 Found 39 hits of ph data with Target = 'MAP kinase-activated protein kinase 2'
Found 39 hits of ph data with Target = 'MAP kinase-activated protein kinase 2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM27344
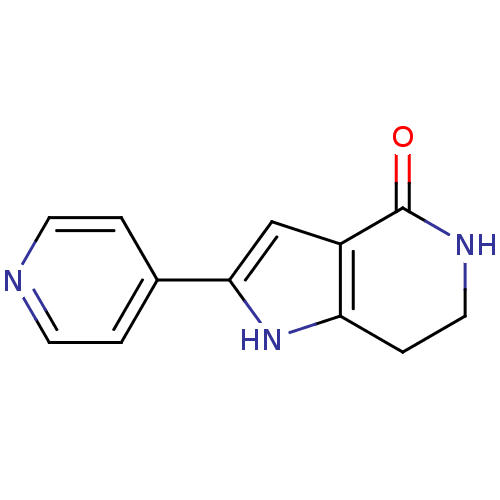
(2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...)Show InChI InChI=1S/C12H11N3O/c16-12-9-7-11(8-1-4-13-5-2-8)15-10(9)3-6-14-12/h1-2,4-5,7,15H,3,6H2,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM236557
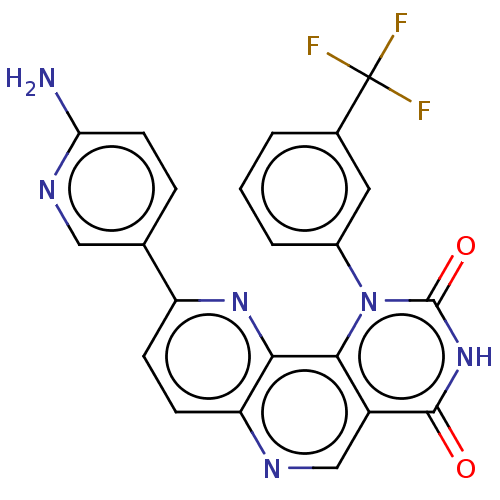
(US9365572, 5)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3c(n(-c4cccc(c4)C(F)(F)F)c(=O)[nH]c3=O)c2n1 Show InChI InChI=1S/C22H13F3N6O2/c23-22(24,25)12-2-1-3-13(8-12)31-19-14(20(32)30-21(31)33)10-27-16-6-5-15(29-18(16)19)11-4-7-17(26)28-9-11/h1-10H,(H2,26,28)(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 28 |
Xuanzhu Pharma Co., Ltd.
US Patent
| Assay Description
Agents: 1-fold kinase buffer without MnCl2: 50 mM HEPES, pH 7.5, 0.0015% Brij-35, 10 mM MgCl2, 2 mM DTT. 1-fold kinase buffer with MnCl2: 50 mM HEPES... |
US Patent US9365572 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TNC |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30178
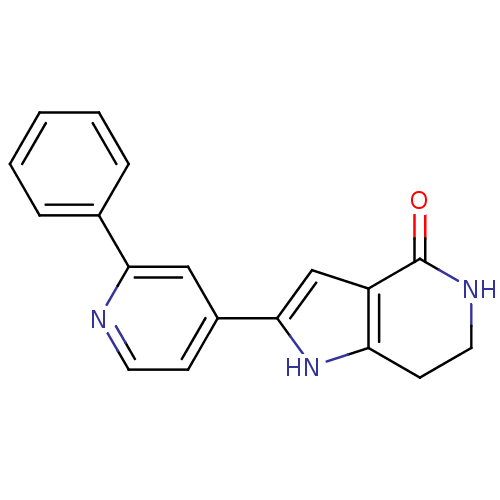
(Pyrrolopyridine, 9)Show InChI InChI=1S/C18H15N3O/c22-18-14-11-17(21-15(14)7-9-20-18)13-6-8-19-16(10-13)12-4-2-1-3-5-12/h1-6,8,10-11,21H,7,9H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30179

(Pyrrolopyridine, 10)Show InChI InChI=1S/C17H14N4O/c22-17-13-10-16(21-14(13)4-8-20-17)12-3-7-19-15(9-12)11-1-5-18-6-2-11/h1-3,5-7,9-10,21H,4,8H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30180
