

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391363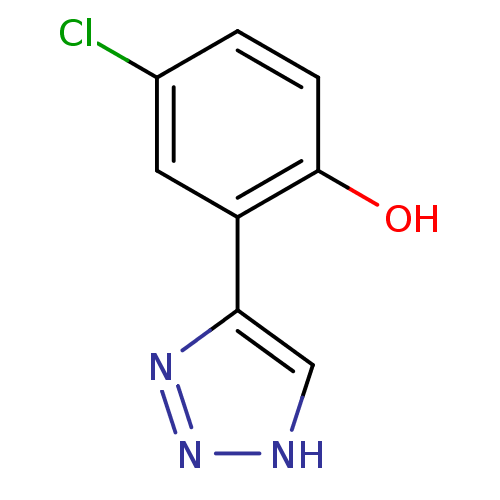 (CHEMBL2148074) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391358 (CHEMBL2147989) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391359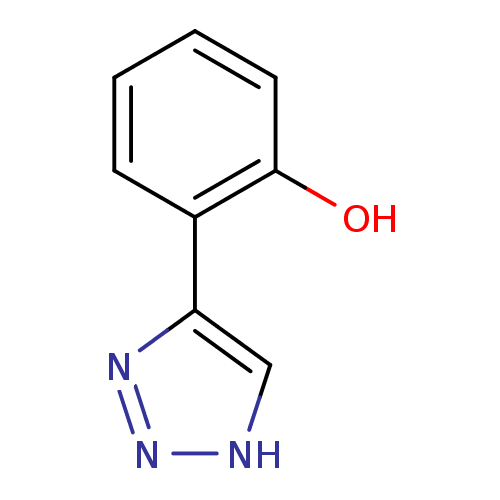 (CHEMBL2147998) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17467 (1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391365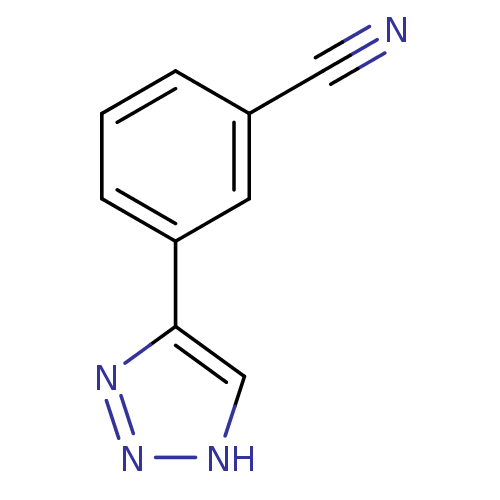 (CHEMBL2147990) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391372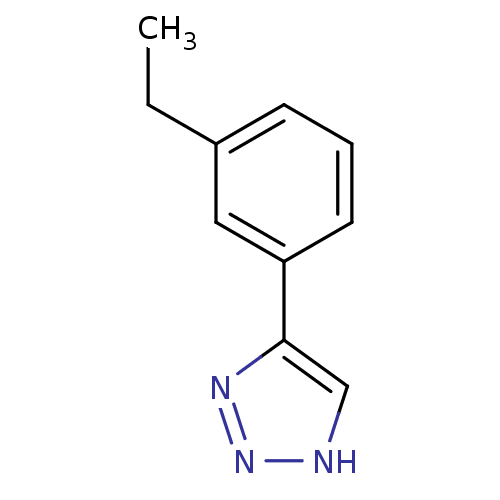 (CHEMBL2147992) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50355861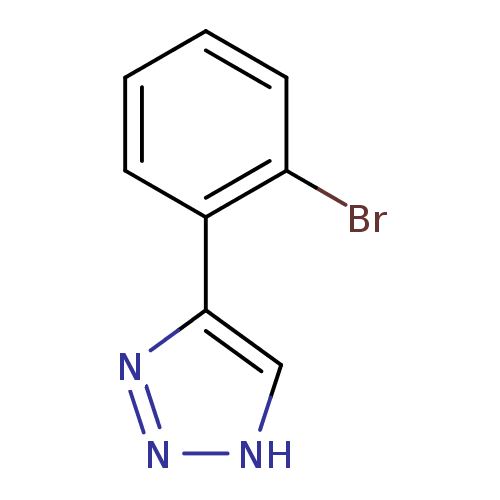 (CHEMBL1909733) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391360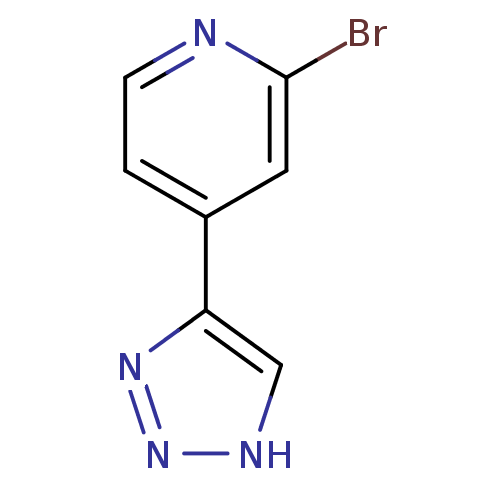 (CHEMBL2147995) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391374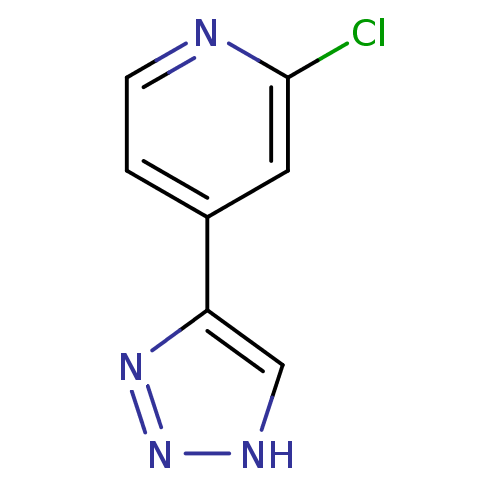 (CHEMBL2146496) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300305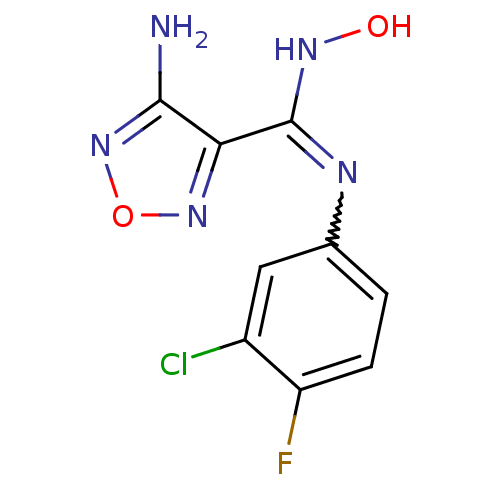 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391366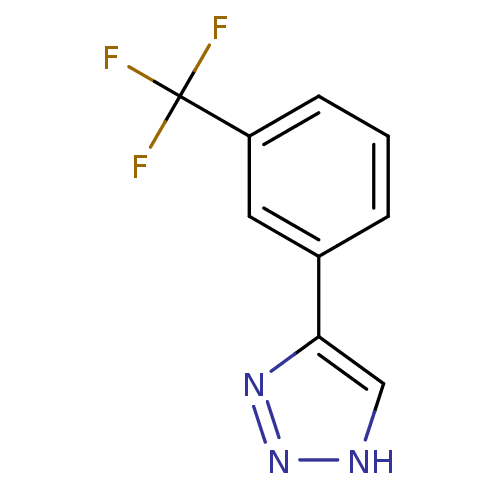 (CHEMBL2147991) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 7.4 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391375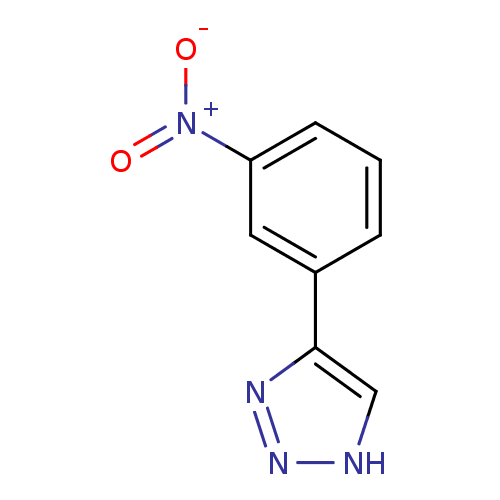 (CHEMBL2147993) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391369 (CHEMBL2148075) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17462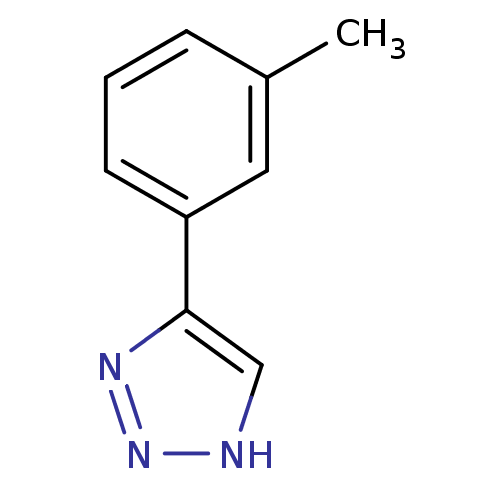 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391361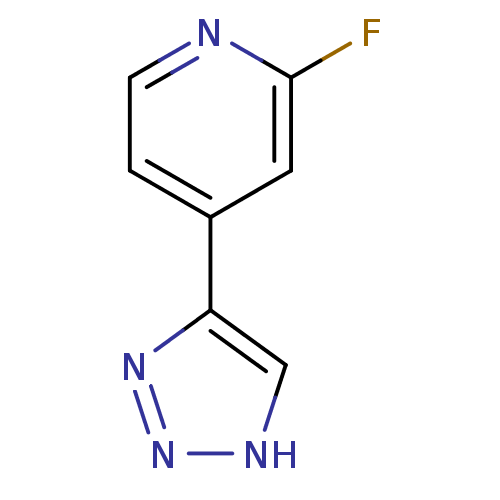 (CHEMBL2147996) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391373 (CHEMBL2148076) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17448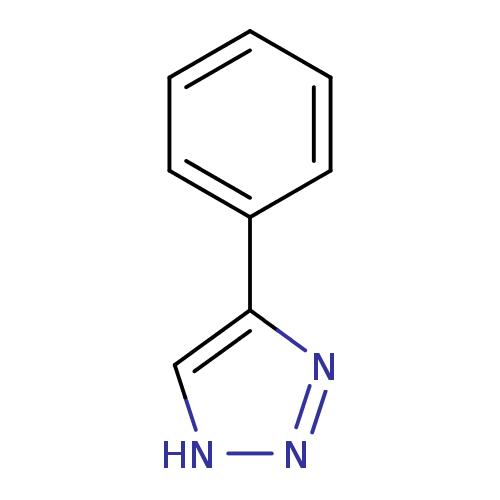 