 Found 1142 hits of ic50 data for polymerid = 3611,2223,3611
Found 1142 hits of ic50 data for polymerid = 3611,2223,3611 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50098959

(5-Ethynyl-4,5-dihydro-thieno[2,3-c]pyridin-7-ylami...)Show InChI InChI=1S/C9H8N2S/c1-2-7-5-6-3-4-12-8(6)9(10)11-7/h1,3-4,7H,5H2,(H2,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by n-NOS from rat cerebellum |
Bioorg Med Chem Lett 11: 1027-30 (2001)
BindingDB Entry DOI: 10.7270/Q2F76BT7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091817
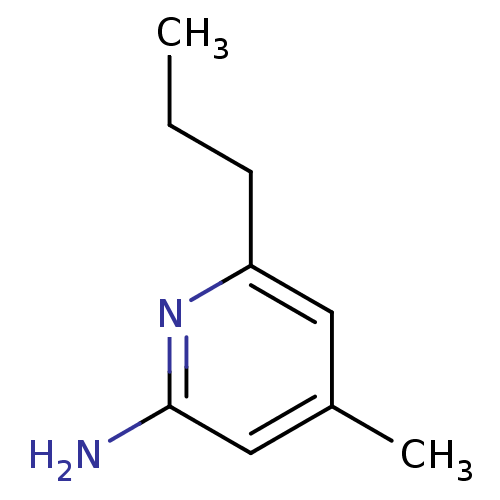
(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase. |
J Med Chem 47: 3320-3 (2004)
Article DOI: 10.1021/jm031035n
BindingDB Entry DOI: 10.7270/Q25M656D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50138977

(CHEMBL419740 | N-(3-Aminomethyl-phenyl)-acetamidin...)Show InChI InChI=1S/C9H13N3/c1-7(11)12-9-4-2-3-8(5-9)6-10/h2-5H,6,10H2,1H3,(H2,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase (nNOS) |
J Med Chem 47: 703-10 (2004)
Article DOI: 10.1021/jm030297m
BindingDB Entry DOI: 10.7270/Q2MC90SQ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50098962
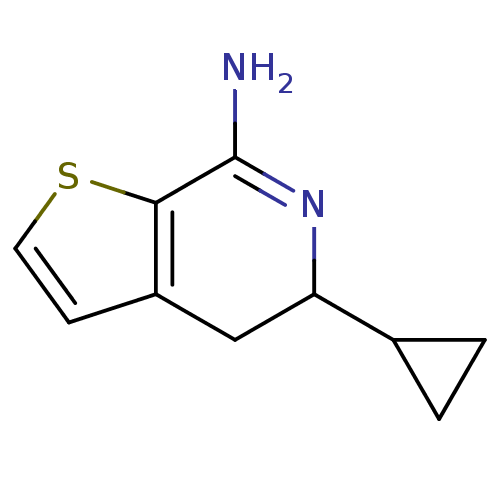
(5-Cyclopropyl-4,5-dihydro-thieno[2,3-c]pyridin-7-y...)Show InChI InChI=1S/C10H12N2S/c11-10-9-7(3-4-13-9)5-8(12-10)6-1-2-6/h3-4,6,8H,1-2,5H2,(H2,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by n-NOS from rat cerebellum |
Bioorg Med Chem Lett 11: 1027-30 (2001)
BindingDB Entry DOI: 10.7270/Q2F76BT7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50363262