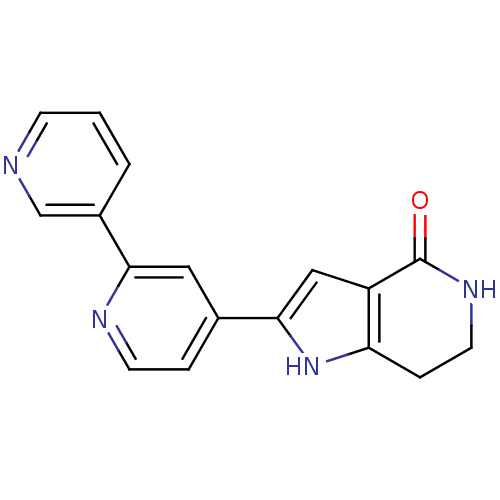
(Pyrrolopyridine, 11)Show InChI InChI=1S/C17H14N4O/c22-17-13-9-16(21-14(13)4-7-20-17)11-3-6-19-15(8-11)12-2-1-5-18-10-12/h1-3,5-6,8-10,21H,4,7H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30181

(Pyrrolopyridine, 12)Show InChI InChI=1S/C16H13N5O/c22-16-12-6-15(21-13(12)2-4-20-16)10-1-3-19-14(5-10)11-7-17-9-18-8-11/h1,3,5-9,21H,2,4H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30182

(Pyrrolopyridine, 13)Show InChI InChI=1S/C16H13N3OS/c20-16-12-8-15(19-13(12)2-5-18-16)10-1-4-17-14(7-10)11-3-6-21-9-11/h1,3-4,6-9,19H,2,5H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30183
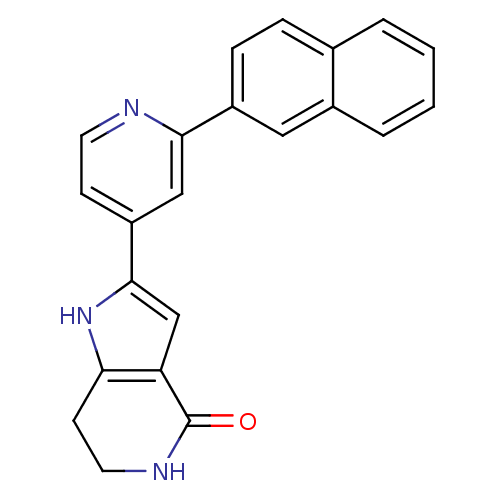
(Pyrrolopyridine, 14)Show SMILES O=C1NCCc2[nH]c(cc12)-c1ccnc(c1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C22H17N3O/c26-22-18-13-21(25-19(18)8-10-24-22)17-7-9-23-20(12-17)16-6-5-14-3-1-2-4-15(14)11-16/h1-7,9,11-13,25H,8,10H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30184
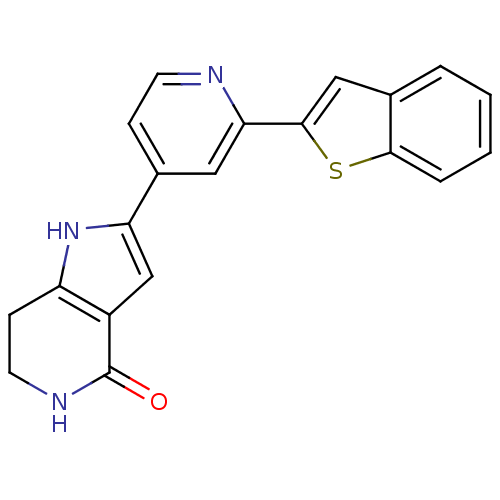
(Pyrrolopyridine, 15)Show SMILES O=C1NCCc2[nH]c(cc12)-c1ccnc(c1)-c1cc2ccccc2s1 Show InChI InChI=1S/C20H15N3OS/c24-20-14-11-16(23-15(14)6-8-22-20)12-5-7-21-17(9-12)19-10-13-3-1-2-4-18(13)25-19/h1-5,7,9-11,23H,6,8H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30185
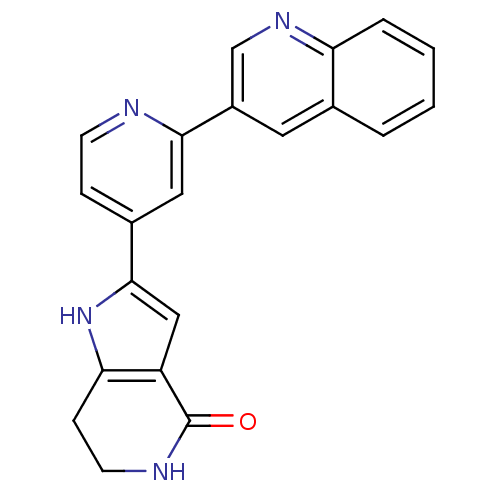
(CHEMBL226403 | Pyrrolopyridine, 16)Show SMILES O=C1NCCc2[nH]c(cc12)-c1ccnc(c1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C21H16N4O/c26-21-16-11-20(25-18(16)6-8-23-21)14-5-7-22-19(10-14)15-9-13-3-1-2-4-17(13)24-12-15/h1-5,7,9-12,25H,6,8H2,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30186