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391368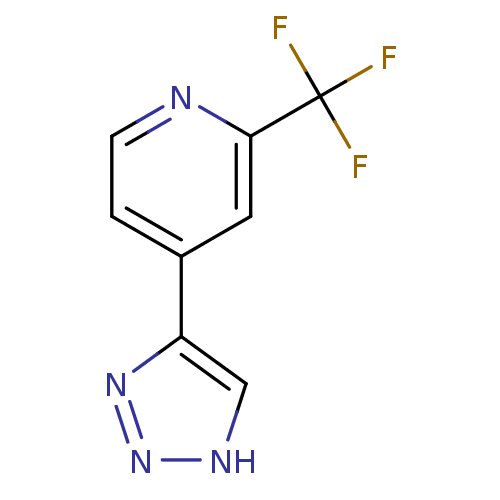 (CHEMBL2147997) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391358 (CHEMBL2147989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391362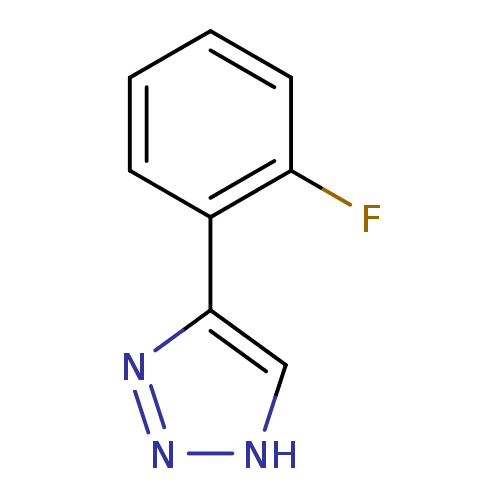 (CHEMBL2148000) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17467 (1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391367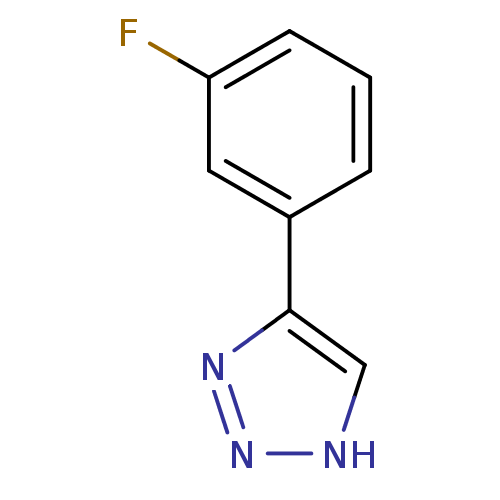 (CHEMBL2147994) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391372 (CHEMBL2147992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391358 (CHEMBL2147989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17463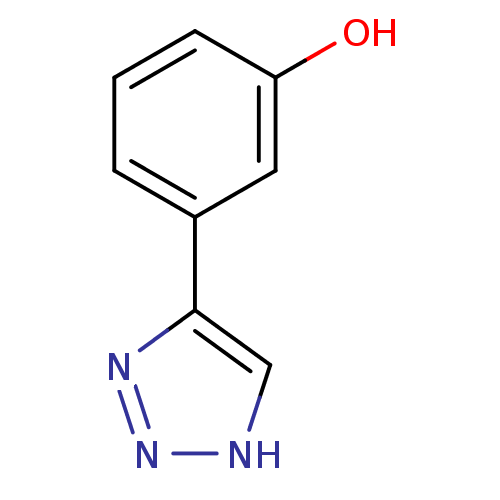 (1,2,3-triazole analogue, 19 | 3-(1H-1,2,3-triazol-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50355862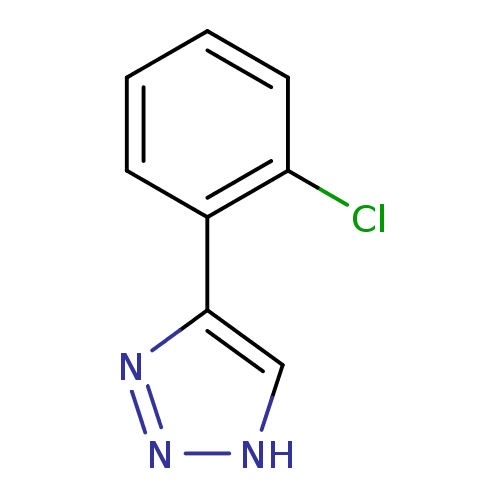 (CHEMBL1909734) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391364 (CHEMBL2148077) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391370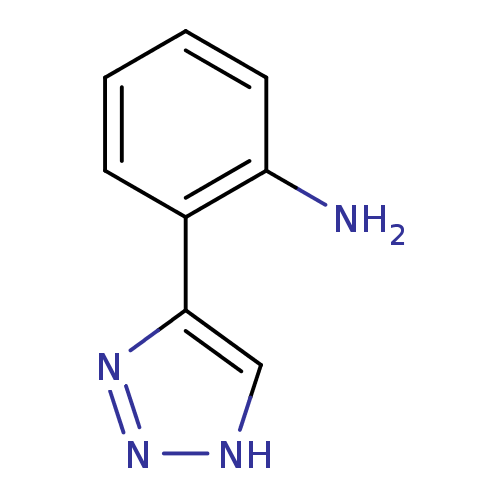 (CHEMBL2147999) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391359 (CHEMBL2147998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17452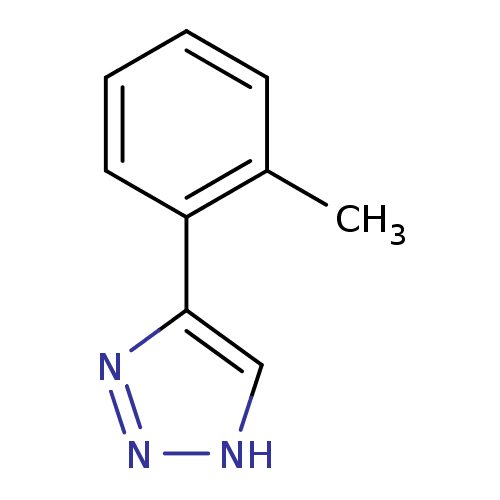 (1,2,3-triazole analogue, 8 | 5-(2-methylphenyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17467 (1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50355867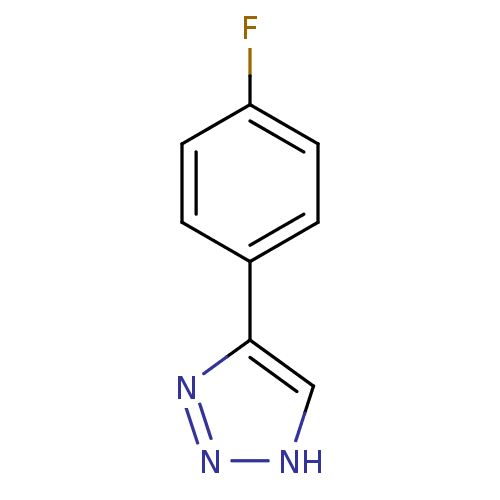 (CHEMBL1909735) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355861 (CHEMBL1909733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391365 (CHEMBL2147990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391374 (CHEMBL2146496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391365 (CHEMBL2147990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391360 (CHEMBL2147995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391360 (CHEMBL2147995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391374 (CHEMBL2146496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17464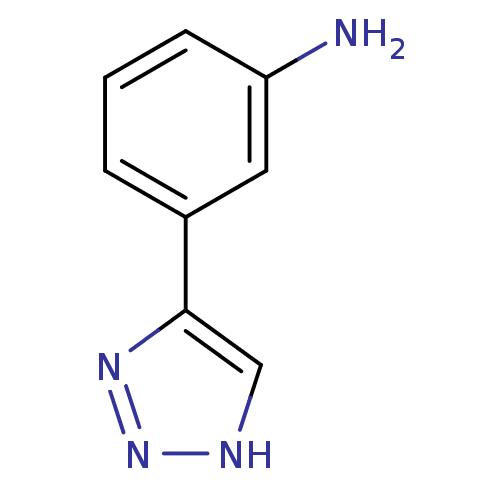 (1,2,3-triazole analogue, 20 | 3-(1H-1,2,3-triazol-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391369 (CHEMBL2148075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391366 (CHEMBL2147991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391373 (CHEMBL2148076) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50355861 (CHEMBL1909733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391361 (CHEMBL2147996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |