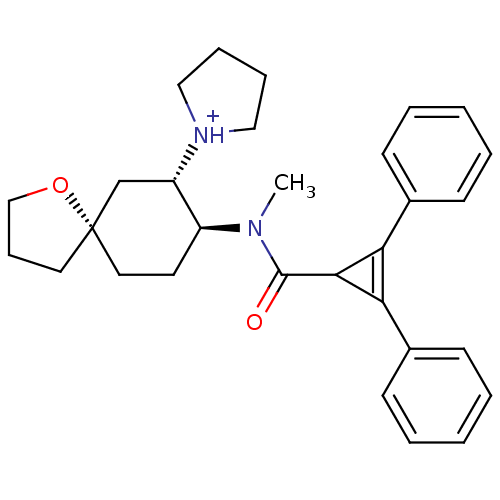 (1-{8-[(2,3-Diphenyl-cycloprop-2-enecarbonyl)-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-U-69,593 to cloned rat Opioid receptor kappa 1 expressed in CHO cell line | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-DAGO to rat brain mu opioid receptor | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50088375 (1-{8-[(2,3-Diphenyl-cycloprop-2-enecarbonyl)-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-DAGO to rat brain mu opioid receptor | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290972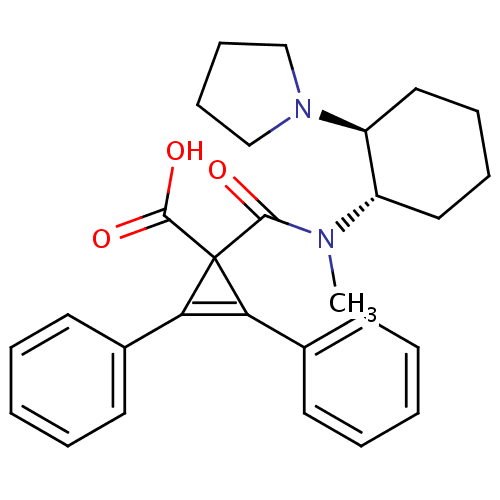 (1-[Methyl-((1S,2S)-2-pyrrolidin-1-yl-cyclohexyl)-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290973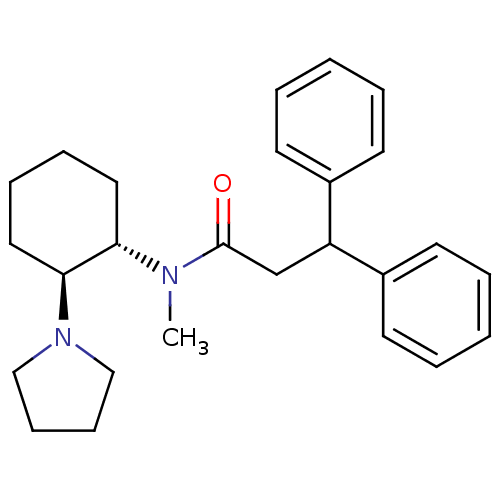 (CHEMBL318430 | N-Methyl-3,3-diphenyl-N-((1S,2S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290974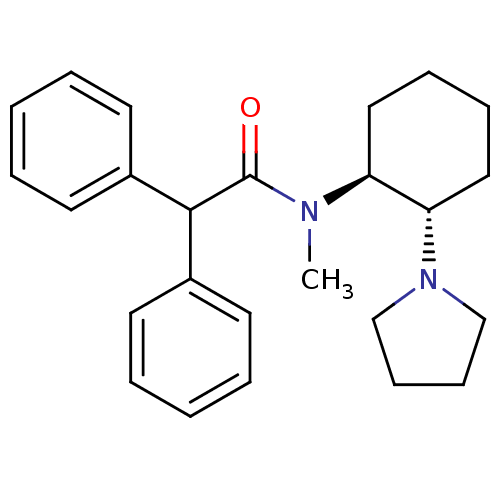 (CHEMBL327944 | N-Methyl-2,2-diphenyl-N-((1S,2S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290975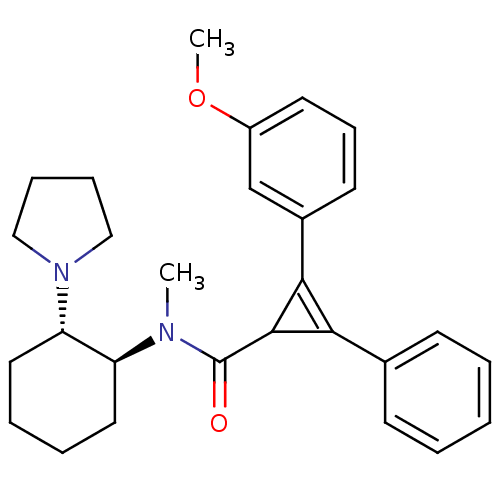 (2-(3-Methoxy-phenyl)-3-phenyl-cycloprop-2-enecarbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290976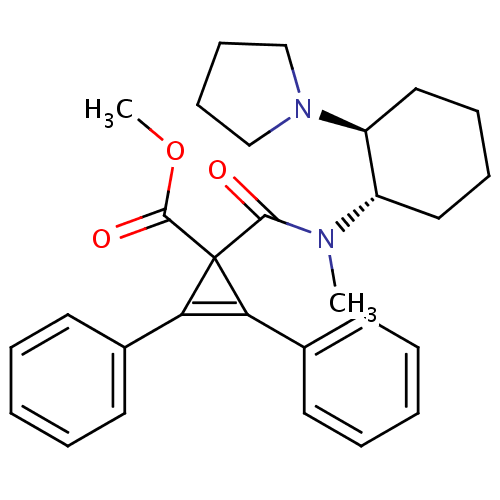 (1-[Methyl-((1S,2S)-2-pyrrolidin-1-yl-cyclohexyl)-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290977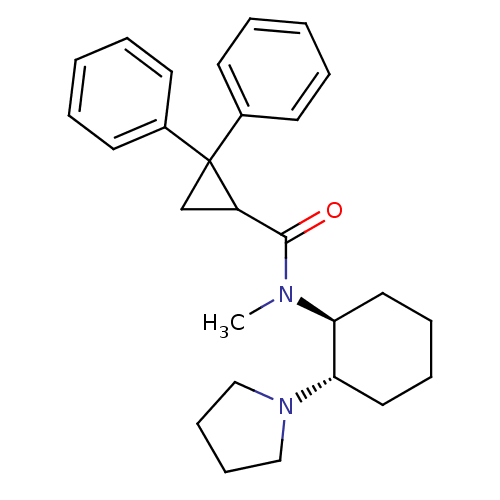 (2,2-Diphenyl-cyclopropanecarboxylic acid methyl-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290978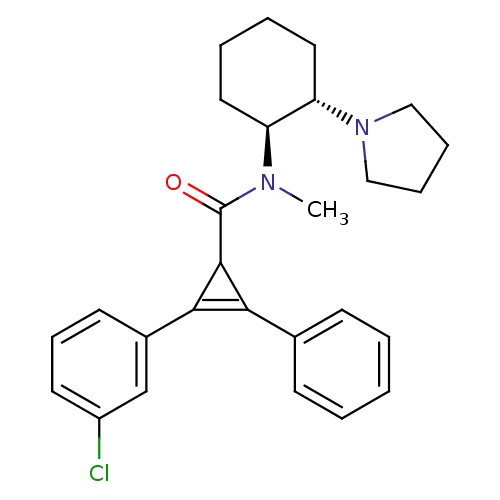 (2-(3-Chloro-phenyl)-3-phenyl-cycloprop-2-enecarbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290971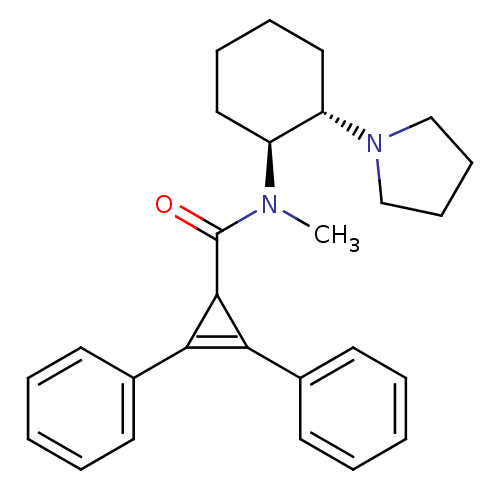 (2,3-Diphenyl-cycloprop-2-enecarboxylic acid methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290970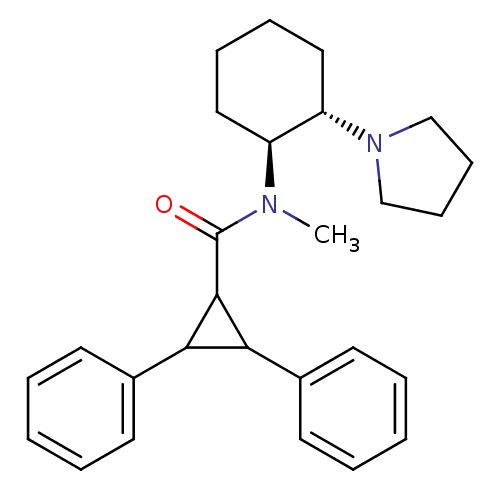 (2,3-Diphenyl-cyclopropanecarboxylic acid methyl-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50088375 (1-{8-[(2,3-Diphenyl-cycloprop-2-enecarbonyl)-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290969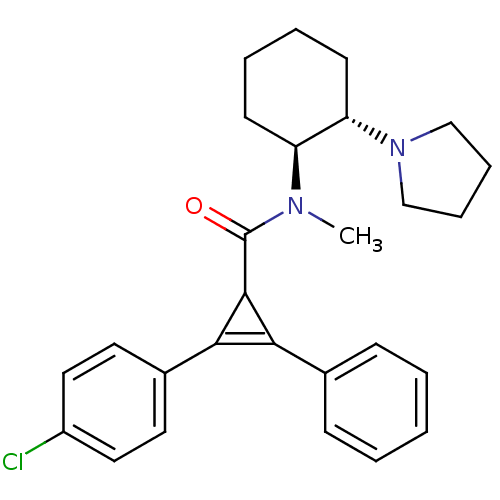 (2-(4-Chloro-phenyl)-3-phenyl-cycloprop-2-enecarbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290968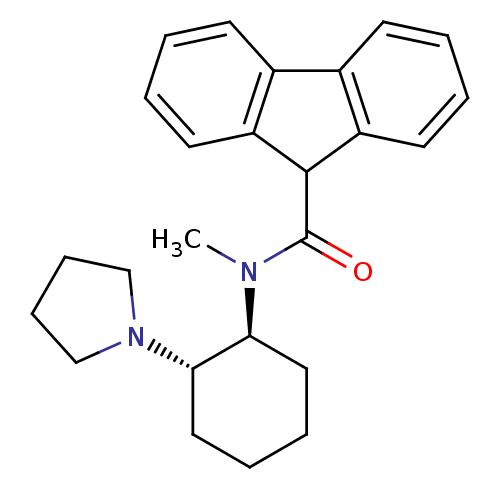 (9H-Fluorene-9-carboxylic acid methyl-((1S,2S)-2-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||