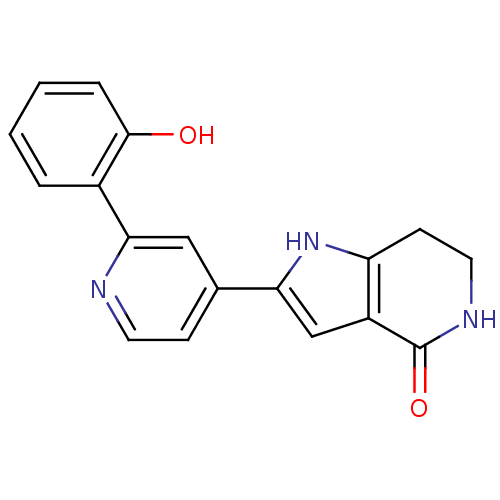
(Pyrrolopyridine, 17)Show InChI InChI=1S/C18H15N3O2/c22-17-4-2-1-3-12(17)16-9-11(5-7-19-16)15-10-13-14(21-15)6-8-20-18(13)23/h1-5,7,9-10,21-22H,6,8H2,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30187
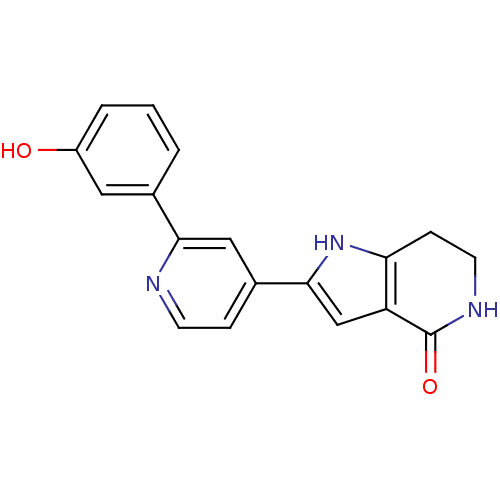
(Pyrrolopyridine, 18)Show SMILES Oc1cccc(c1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C18H15N3O2/c22-13-3-1-2-11(8-13)16-9-12(4-6-19-16)17-10-14-15(21-17)5-7-20-18(14)23/h1-4,6,8-10,21-22H,5,7H2,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30188
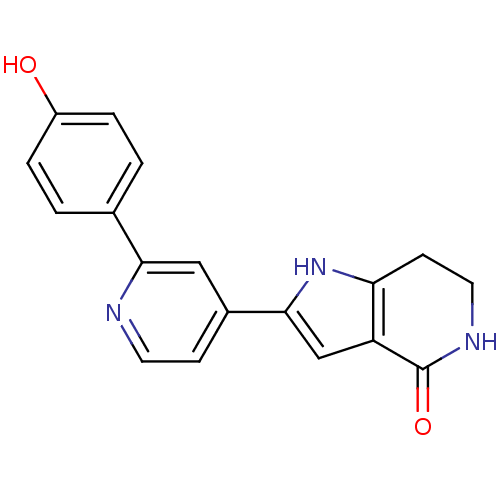
(Pyrrolopyridine, 19)Show SMILES Oc1ccc(cc1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C18H15N3O2/c22-13-3-1-11(2-4-13)16-9-12(5-7-19-16)17-10-14-15(21-17)6-8-20-18(14)23/h1-5,7,9-10,21-22H,6,8H2,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30189
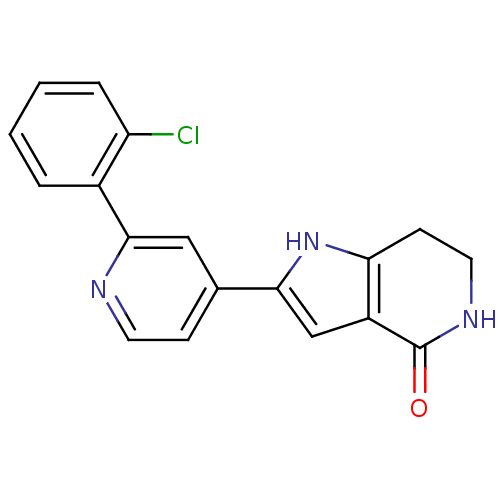
(Pyrrolopyridine, 20)Show InChI InChI=1S/C18H14ClN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 606 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30190
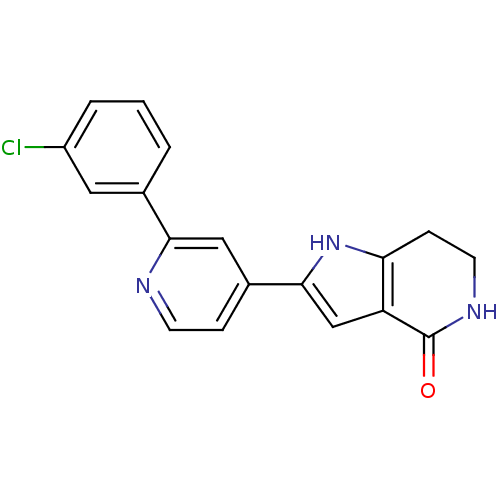
(Pyrrolopyridine, 21)Show SMILES Clc1cccc(c1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C18H14ClN3O/c19-13-3-1-2-11(8-13)16-9-12(4-6-20-16)17-10-14-15(22-17)5-7-21-18(14)23/h1-4,6,8-10,22H,5,7H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30191

(Pyrrolopyridine, 22)Show SMILES Clc1ccc(cc1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C18H14ClN3O/c19-13-3-1-11(2-4-13)16-9-12(5-7-20-16)17-10-14-15(22-17)6-8-21-18(14)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30192