(CHEMBL1944717)Show SMILES Fc1ccc2c(CN(c3cccc(Cl)c3)c3cnccn3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H13ClF2N4O/c21-13-2-1-3-14(9-13)27(17-10-24-6-7-25-17)11-12-8-18(28)26-20-15(12)4-5-16(22)19(20)23/h1-10H,11H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Afraxis
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as NO production by 2,3-diaminonapthalene-based fluorescence assay |
Bioorg Med Chem Lett 22: 1237-41 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.073
BindingDB Entry DOI: 10.7270/Q26W9BJ5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM111496
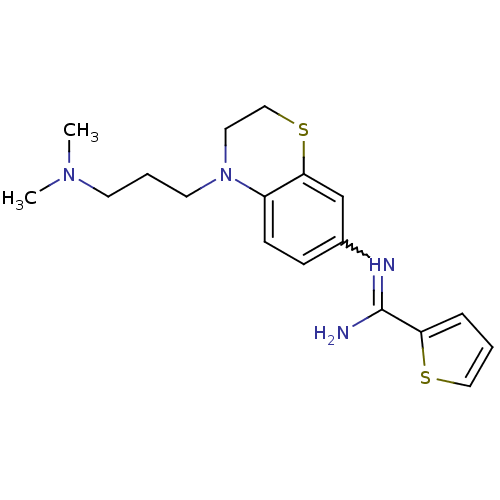
(US8618286, 22)Show SMILES CN(C)CCCN1CCSc2cc(ccc12)N=C(N)c1cccs1 |w:16.17| Show InChI InChI=1S/C18H24N4S2/c1-21(2)8-4-9-22-10-12-24-17-13-14(6-7-15(17)22)20-18(19)16-5-3-11-23-16/h3,5-7,11,13H,4,8-10,12H2,1-2H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc.
US Patent
| Assay Description
Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... |
US Patent US8618286 (2013)
BindingDB Entry DOI: 10.7270/Q2C53JH7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062133
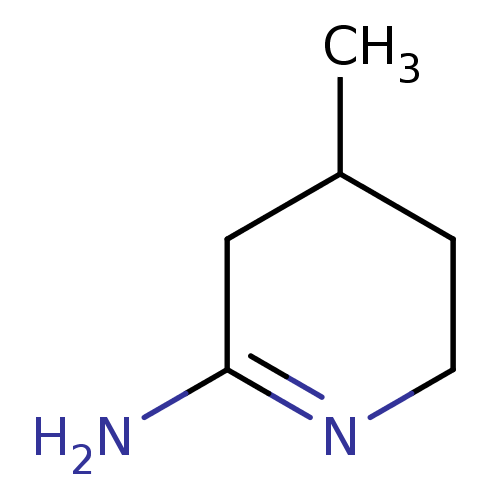
(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50098961
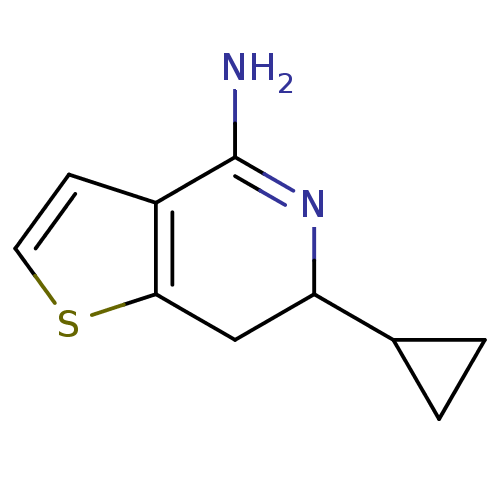
(6-Cyclopropyl-6,7-dihydro-thieno[3,2-c]pyridin-4-y...)Show InChI InChI=1S/C10H12N2S/c11-10-7-3-4-13-9(7)5-8(12-10)6-1-2-6/h3-4,6,8H,1-2,5H2,(H2,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by n-NOS from rat cerebellum |
Bioorg Med Chem Lett 11: 1027-30 (2001)
BindingDB Entry DOI: 10.7270/Q2F76BT7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50352593

(CHEMBL1825174)Show SMILES CN1CCC[C@H]1CCn1ccc2cc(ccc12)N=C(N)c1cccs1 |r,w:17.19| Show InChI InChI=1S/C20H24N4S/c1-23-10-2-4-17(23)9-12-24-11-8-15-14-16(6-7-18(15)24)22-20(21)19-5-3-13-25-19/h3,5-8,11,13-14,17H,2,4,9-10,12H2,1H3,(H2,21,22)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method |
Bioorg Med Chem Lett 21: 5301-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.022
BindingDB Entry DOI: 10.7270/Q2FN16KP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141086

(6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...)Show InChI InChI=1S/C18H23N3O/c1-21(2)11-12-22-17-10-9-14(13-5-3-6-15(13)17)16-7-4-8-18(19)20-16/h4,7-10H,3,5-6,11-12H2,1-2H3,(H2,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091817

(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Chem Biol 4: 700-7 (2008)
Article DOI: 10.1038/nchembio.115
BindingDB Entry DOI: 10.7270/Q2SB443Q |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50098951
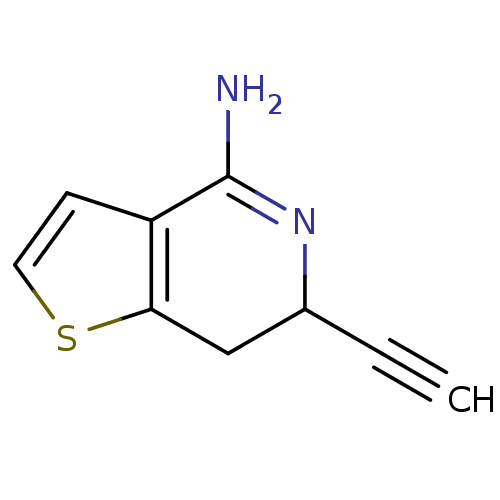
(6-Ethynyl-6,7-dihydro-thieno[3,2-c]pyridin-4-ylami...)Show InChI InChI=1S/C9H8N2S/c1-2-6-5-8-7(3-4-12-8)9(10)11-6/h1,3-4,6H,5H2,(H2,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by n-NOS from rat cerebellum |
Bioorg Med Chem Lett 11: 1027-30 (2001)
BindingDB Entry DOI: 10.7270/Q2F76BT7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM111497
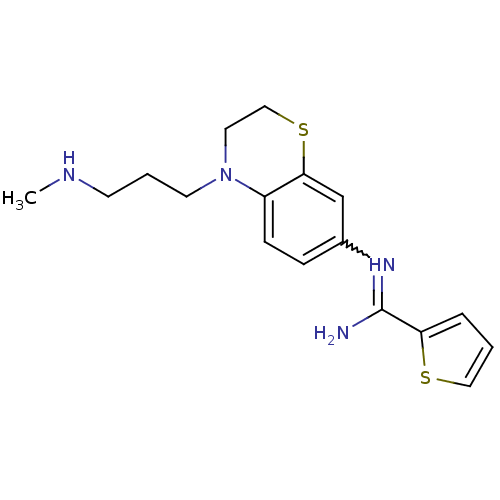
(US8618286, 23)Show SMILES CNCCCN1CCSc2cc(ccc12)N=C(N)c1cccs1 |w:15.16| Show InChI InChI=1S/C17H22N4S2/c1-19-7-3-8-21-9-11-23-16-12-13(5-6-14(16)21)20-17(18)15-4-2-10-22-15/h2,4-6,10,12,19H,3,7-9,11H2,1H3,(H2,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc.
US Patent
| Assay Description
Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... |
US Patent US8618286 (2013)
BindingDB Entry DOI: 10.7270/Q2C53JH7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50206074

