

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50261233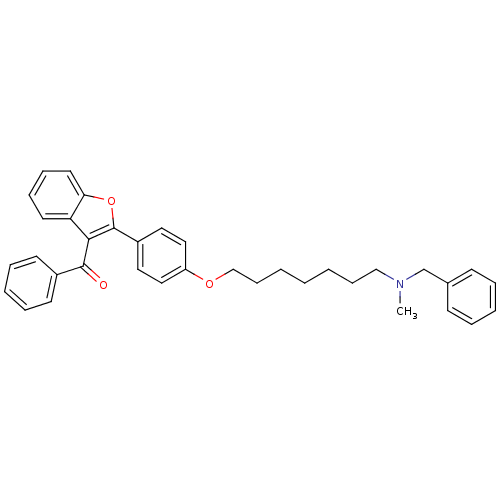 ((2-{4-[7-(benzylmethylamino)heptyloxy]phenyl}benzo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394567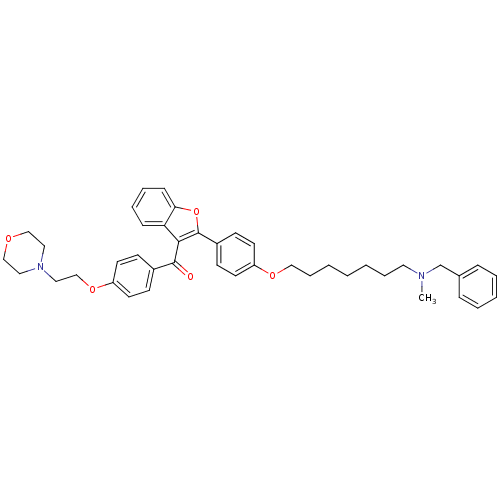 (CHEMBL2160225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394567 (CHEMBL2160225) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394568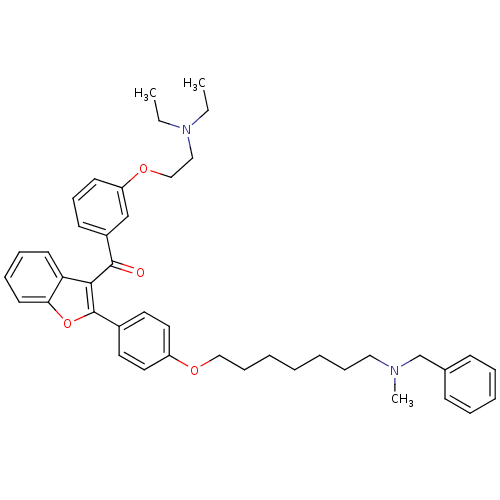 (CHEMBL2160223) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394569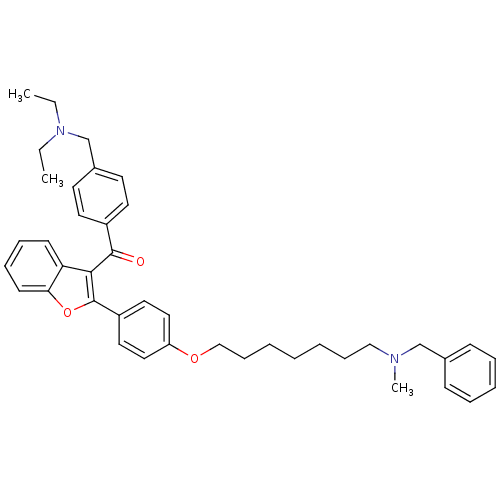 (CHEMBL2160222) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394570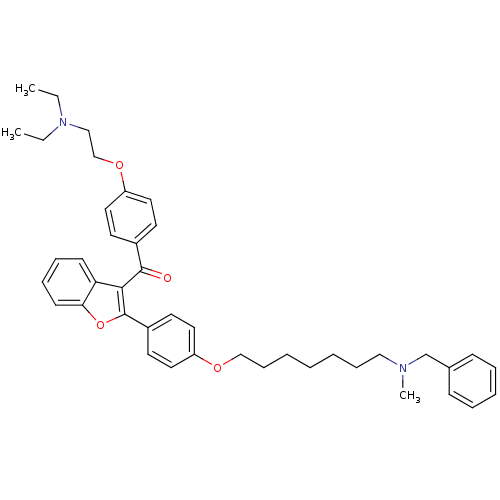 (CHEMBL2160224) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394572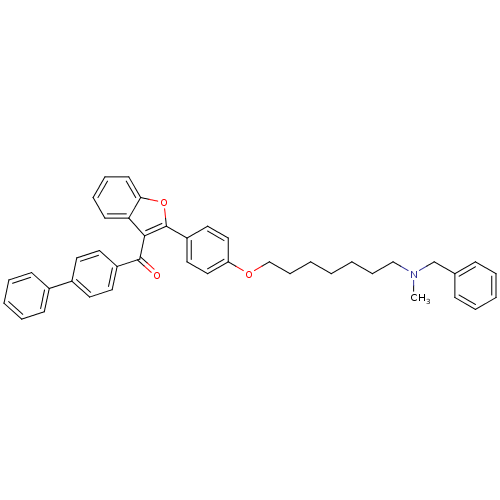 (CHEMBL2160219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394568 (CHEMBL2160223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50261202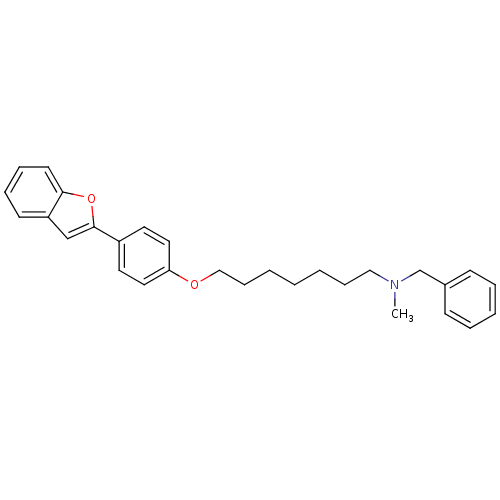 (CHEMBL497755 | [7-(4-Benzofuran-2-yl-phenoxy)hepht...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394572 (CHEMBL2160219) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50261202 (CHEMBL497755 | [7-(4-Benzofuran-2-yl-phenoxy)hepht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50261233 ((2-{4-[7-(benzylmethylamino)heptyloxy]phenyl}benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394577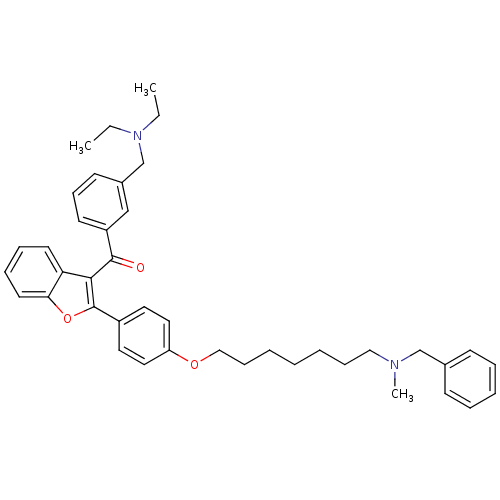 (CHEMBL2160221) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394569 (CHEMBL2160222) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394582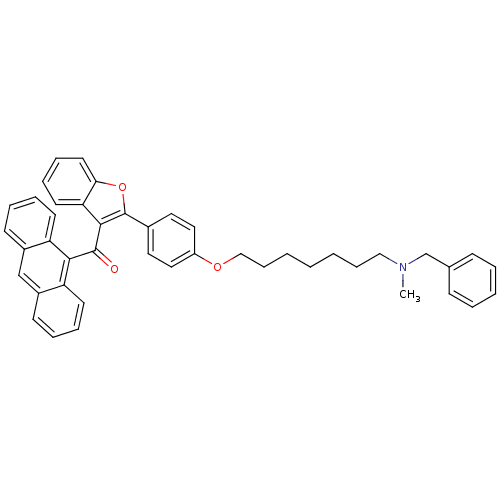 (CHEMBL2160220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394581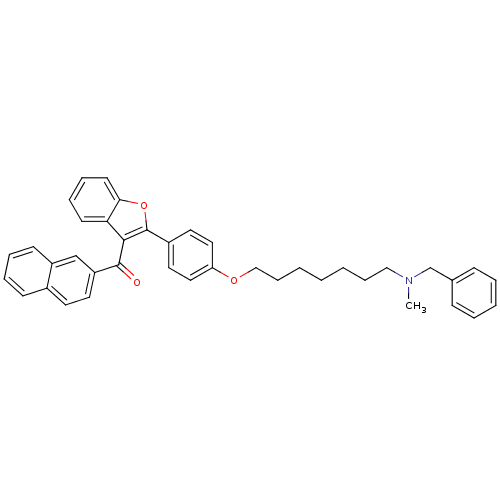 (CHEMBL2160218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50394580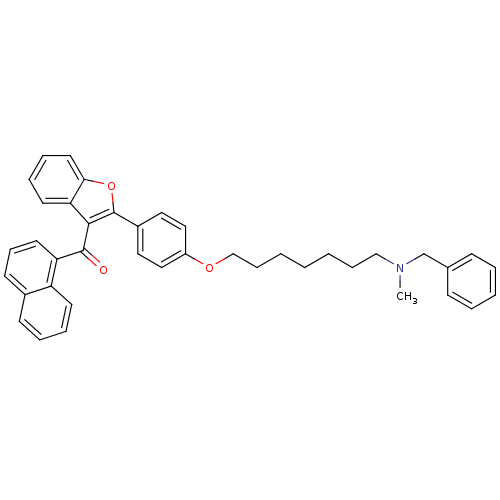 (CHEMBL2160217) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394570 (CHEMBL2160224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394577 (CHEMBL2160221) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394582 (CHEMBL2160220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394581 (CHEMBL2160218) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50394580 (CHEMBL2160217) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394563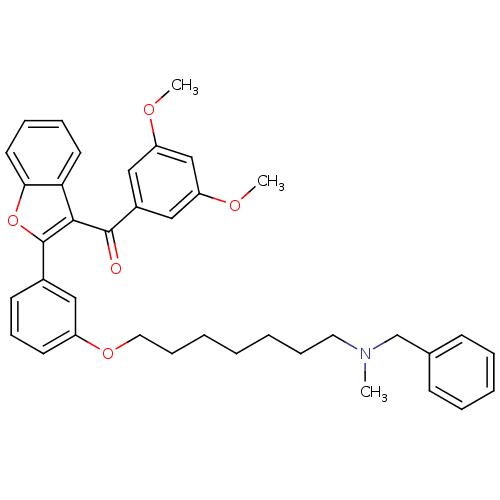 (CHEMBL2160234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394564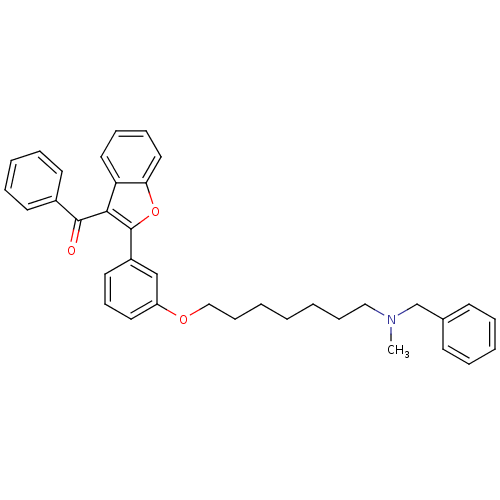 (CHEMBL2160227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394565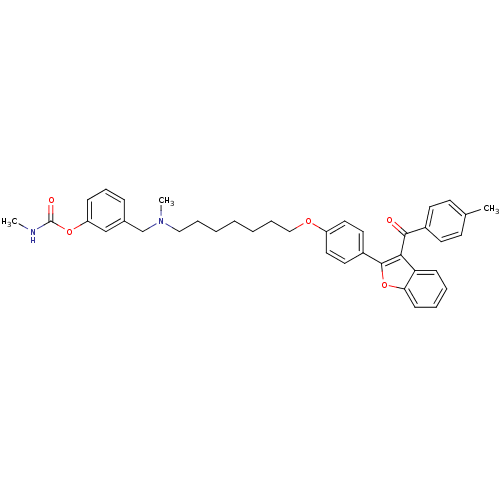 (CHEMBL2160226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394566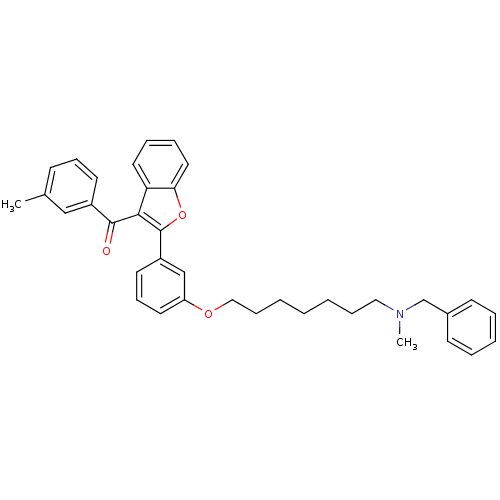 (CHEMBL2160228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394565 (CHEMBL2160226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394571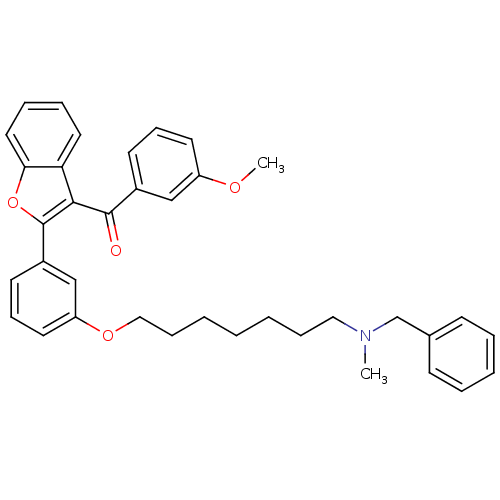 (CHEMBL2160231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394568 (CHEMBL2160223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394573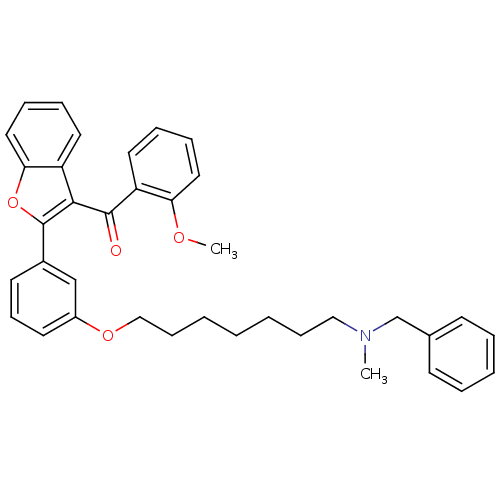 (CHEMBL2160230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394574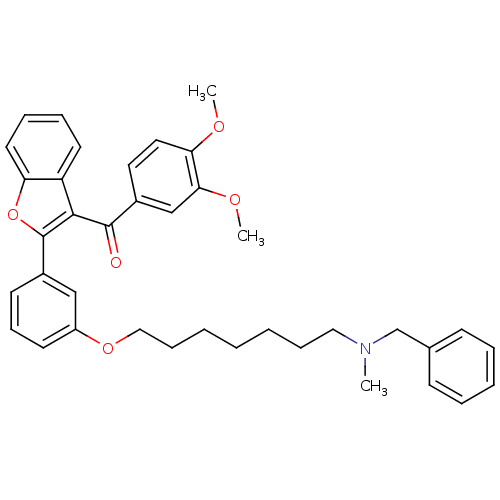 (CHEMBL2160233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394564 (CHEMBL2160227) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394575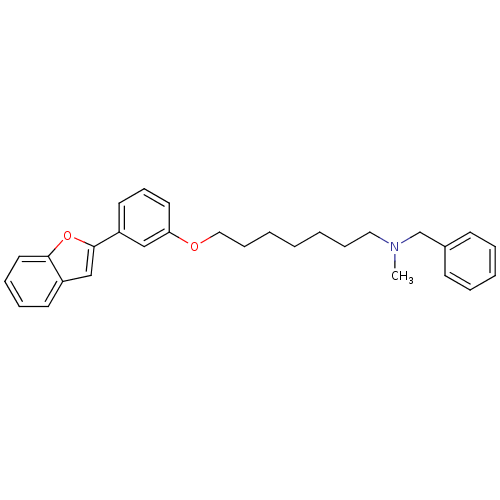 (CHEMBL2160216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394576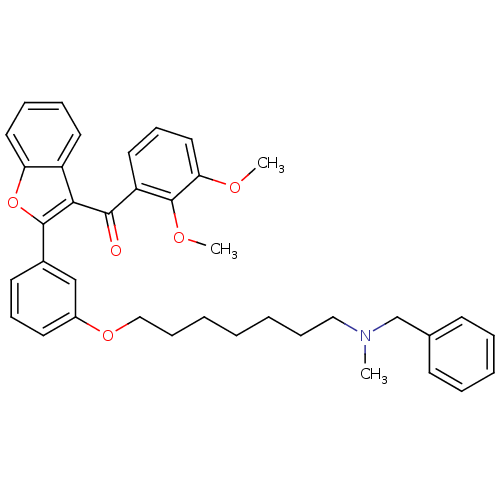 (CHEMBL2160232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394577 (CHEMBL2160221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394578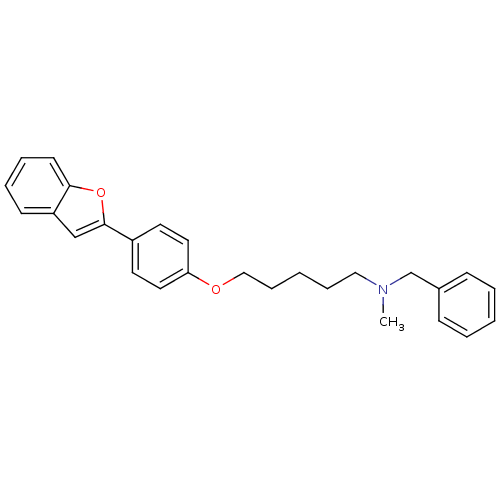 (CHEMBL2160213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394579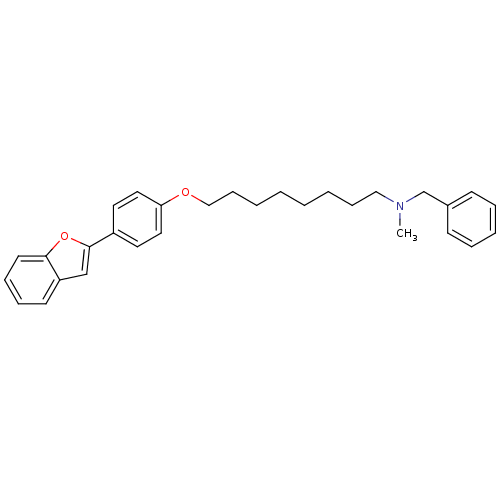 (CHEMBL2160214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394569 (CHEMBL2160222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394567 (CHEMBL2160225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394570 (CHEMBL2160224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394568 (CHEMBL2160223) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394567 (CHEMBL2160225) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394583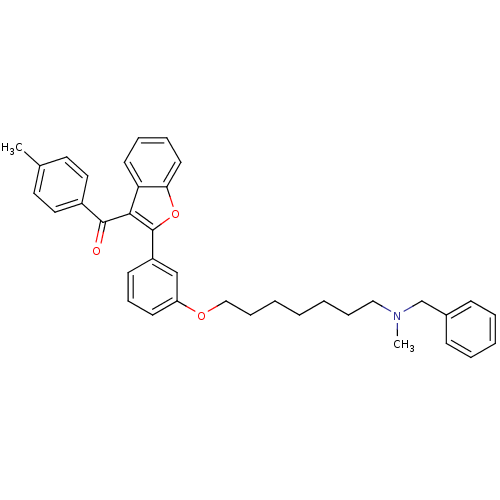 (CHEMBL2160229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394577 (CHEMBL2160221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394584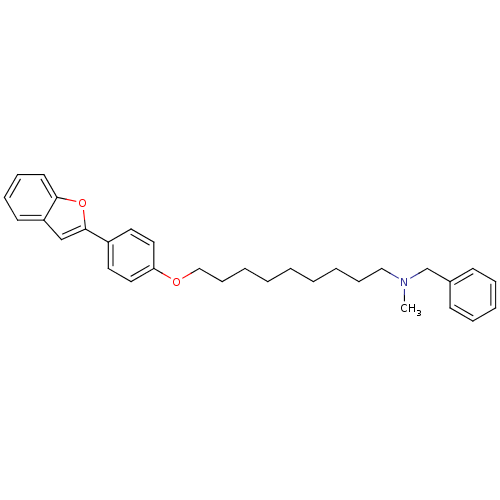 (CHEMBL2160215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394580 (CHEMBL2160217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394582 (CHEMBL2160220) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394570 (CHEMBL2160224) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's met... | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394582 (CHEMBL2160220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50394572 (CHEMBL2160219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior to substrate addition by Ellman's method | Eur J Med Chem 58: 519-32 (2012) Article DOI: 10.1016/j.ejmech.2012.10.045 BindingDB Entry DOI: 10.7270/Q2XW4KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 78 total ) | Next | Last >> |