

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for human fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for rat fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171297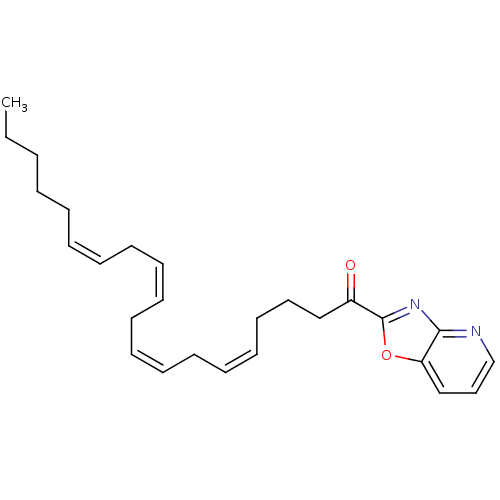 ((5Z,8Z,11Z,14Z)-1-(oxazolo[4,5-b]pyridin-2-yl)icos...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50069273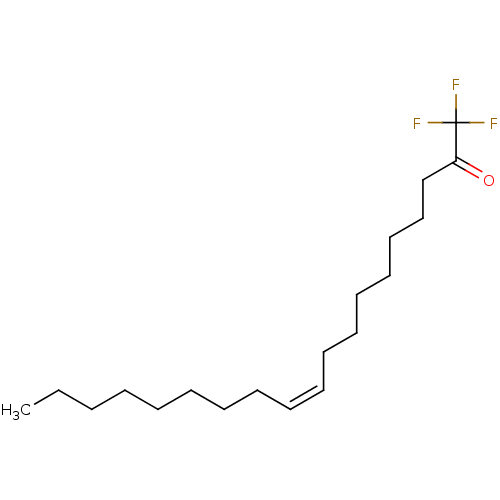 ((Z)-1,1,1-Trifluoro-nonadec-10-en-2-one | 1,1,1-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50100865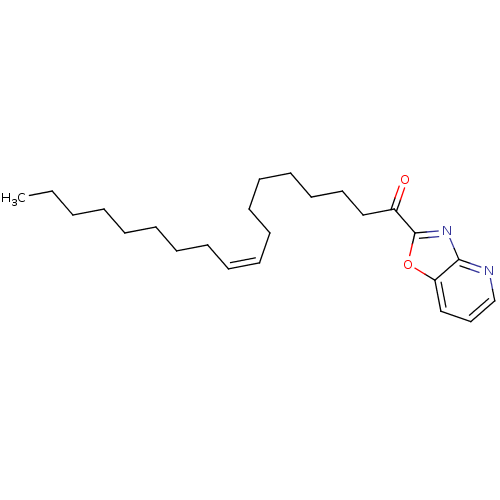 ((Z)-1-(oxazolo[4,5-b]pyridin-2-yl)octadec-9-en-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50171290 (5-(4-Bromo-phenyl)-1-(2,4-dichloro-phenyl)-4-ethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to human cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171295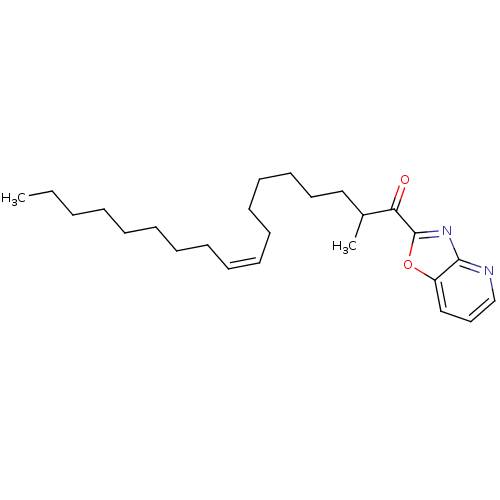 ((Z)-2-Methyl-1-oxazolo[4,5-b]pyridin-2-yl-octadec-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50171296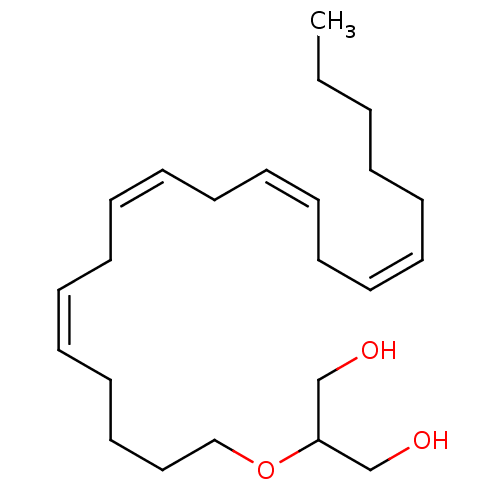 (2-[((11Z,14Z)-Icosa-5,8,11,14-tetraenyl)oxy]-propa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50073987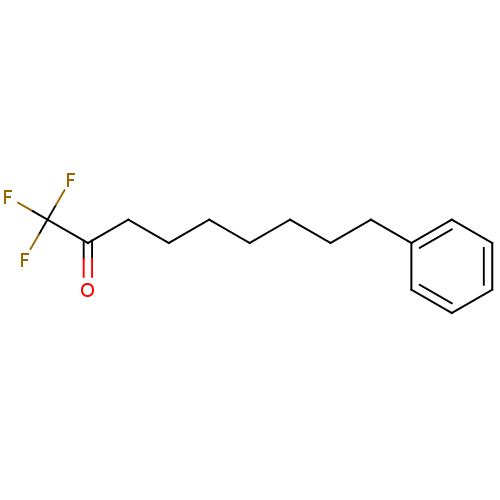 (1,1,1-Trifluoro-9-phenyl-nonan-2-one | 1,1,1-trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM85675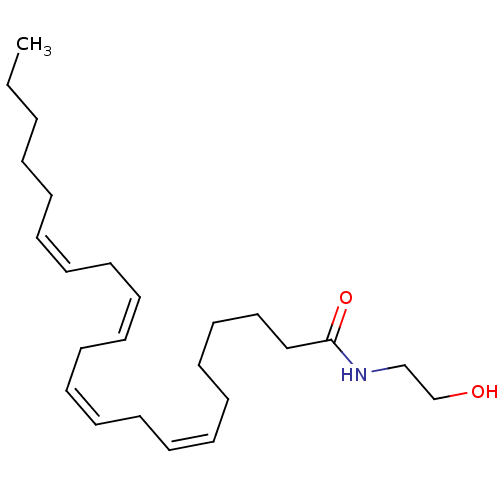 (Anandamide + PMSF | CHEMBL321585) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for human cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50056457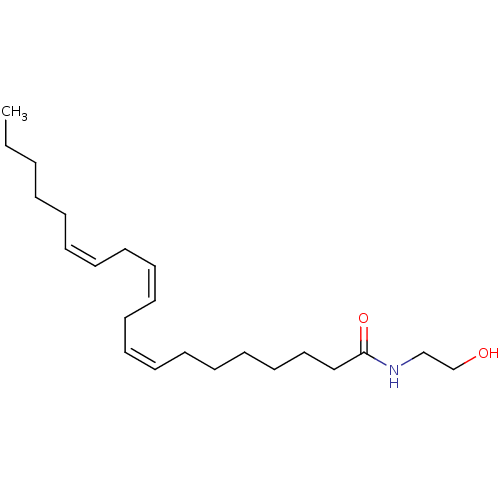 ((8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-8,11,14-trien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50073986 (1,1,1-Trifluoro-8-(4-heptyl-phenyl)-octan-2-one | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Apparent binding affinity for fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM20462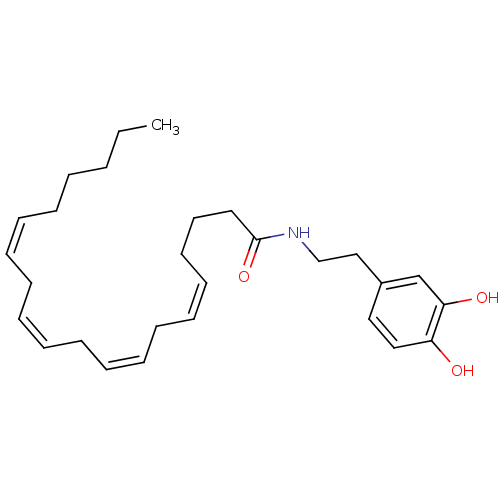 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50171290 (5-(4-Bromo-phenyl)-1-(2,4-dichloro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to human cannabinoid receptor 2 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Homo sapiens (Human)) | BDBM50069269 ((R)-octan-2-yl 4-bromo-3-oxobutanoate | 4-Bromo-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for Cannabinoid receptor | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM23028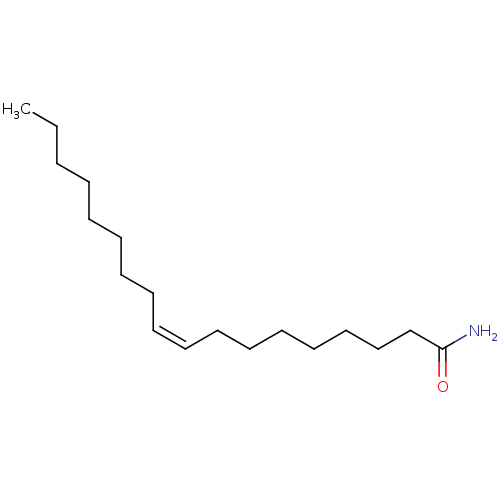 ((9Z)-octadec-9-enamide | 14C-labeled oleamide | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to rat cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50171285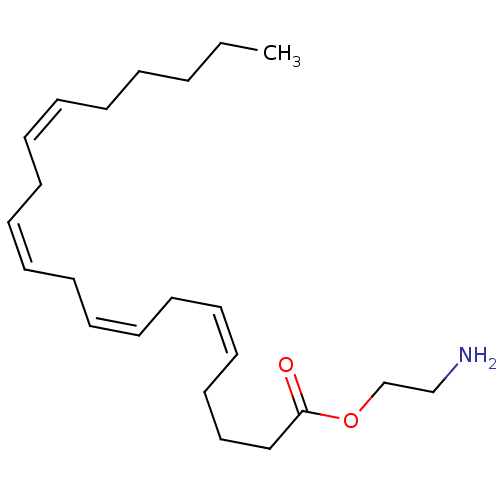 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Binding affinity for cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50132714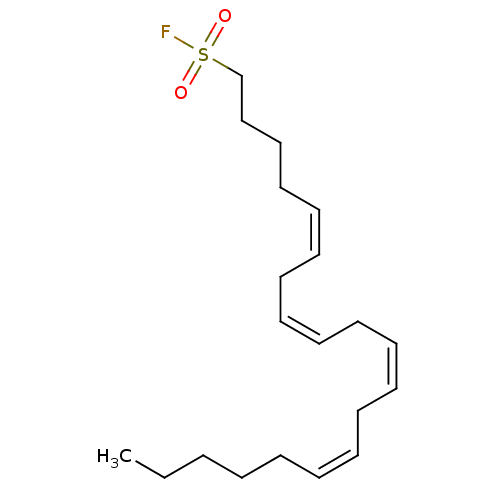 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenesulfonyl f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23316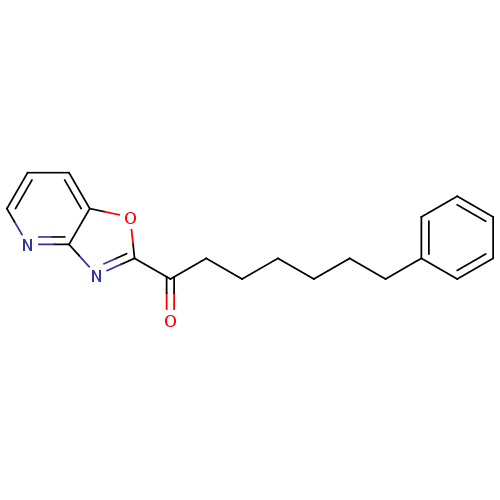 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50132715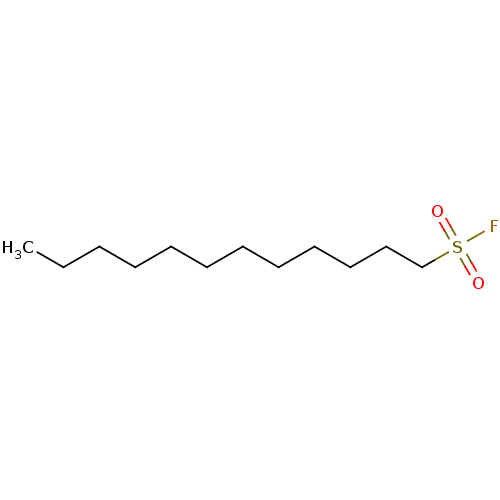 (CHEMBL97038 | Dodecanesulfonyl fluoride | dodecane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50132716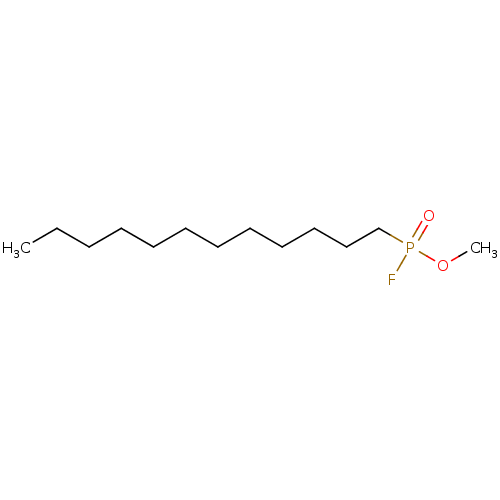 (CHEMBL113263 | methyl