((+/-)-N-{2-[2-(1-methyl-pyrrolidin-2-yl)-ethylamin...)Show SMILES CN1CCCC1CCNc1nc2cc(ccc2s1)N=C(N)c1cccs1 |w:18.20| Show InChI InChI=1S/C19H23N5S2/c1-24-10-2-4-14(24)8-9-21-19-23-15-12-13(6-7-16(15)26-19)22-18(20)17-5-3-11-25-17/h3,5-7,11-12,14H,2,4,8-10H2,1H3,(H2,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal NOS activity |
Bioorg Med Chem Lett 17: 2540-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.011
BindingDB Entry DOI: 10.7270/Q2WM1F6B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777
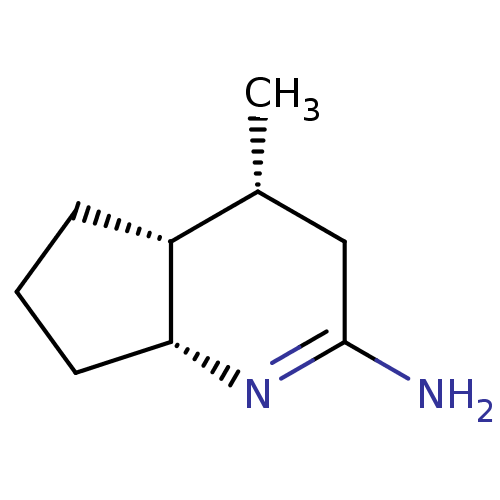
((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM111490
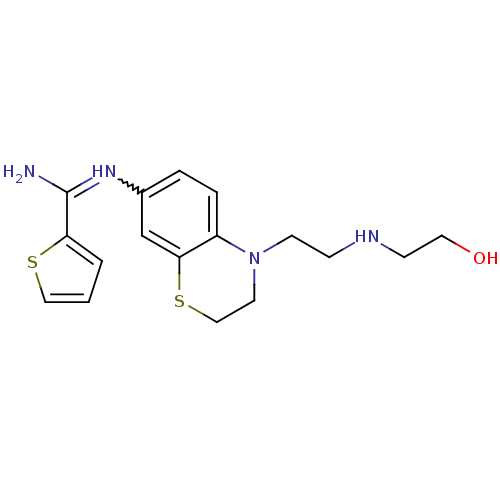
(US8618286, 16)Show SMILES NC(=Nc1ccc2N(CCNCCO)CCSc2c1)c1cccs1 |w:2.2| Show InChI InChI=1S/C17H22N4OS2/c18-17(15-2-1-10-23-15)20-13-3-4-14-16(12-13)24-11-8-21(14)7-5-19-6-9-22/h1-4,10,12,19,22H,5-9,11H2,(H2,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc.
US Patent
| Assay Description
Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... |
US Patent US8618286 (2013)
BindingDB Entry DOI: 10.7270/Q2C53JH7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141082
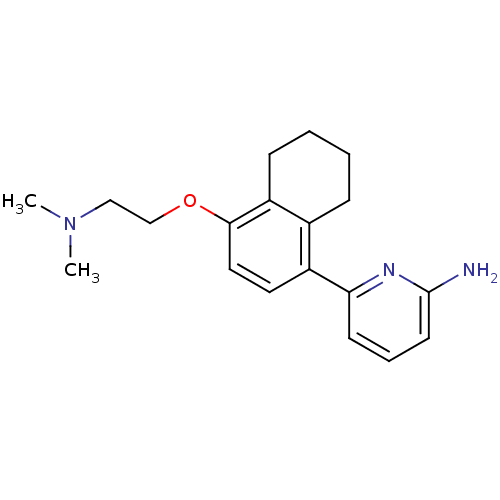
(6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C19H25N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h5,8-11H,3-4,6-7,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50122307
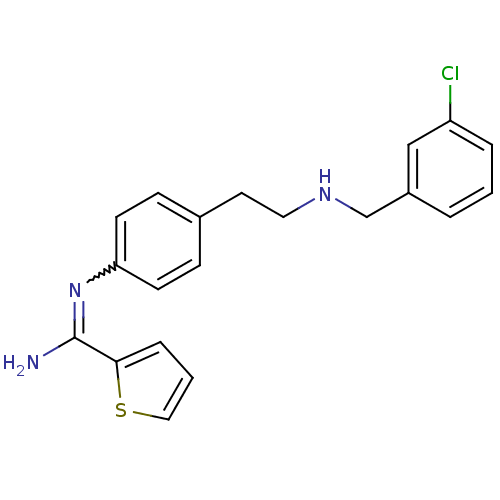
(CHEMBL293212 | N-{4-[2-(3-Chloro-benzylamino)-ethy...)Show SMILES NC(=Nc1ccc(CCNCc2cccc(Cl)c2)cc1)c1cccs1 |w:2.2| Show InChI InChI=1S/C20H20ClN3S/c21-17-4-1-3-16(13-17)14-23-11-10-15-6-8-18(9-7-15)24-20(22)19-5-2-12-25-19/h1-9,12-13,23H,10-11,14H2,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human (neuronal nitric oxide synthase) n-NOS |
Bioorg Med Chem Lett 13: 1981-4 (2003)
BindingDB Entry DOI: 10.7270/Q2FN16QG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50430743
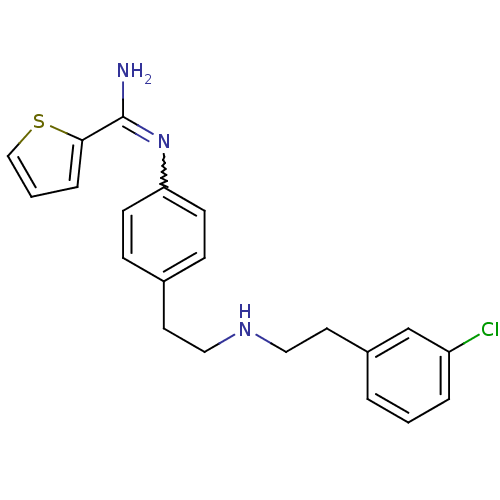
(CHEMBL2333953)Show SMILES NC(=Nc1ccc(CCNCCc2cccc(Cl)c2)cc1)c1cccs1 |w:2.2| Show InChI InChI=1S/C21H22ClN3S/c22-18-4-1-3-17(15-18)11-13-24-12-10-16-6-8-19(9-7-16)25-21(23)20-5-2-14-26-20/h1-9,14-15,24H,10-13H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS |
J Med Chem 56: 3121-47 (2013)
Article DOI: 10.1021/jm3015926
BindingDB Entry DOI: 10.7270/Q23T9JKG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50122307