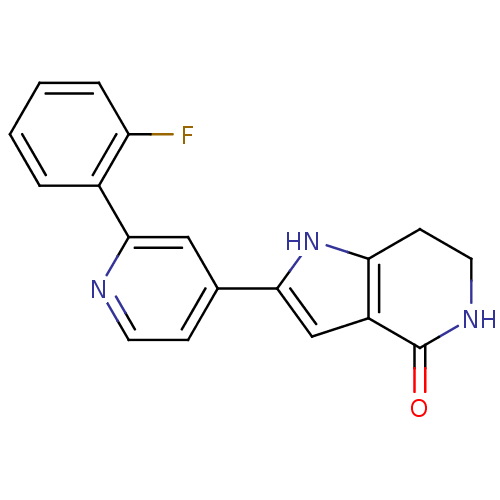
(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30193
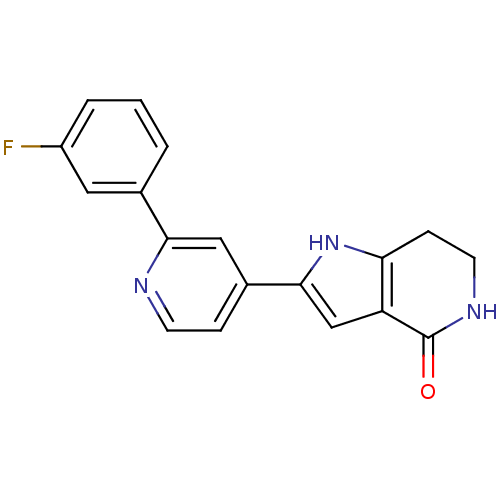
(Pyrrolopyridine, 24)Show SMILES Fc1cccc(c1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C18H14FN3O/c19-13-3-1-2-11(8-13)16-9-12(4-6-20-16)17-10-14-15(22-17)5-7-21-18(14)23/h1-4,6,8-10,22H,5,7H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30194

(Pyrrolopyridine, 25)Show SMILES Fc1ccc(cc1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C18H14FN3O/c19-13-3-1-11(2-4-13)16-9-12(5-7-20-16)17-10-14-15(22-17)6-8-21-18(14)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30195

(Pyrrolopyridine, 26)Show SMILES FC(F)(F)c1ccc(cc1)-c1cc(ccn1)-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C19H14F3N3O/c20-19(21,22)13-3-1-11(2-4-13)16-9-12(5-7-23-16)17-10-14-15(25-17)6-8-24-18(14)26/h1-5,7,9-10,25H,6,8H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30309

(Pyrrolo-pyrimidone, 2)Show SMILES Fc1ccc(cc1)-c1cc(ccn1)-c1cc2c(nc[nH]c2=O)[nH]1 Show InChI InChI=1S/C17H11FN4O/c18-12-3-1-10(2-4-12)14-7-11(5-6-19-14)15-8-13-16(22-15)20-9-21-17(13)23/h1-9H,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30310
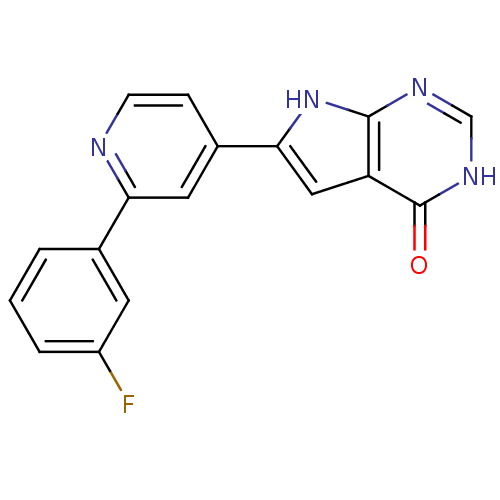
(Pyrrolo-pyrimidone, 3)Show SMILES Fc1cccc(c1)-c1cc(ccn1)-c1cc2c(nc[nH]c2=O)[nH]1 Show InChI InChI=1S/C17H11FN4O/c18-12-3-1-2-10(6-12)14-7-11(4-5-19-14)15-8-13-16(22-15)20-9-21-17(13)23/h1-9H,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30311