dodecylphosphonofluoridoate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase; range=1-3 nM | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171287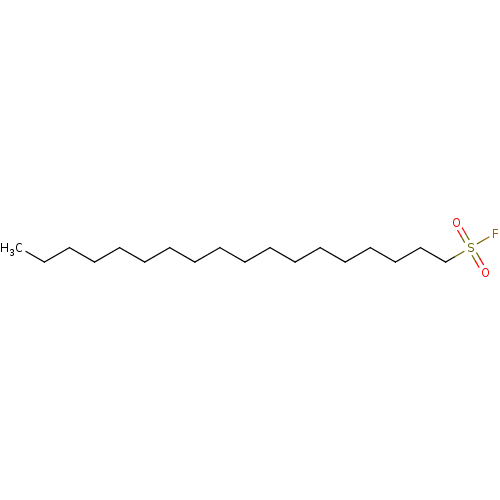 (CHEMBL191481 | Octadecanesulfonyl fluoride) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171293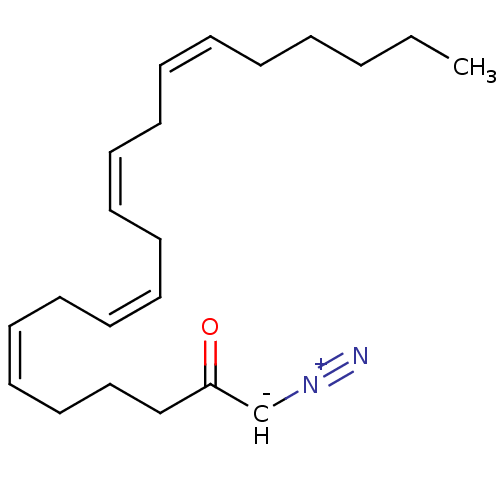 ((6Z,9Z,12Z,15Z)-1-Diazo-henicosa-6,9,12,15-tetraen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase; range= .5-6 nM | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171292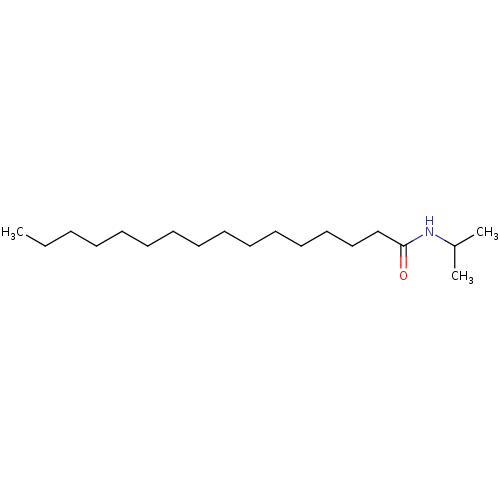 (CHEMBL32827 | Hexadecanoic acid isopropylamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50132714 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenesulfonyl f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to mouse Cannabinoid receptor 1 | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50046268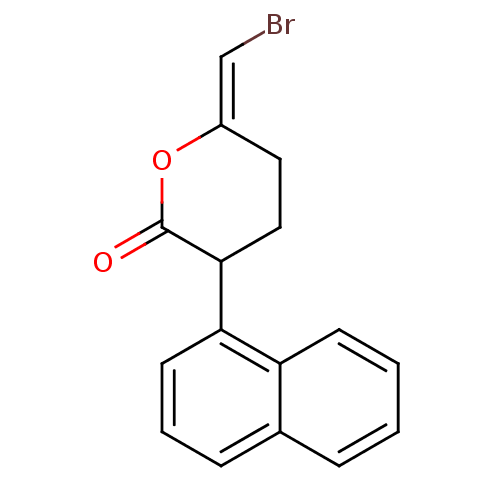 (6-Bromomethylene-3-naphthalen-1-yl-tetrahydro-pyra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50069269 ((R)-octan-2-yl 4-bromo-3-oxobutanoate | 4-Bromo-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50059523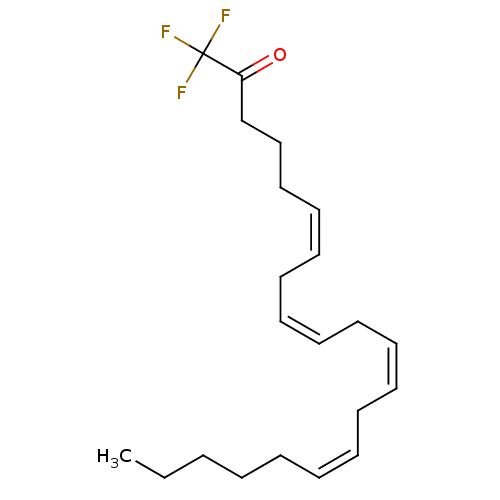 ((6Z,9Z,12Z,15Z)-1,1,1-trifluorohenicosa-6,9,12,15-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of fatty acid amide hydrolase; range = .23-3 uM | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50171299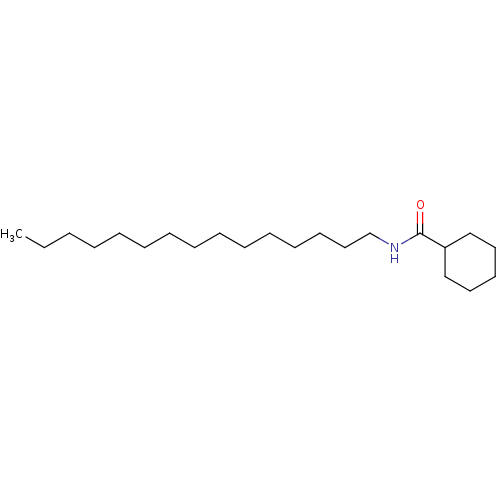 (CHEMBL190662 | Cyclohexanecarboxylic acid pentadec...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against N-palmitoylethanolamine acid amidase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50056486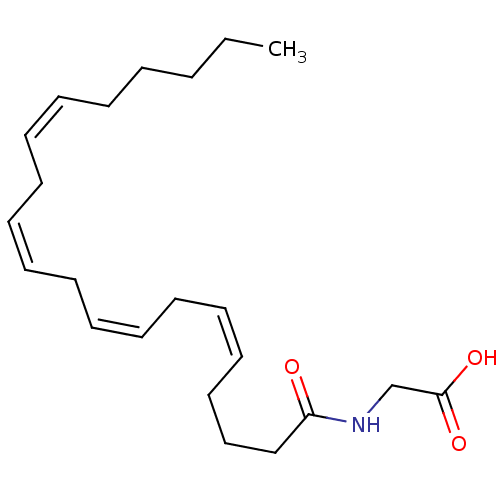 (((5Z,8Z)-Icosa-5,8,11,14-tetraenoylamino)-acetic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of fatty acid amide hydrolase; range = 4.1-7 uM | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171288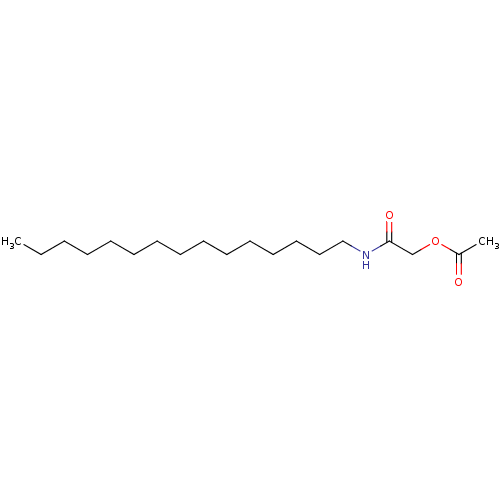 (Acetic acid pentadecylcarbamoylmethyl ester | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171298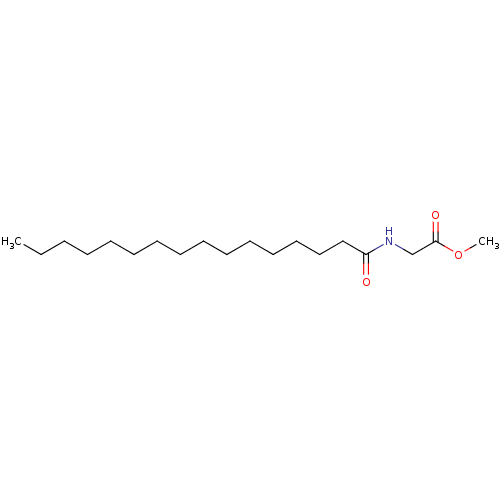 (CHEMBL286321 | Hexadecanoylamino-acetic acid methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM22987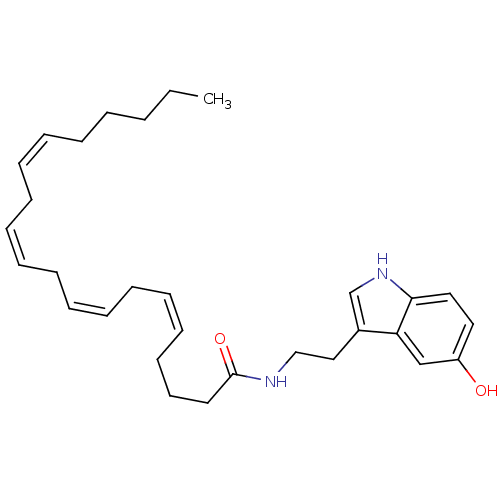 ((5Z,8Z,11Z,14Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50171289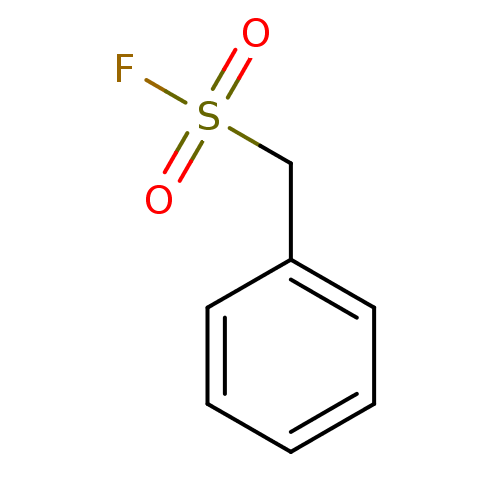 (Benzenemethanesulfonyl fluoride | CHEMBL190503 | P...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Irreversible inhibition of fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50171291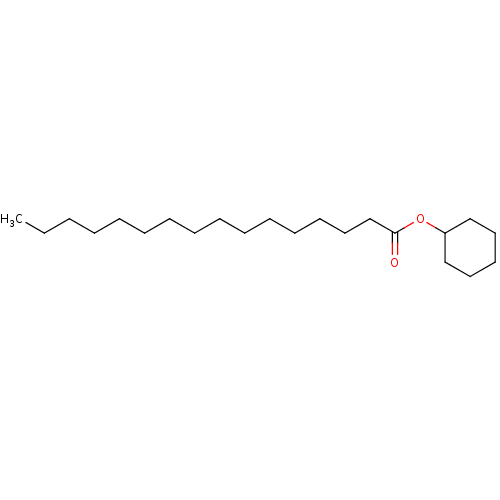 (CHEMBL139056 | Hexadecanoic acid cyclohexyl ester ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against N-palmitoylethanolamine acid amidase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50096882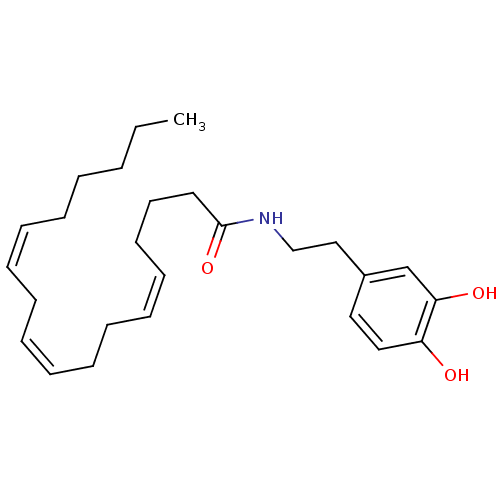 ((5Z,9Z,12Z)-Octadeca-5,9,12-trienoic acid [2-(3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50128913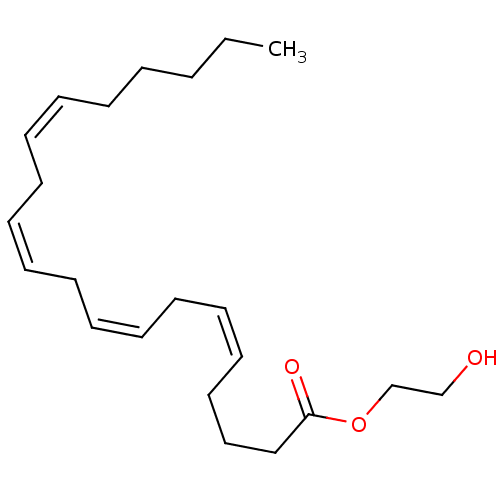 ((5Z,8Z,11Z)-Icosa-5,8,11,14-tetraenoic acid 2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50128913 ((5Z,8Z,11Z)-Icosa-5,8,11,14-tetraenoic acid 2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against monoacylglycerol lipase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50171294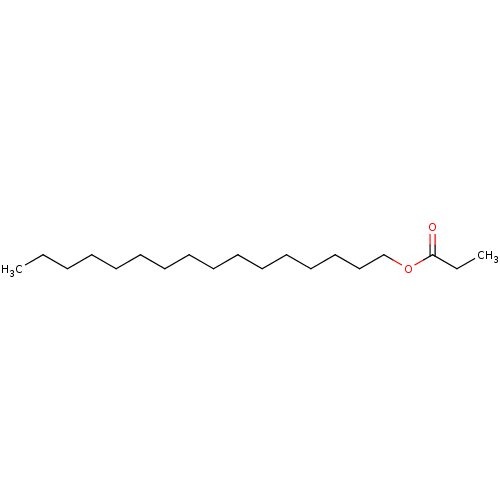 (CHEMBL138543 | HEXADECYLPROPIONATE | Propionic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against N-palmitoylethanolamine acid amidase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50058046 (CHEMBL526 | propofol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against fatty acid amide hydrolase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50171286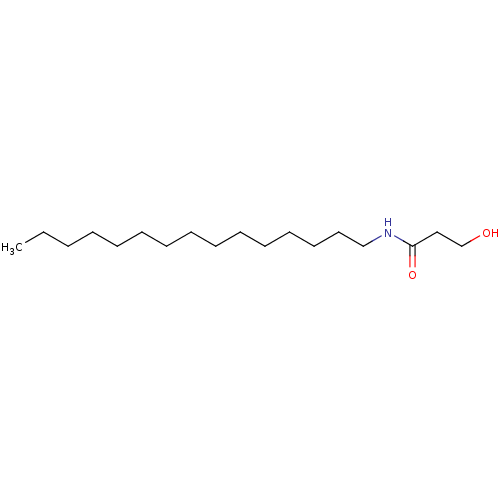 (3-Hydroxy-N-pentadecyl-propionamide | CHEMBL423904...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibitory concentration against N-palmitoylethanolamine acid amidase | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23028 ((9Z)-octadec-9-enamide | 14C-labeled oleamide | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of fatty acid amide hydrolase; range = 22-68 uM | J Med Chem 48: 5059-87 (2005) Article DOI: 10.1021/jm058183t BindingDB Entry DOI: 10.7270/Q2J96753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||