(CHEMBL293212 | N-{4-[2-(3-Chloro-benzylamino)-ethy...)Show SMILES NC(=Nc1ccc(CCNCc2cccc(Cl)c2)cc1)c1cccs1 |w:2.2| Show InChI InChI=1S/C20H20ClN3S/c21-17-4-1-3-16(13-17)14-23-11-10-15-6-8-18(9-7-15)24-20(22)19-5-2-12-25-19/h1-9,12-13,23H,10-11,14H2,(H2,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as NO production by hemoglobin capture assay |
J Med Chem 57: 1513-30 (2014)
Article DOI: 10.1021/jm401838x
BindingDB Entry DOI: 10.7270/Q2MP54SZ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM196649
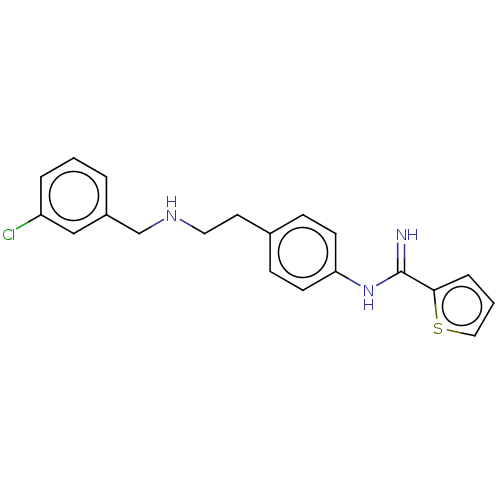
(US9212144, 4)Show InChI InChI=1S/C20H20ClN3S/c21-17-4-1-3-16(13-17)14-23-11-10-15-6-8-18(9-7-15)24-20(22)19-5-2-12-25-19/h1-9,12-13,23H,10-11,14H2,(H2,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| DrugBank
PDB
US Patent
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Northwestern University
US Patent
| Assay Description
To test for enzyme inhibition, the hemoglobin capture assay was used to measure nitric oxide production. The assay was performed at 37° C. in HEPES b... |
US Patent US9212144 (2015)
BindingDB Entry DOI: 10.7270/Q2K0732P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164784
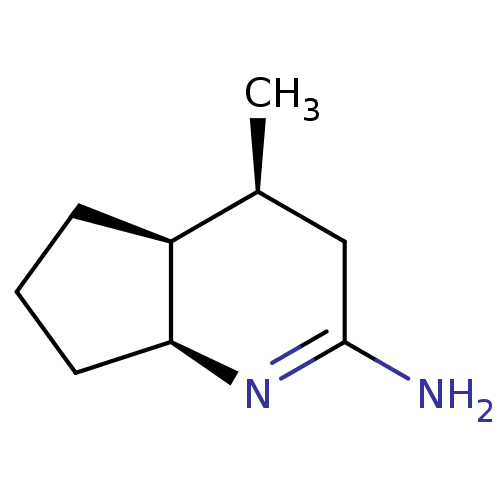
((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50098950
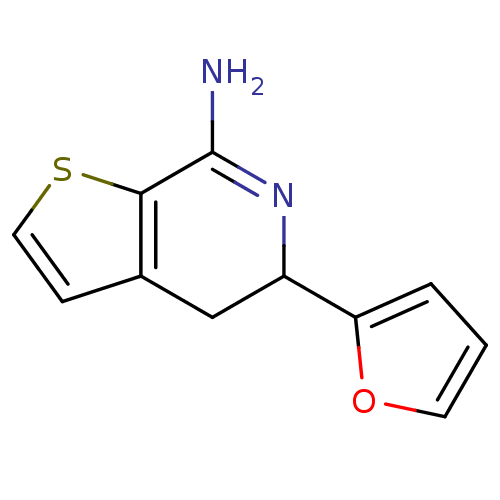
(5-Furan-2-yl-4,5-dihydro-thieno[2,3-c]pyridin-7-yl...)Show InChI InChI=1S/C11H10N2OS/c12-11-10-7(3-5-15-10)6-8(13-11)9-2-1-4-14-9/h1-5,8H,6H2,(H2,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by n-NOS from rat cerebellum |
Bioorg Med Chem Lett 11: 1027-30 (2001)
BindingDB Entry DOI: 10.7270/Q2F76BT7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50138977

(CHEMBL419740 | N-(3-Aminomethyl-phenyl)-acetamidin...)Show InChI InChI=1S/C9H13N3/c1-7(11)12-9-4-2-3-8(5-9)6-10/h2-5H,6,10H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS |
J Med Chem 56: 3121-47 (2013)
Article DOI: 10.1021/jm3015926
BindingDB Entry DOI: 10.7270/Q23T9JKG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50401280
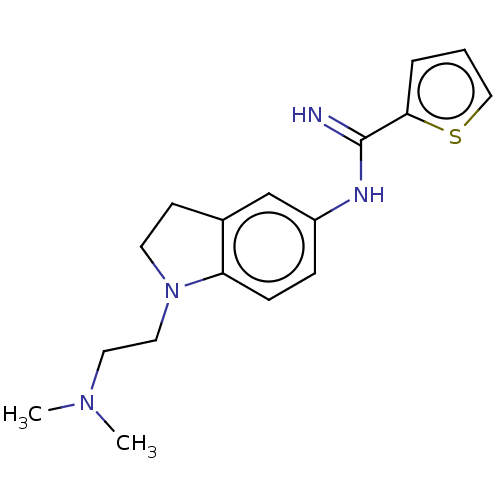
(CHEMBL3216124)Show InChI InChI=1S/C17H22N4S/c1-20(2)9-10-21-8-7-13-12-14(5-6-15(13)21)19-17(18)16-4-3-11-22-16/h3-6,11-12H,7-10H2,1-2H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human nNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of [3H]-larginine to [3H]-L-citrulline preinc... |
Eur J Med Chem 55: 94-107 (2012)
Article DOI: 10.1016/j.ejmech.2012.07.006
BindingDB Entry DOI: 10.7270/Q2GQ6ZWQ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50091800
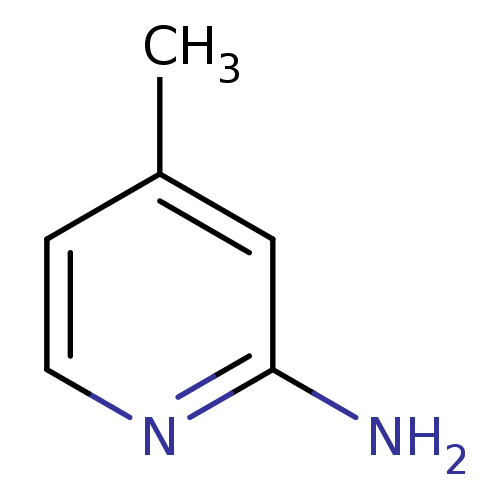
(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50128920
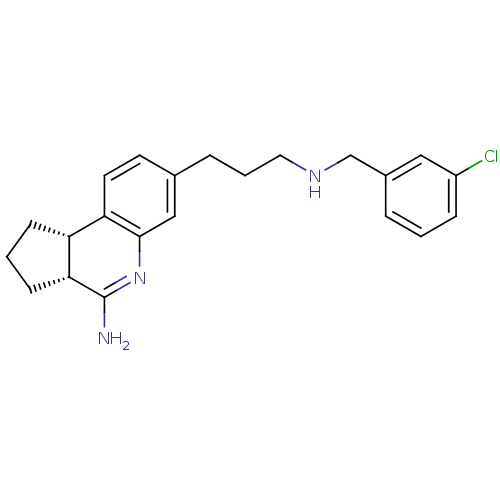
((3R,3aS)-7-[3-(3-Chloro-benzylamino)-propyl]-2,3,3...)Show SMILES NC1=Nc2cc(CCCNCc3cccc(Cl)c3)ccc2[C@H]2CCC[C@@H]12 |t:1| Show InChI InChI=1S/C22H26ClN3/c23-17-6-1-4-16(12-17)14-25-11-3-5-15-9-10-19-18-7-2-8-20(18)22(24)26-21(19)13-15/h1,4,6,9-10,12-13,18,20,25H,2-3,5,7-8,11,14H2,(H2,24,26)/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 743-6 (2004)
BindingDB Entry DOI: 10.7270/Q2GB23F7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50128920

((3R,3aS)-7-[3-(3-Chloro-benzylamino)-propyl]-2,3,3...)Show SMILES NC1=Nc2cc(CCCNCc3cccc(Cl)c3)ccc2[C@H]2CCC[C@@H]12 |t:1| Show InChI InChI=1S/C22H26ClN3/c23-17-6-1-4-16(12-17)14-25-11-3-5-15-9-10-19-18-7-2-8-20(18)22(24)26-21(19)13-15/h1,4,6,9-10,12-13,18,20,25H,2-3,5,7-8,11,14H2,(H2,24,26)/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human (neuronal nitric oxide synthase) n-NOS |
Bioorg Med Chem Lett 13: 1981-4 (2003)
BindingDB Entry DOI: 10.7270/Q2FN16QG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50363270
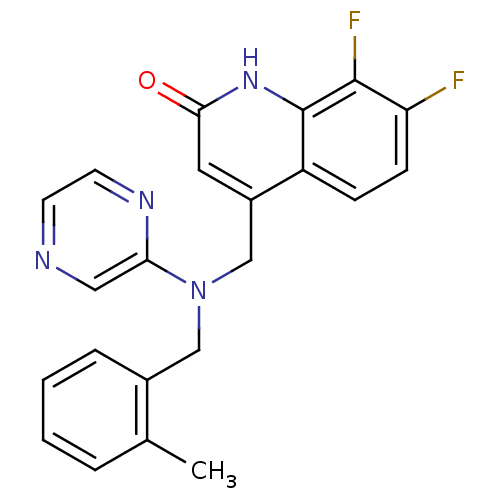
(CHEMBL1944884)Show SMILES Cc1ccccc1CN(Cc1cc(=O)[nH]c2c(F)c(F)ccc12)c1cnccn1 Show InChI InChI=1S/C22H18F2N4O/c1-14-4-2-3-5-15(14)12-28(19-11-25-8-9-26-19)13-16-10-20(29)27-22-17(16)6-7-18(23)21(22)24/h2-11H,12-13H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Afraxis
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as NO production by 2,3-diaminonapthalene-based fluorescence assay |
Bioorg Med Chem Lett 22: 1237-41 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.073
BindingDB Entry DOI: 10.7270/Q26W9BJ5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141081

(6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...)Show InChI InChI=1S/C20H21N3O/c1-23-12-11-14(13-23)24-19-10-9-16(15-5-2-3-6-17(15)19)18-7-4-8-20(21)22-18/h2-10,14H,11-13H2,1H3,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50128916
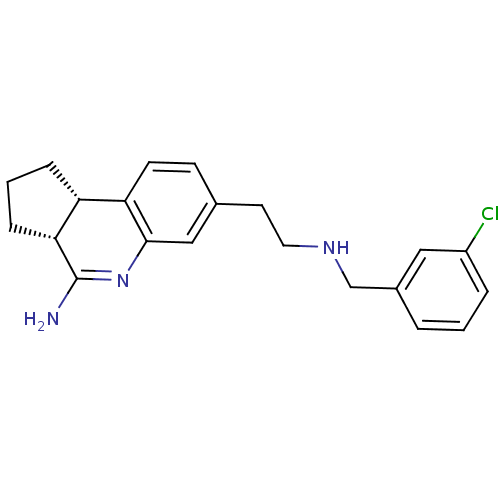
((3aR,9bS)-7-[2-(3-Chloro-benzylamino)-ethyl]-2,3,3...)Show SMILES NC1=Nc2cc(CCNCc3cccc(Cl)c3)ccc2[C@H]2CCC[C@@H]12 |t:1| Show InChI InChI=1S/C21H24ClN3/c22-16-4-1-3-15(11-16)13-24-10-9-14-7-8-18-17-5-2-6-19(17)21(23)25-20(18)12-14/h1,3-4,7-8,11-12,17,19,24H,2,5-6,9-10,13H2,(H2,23,25)/t17-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human (neuronal nitric oxide synthase) n-NOS |
Bioorg Med Chem Lett 13: 1981-4 (2003)
BindingDB Entry DOI: 10.7270/Q2FN16QG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50098953
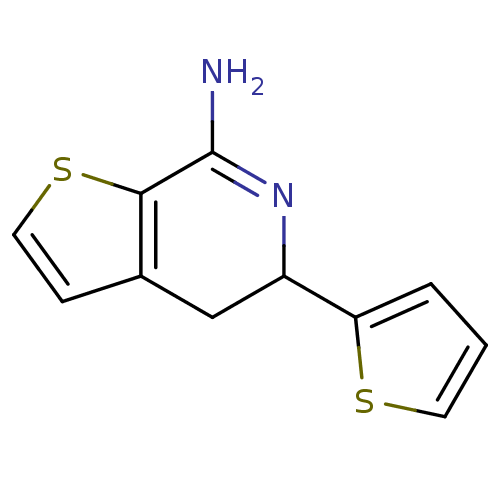
(5-Thiophen-2-yl-4,5-dihydro-thieno[2,3-c]pyridin-7...)Show InChI InChI=1S/C11H10N2S2/c12-11-10-7(3-5-15-10)6-8(13-11)9-2-1-4-14-9/h1-5,8H,6H2,(H2,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Ability to inhibit the conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by n-NOS from rat cerebellum |
Bioorg Med Chem Lett 11: 1027-30 (2001)
BindingDB Entry DOI: 10.7270/Q2F76BT7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM142057
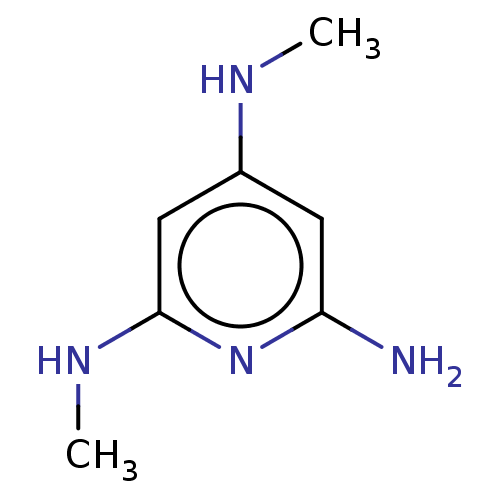
(US8927730, Aminopyridinea)Show InChI InChI=1S/C7H12N4/c1-9-5-3-6(8)11-7(4-5)10-2/h3-4H,1-2H3,(H4,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Northwestern University
US Patent
| Assay Description
The three NOS isoforms, rat nNOS, murine iNOS and bovine eNOS were recombinant enzymes overexpressed in E. coli and purified as reported in the liter... |
US Patent US8927730 (2015)
BindingDB Entry DOI: 10.7270/Q2610Z1V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM36402

((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...)Show InChI InChI=1S/C10H14N2OS/c1-2-3-7-6-13-8-4-5-14-9(8)10(11)12-7/h4-5,7H,2-3,6H2,1H3,(H2,11,12)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Chem Biol 4: 700-7 (2008)
Article DOI: 10.1038/nchembio.115
BindingDB Entry DOI: 10.7270/Q2SB443Q |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50206073

(CHEMBL233652 | N-[2-(2-pyridin-2-yl-ethylamino)-be...)Show SMILES NC(=Nc1ccc2nc(NCCc3ccccn3)sc2c1)c1cccs1 |w:2.2| Show InChI InChI=1S/C19H17N5S2/c20-18(16-5-3-11-25-16)23-14-6-7-15-17(12-14)26-19(24-15)22-10-8-13-4-1-2-9-21-13/h1-7,9,11-12H,8,10H2,(H2,20,23)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat neuronal NOS activity |
Bioorg Med Chem Lett 17: 2540-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.011
BindingDB Entry DOI: 10.7270/Q2WM1F6B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50065843
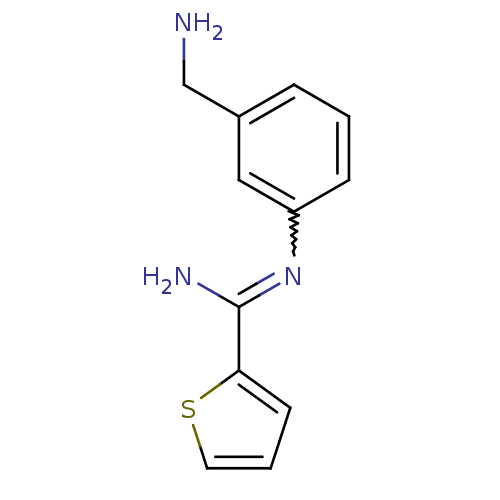
(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum |
Bioorg Med Chem Lett 13: 209-12 (2002)
BindingDB Entry DOI: 10.7270/Q2K9382B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat nNOS expressed in Escherichia coli by hemoglobin capture assay |
Bioorg Med Chem 20: 2435-43 (2012)
Article DOI: 10.1016/j.bmc.2012.01.037
BindingDB Entry DOI: 10.7270/Q26110TZ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM111498
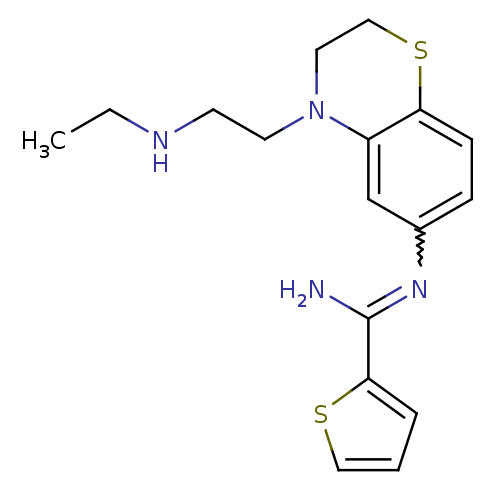
(US8618286, 24)Show SMILES CCNCCN1CCSc2ccc(cc12)N=C(N)c1cccs1 |w:15.16| Show InChI InChI=1S/C17H22N4S2/c1-2-19-7-8-21-9-11-23-15-6-5-13(12-14(15)21)20-17(18)16-4-3-10-22-16/h3-6,10,12,19H,2,7-9,11H2,1H3,(H2,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc.
US Patent
| Assay Description
Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... |
US Patent US8618286 (2013)
BindingDB Entry DOI: 10.7270/Q2C53JH7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141086

(6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...)Show InChI InChI=1S/C18H23N3O/c1-21(2)11-12-22-17-10-9-14(13-5-3-6-15(13)17)16-7-4-8-18(19)20-16/h4,7-10H,3,5-6,11-12H2,1-2H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150885
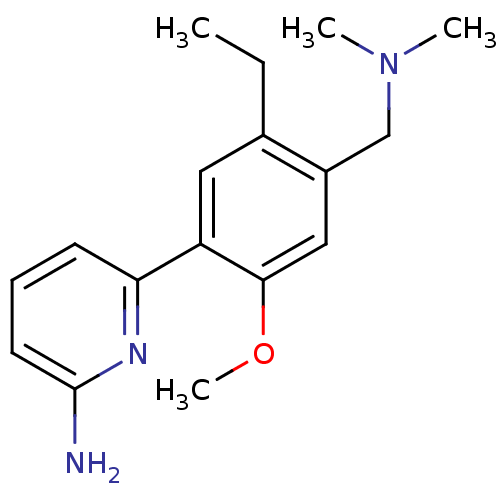
(6-(4-Dimethylaminomethyl-5-ethyl-2-methoxy-phenyl)...)Show InChI InChI=1S/C17H23N3O/c1-5-12-9-14(15-7-6-8-17(18)19-15)16(21-4)10-13(12)11-20(2)3/h6-10H,5,11H2,1-4H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150887
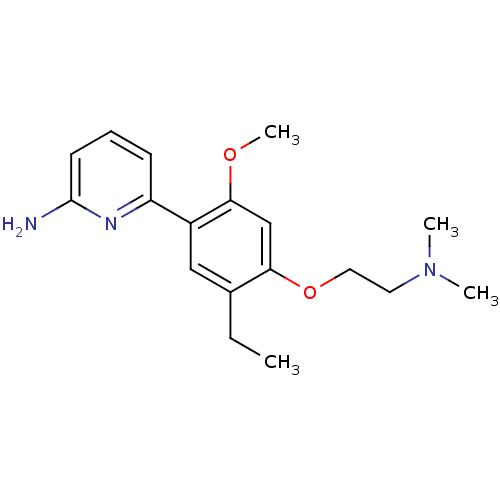
(6-[4-(2-Dimethylamino-ethoxy)-5-ethyl-2-methoxy-ph...)Show InChI InChI=1S/C18H25N3O2/c1-5-13-11-14(15-7-6-8-18(19)20-15)17(22-4)12-16(13)23-10-9-21(2)3/h6-8,11-12H,5,9-10H2,1-4H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141089

(6-[7-(2-Pyrrolidin-1-yl-ethoxy)-indan-4-yl]-pyridi...)Show InChI InChI=1S/C20H25N3O/c21-20-8-4-7-18(22-20)16-9-10-19(17-6-3-5-15(16)17)24-14-13-23-11-1-2-12-23/h4,7-10H,1-3,5-6,11-14H2,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50363266

(CHEMBL1944721)Show SMILES Fc1ccc2c(CN(Cc3ccccc3Cl)c3cnccn3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C21H15ClF2N4O/c22-16-4-2-1-3-13(16)11-28(18-10-25-7-8-26-18)12-14-9-19(29)27-21-15(14)5-6-17(23)20(21)24/h1-10H,11-12H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Afraxis
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as NO production by 2,3-diaminonapthalene-based fluorescence assay |
Bioorg Med Chem Lett 22: 1237-41 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.073
BindingDB Entry DOI: 10.7270/Q26W9BJ5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50352592
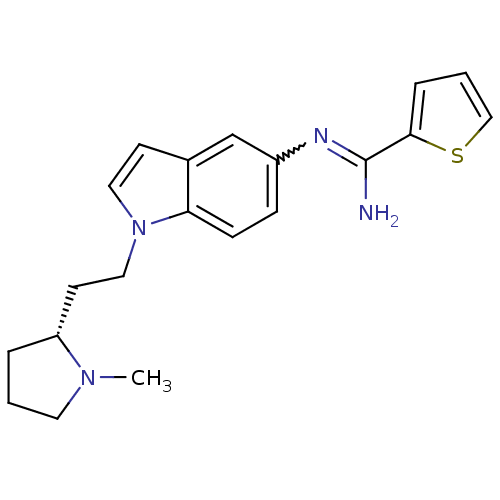
(CHEMBL1825173)Show SMILES CN1CCC[C@@H]1CCn1ccc2cc(ccc12)N=C(N)c1cccs1 |r,w:17.19| Show InChI InChI=1S/C20H24N4S/c1-23-10-2-4-17(23)9-12-24-11-8-15-14-16(6-7-18(15)24)22-20(21)19-5-3-13-25-19/h3,5-8,11,13-14,17H,2,4,9-10,12H2,1H3,(H2,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method |
Bioorg Med Chem Lett 21: 5301-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.022
BindingDB Entry DOI: 10.7270/Q2FN16KP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50138983

((S)-2-Amino-5-(2-methyl-isothioureido)-pentanoic a...)Show InChI InChI=1S/C7H15N3O2S/c1-13-7(9)10-4-2-3-5(8)6(11)12/h5H,2-4,8H2,1H3,(H2,9,10)(H,11,12)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human neuronal nitric oxide synthase expressed in Sf-9 cells |
Bioorg Med Chem Lett 15: 2881-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.078
BindingDB Entry DOI: 10.7270/Q2PK0FP1 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM111482

(US8618286, 8)Show SMILES CNCCN1CCSc2cc(ccc12)N=C(N)c1cccs1 |w:14.15| Show InChI InChI=1S/C16H20N4S2/c1-18-6-7-20-8-10-22-15-11-12(4-5-13(15)20)19-16(17)14-3-2-9-21-14/h2-5,9,11,18H,6-8,10H2,1H3,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc.
US Patent
| Assay Description
Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... |
US Patent US8618286 (2013)
BindingDB Entry DOI: 10.7270/Q2C53JH7 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50124519

(5,8-Difluoro-2-furan-2-yl-1,2-dihydro-quinazolin-4...)Show InChI InChI=1S/C12H9F2N3O/c13-6-3-4-7(14)10-9(6)11(15)17-12(16-10)8-2-1-5-18-8/h1-5,12,16H,(H2,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data