(Pyrrolo-pyrimidone, 4)Show SMILES COc1ccc(cc1)-c1cc(ccn1)-c1cc2c(nc[nH]c2=O)[nH]1 Show InChI InChI=1S/C18H14N4O2/c1-24-13-4-2-11(3-5-13)15-8-12(6-7-19-15)16-9-14-17(22-16)20-10-21-18(14)23/h2-10H,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30312

(Pyrrolo-pyrimidone, 5)Show SMILES COc1cccc(c1)-c1cc(ccn1)-c1cc2c(nc[nH]c2=O)[nH]1 Show InChI InChI=1S/C18H14N4O2/c1-24-13-4-2-3-11(7-13)15-8-12(5-6-19-15)16-9-14-17(22-16)20-10-21-18(14)23/h2-10H,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30313
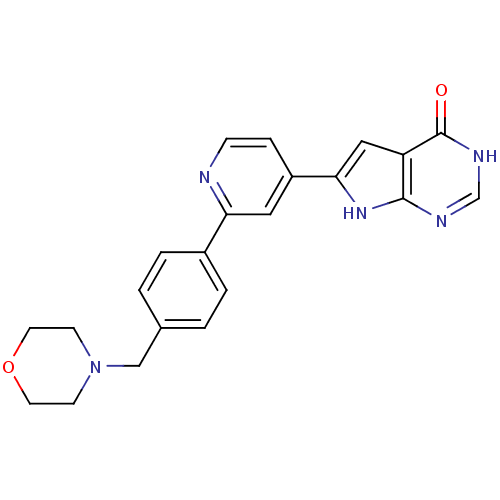
(Pyrrolo-pyrimidone, 6)Show SMILES O=c1[nH]cnc2[nH]c(cc12)-c1ccnc(c1)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C22H21N5O2/c28-22-18-12-20(26-21(18)24-14-25-22)17-5-6-23-19(11-17)16-3-1-15(2-4-16)13-27-7-9-29-10-8-27/h1-6,11-12,14H,7-10,13H2,(H2,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30314
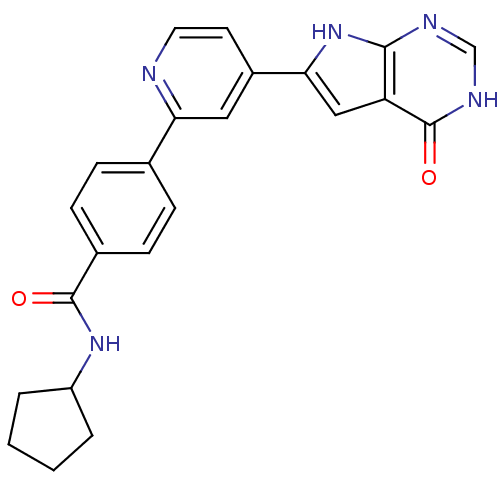
(Pyrrolo-pyrimidone, 7)Show SMILES O=C(NC1CCCC1)c1ccc(cc1)-c1cc(ccn1)-c1cc2c(nc[nH]c2=O)[nH]1 Show InChI InChI=1S/C23H21N5O2/c29-22(27-17-3-1-2-4-17)15-7-5-14(6-8-15)19-11-16(9-10-24-19)20-12-18-21(28-20)25-13-26-23(18)30/h5-13,17H,1-4H2,(H,27,29)(H2,25,26,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30315
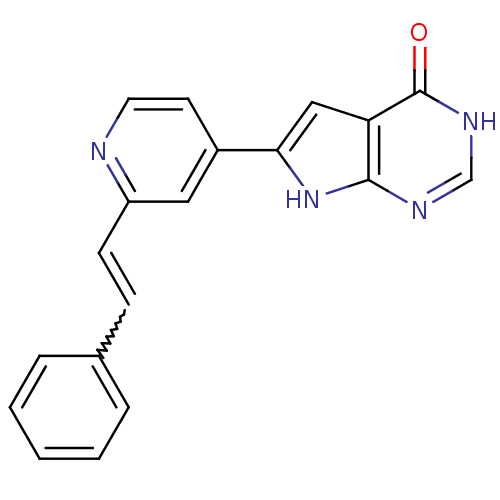
(Pyrrolo-pyrimidone, 8)Show SMILES O=c1[nH]cnc2[nH]c(cc12)-c1ccnc(C=Cc2ccccc2)c1 |w:16.18| Show InChI InChI=1S/C19H14N4O/c24-19-16-11-17(23-18(16)21-12-22-19)14-8-9-20-15(10-14)7-6-13-4-2-1-3-5-13/h1-12H,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30316

(Pyrrolo-pyrimidone, 9)Show SMILES Fc1ccc(C=Cc2cc(ccn2)-c2cc3c(nc[nH]c3=O)[nH]2)cc1 |w:5.4| Show InChI InChI=1S/C19H13FN4O/c20-14-4-1-12(2-5-14)3-6-15-9-13(7-8-21-15)17-10-16-18(24-17)22-11-23-19(16)25/h1-11H,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30317
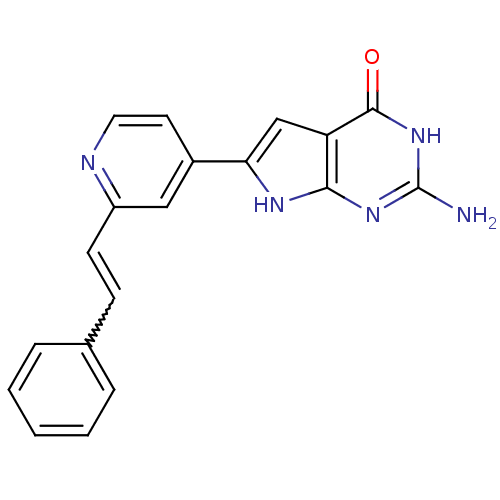
(Pyrrolo-pyrimidone, 10)Show SMILES Nc1nc2[nH]c(cc2c(=O)[nH]1)-c1ccnc(C=Cc2ccccc2)c1 |w:17.19| Show InChI InChI=1S/C19H15N5O/c20-19-23-17-15(18(25)24-19)11-16(22-17)13-8-9-21-14(10-13)7-6-12-4-2-1-3-5-12/h1-11H,(H4,20,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30318
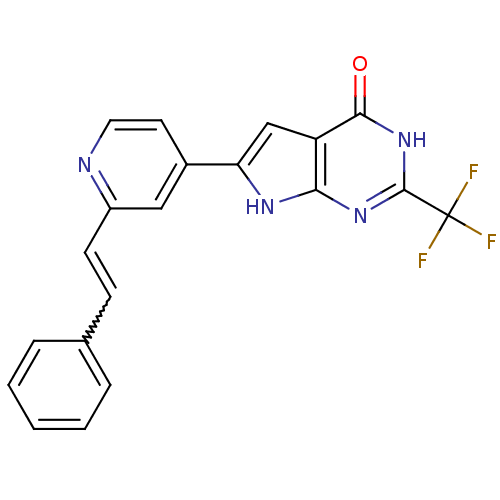
(Pyrrolo-pyrimidone, 11)Show SMILES FC(F)(F)c1nc2[nH]c(cc2c(=O)[nH]1)-c1ccnc(C=Cc2ccccc2)c1 |w:20.22| Show InChI InChI=1S/C20H13F3N4O/c21-20(22,23)19-26-17-15(18(28)27-19)11-16(25-17)13-8-9-24-14(10-13)7-6-12-4-2-1-3-5-12/h1-11H,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30319
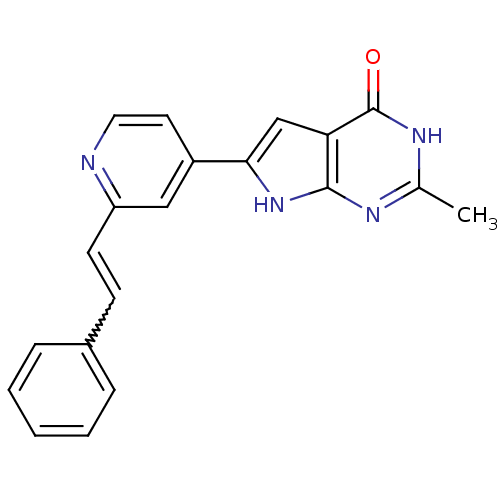
(Pyrrolo-pyrimidone, 12)Show SMILES Cc1nc2[nH]c(cc2c(=O)[nH]1)-c1ccnc(C=Cc2ccccc2)c1 |w:17.19| Show InChI InChI=1S/C20H16N4O/c1-13-22-19-17(20(25)23-13)12-18(24-19)15-9-10-21-16(11-15)8-7-14-5-3-2-4-6-14/h2-12H,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30320

(Pyrrolo-pyrimidone, 13)Show SMILES CCCCc1nc2[nH]c(cc2c(=O)[nH]1)-c1ccnc(C=Cc2ccccc2)c1 |w:20.22| Show InChI InChI=1S/C23H22N4O/c1-2-3-9-21-26-22-19(23(28)27-21)15-20(25-22)17-12-13-24-18(14-17)11-10-16-7-5-4-6-8-16/h4-8,10-15H,2-3,9H2,1H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30321
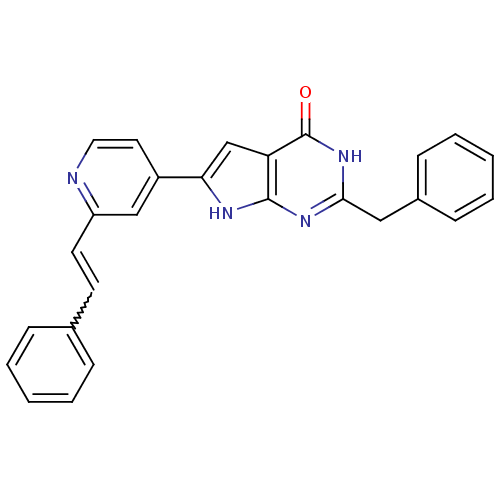
(Pyrrolo-pyrimidone, 14)Show SMILES O=c1[nH]c(Cc2ccccc2)nc2[nH]c(cc12)-c1ccnc(C=Cc2ccccc2)c1 |w:23.26| Show InChI InChI=1S/C26H20N4O/c31-26-22-17-23(28-25(22)29-24(30-26)15-19-9-5-2-6-10-19)20-13-14-27-21(16-20)12-11-18-7-3-1-4-8-18/h1-14,16-17H,15H2,(H2,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30322
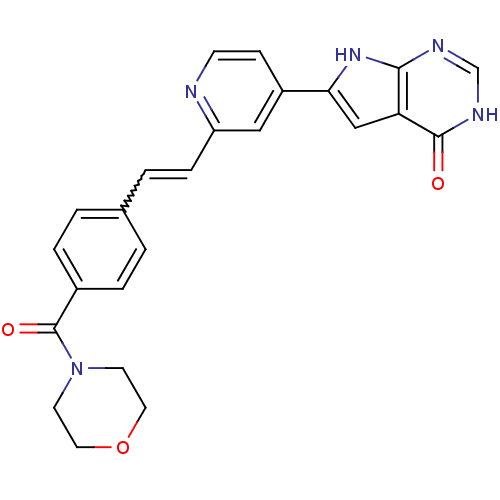
(Pyrrolo-pyrimidone, 15)Show SMILES O=C(N1CCOCC1)c1ccc(C=Cc2cc(ccn2)-c2cc3c(nc[nH]c3=O)[nH]2)cc1 |w:12.12| Show InChI InChI=1S/C24H21N5O3/c30-23-20-14-21(28-22(20)26-15-27-23)18-7-8-25-19(13-18)6-3-16-1-4-17(5-2-16)24(31)29-9-11-32-12-10-29/h1-8,13-15H,9-12H2,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30323
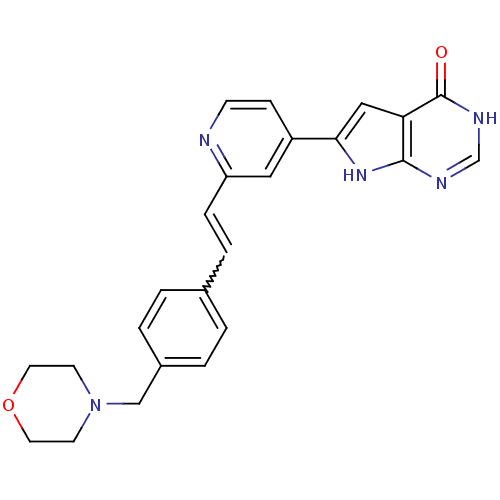
(Pyrrolo-pyrimidone, 16)Show SMILES O=c1[nH]cnc2[nH]c(cc12)-c1ccnc(C=Cc2ccc(CN3CCOCC3)cc2)c1 |w:16.18| Show InChI InChI=1S/C24H23N5O2/c30-24-21-14-22(28-23(21)26-16-27-24)19-7-8-25-20(13-19)6-5-17-1-3-18(4-2-17)15-29-9-11-31-12-10-29/h1-8,13-14,16H,9-12,15H2,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30324

(Pyrrolo-pyrimidone, 17)Show SMILES CN(C)CCOc1ccc(C=Cc2cc(ccn2)-c2cc3c(nc[nH]c3=O)[nH]2)cc1 |w:10.9| Show InChI InChI=1S/C23H23N5O2/c1-28(2)11-12-30-19-7-4-16(5-8-19)3-6-18-13-17(9-10-24-18)21-14-20-22(27-21)25-15-26-23(20)29/h3-10,13-15H,11-12H2,1-2H3,(H2,25,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30325
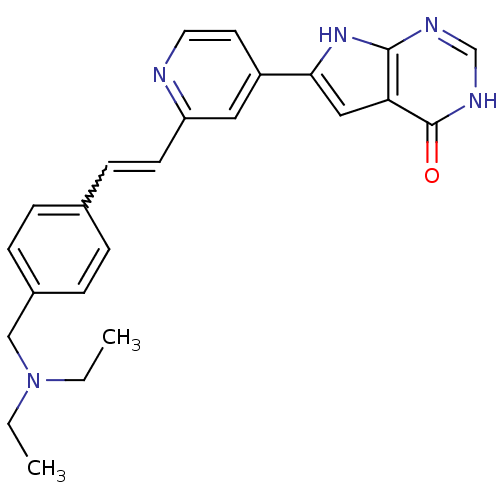
(Pyrrolo-pyrimidone, 18)Show SMILES CCN(CC)Cc1ccc(C=Cc2cc(ccn2)-c2cc3c(nc[nH]c3=O)[nH]2)cc1 |w:10.9| Show InChI InChI=1S/C24H25N5O/c1-3-29(4-2)15-18-7-5-17(6-8-18)9-10-20-13-19(11-12-25-20)22-14-21-23(28-22)26-16-27-24(21)30/h5-14,16H,3-4,15H2,1-2H3,(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
For MK2 inhibition, reactions contained compound or vehicle control, hsp27 peptide substrate and activated MK2 mix containing ATP. After incubation, ... |
Bioorg Med Chem Lett 18: 6142-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.039
BindingDB Entry DOI: 10.7270/Q2V40SKW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM175242
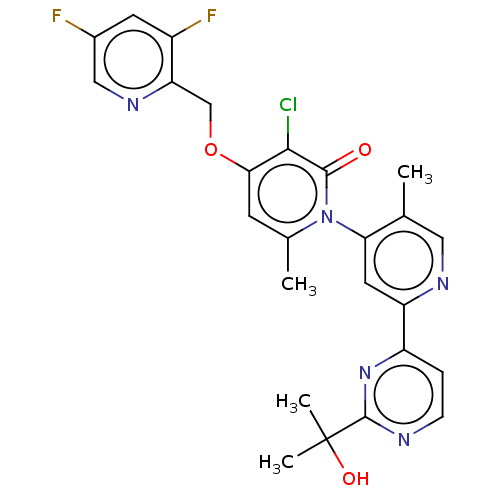
(US11844801, Compound (M)-I | US9115089, 49)Show SMILES Cc1cnc(cc1-n1c(C)cc(OCc2ncc(F)cc2F)c(Cl)c1=O)-c1ccnc(n1)C(C)(C)O |(3.33,-1.54,;2,-2.31,;2,-3.85,;.67,-4.62,;-.67,-3.85,;-.67,-2.31,;.67,-1.54,;.67,,;2,.77,;3.33,,;2,2.31,;.67,3.08,;.67,4.62,;2,5.39,;3.33,4.62,;3.33,3.08,;4.67,2.31,;6,3.08,;7.34,2.31,;6,4.62,;4.67,5.39,;4.67,6.93,;-.67,2.31,;-2,3.08,;-.67,.77,;-2,,;-2,-4.62,;-2,-6.16,;-3.33,-6.93,;-4.67,-6.16,;-4.67,-4.62,;-3.33,-3.85,;-6,-3.85,;-6.77,-2.52,;-5.23,-2.52,;-7.34,-4.62,)| Show InChI InChI=1S/C25H22ClF2N5O3/c1-13-10-30-18(17-5-6-29-24(32-17)25(3,4)35)9-20(13)33-14(2)7-21(22(26)23(33)34)36-12-19-16(28)8-15(27)11-31-19/h5-11,35H,12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | n/a |
CONFLUENCE LIFE SCIENCES, INC.
US Patent
| Assay Description
The ability of compounds to inhibit activated phospho-p38alpha is evaluated using a p38alpha /MK2 and a p38alpha /PRAK cascade assay format. The kina... |
US Patent US9115089 (2015)
BindingDB Entry DOI: 10.7270/Q25M64H1 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM27388

(2-(2-chloropyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,...)Show InChI InChI=1S/C12H10ClN3O/c13-11-5-7(1-3-14-11)10-6-8-9(16-10)2-4-15-12(8)17/h1,3,5-6,16H,2,4H2,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 608 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data