 Found 239 hits with Last Name = 'lingel' and Initial = 'a'
Found 239 hits with Last Name = 'lingel' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM85212
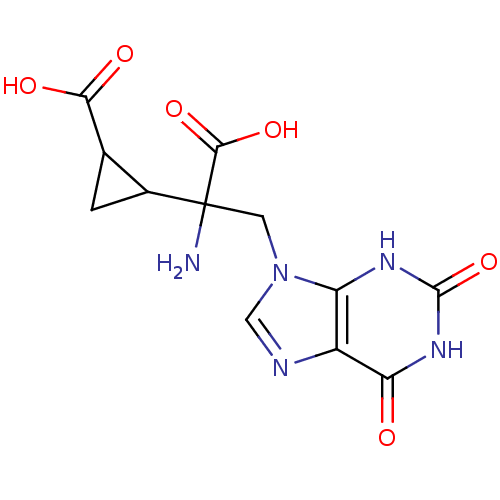
(CAS_5311260 | LY341495 | NSC_5311260)Show SMILES NC(Cn1cnc2c1[nH]c(=O)[nH]c2=O)(C1CC1C(O)=O)C(O)=O Show InChI InChI=1S/C12H13N5O6/c13-12(10(21)22,5-1-4(5)9(19)20)2-17-3-14-6-7(17)15-11(23)16-8(6)18/h3-5H,1-2,13H2,(H,19,20)(H,21,22)(H2,15,16,18,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50034503
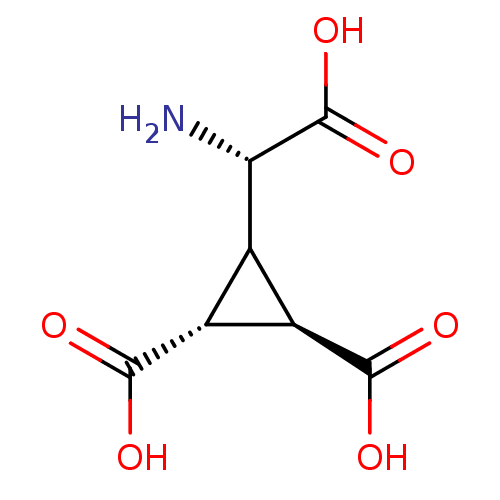
((1R,2R)-3-((S)-Amino-carboxy-methyl)-cyclopropane-...)Show SMILES N[C@@H](C1[C@H]([C@@H]1C(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C7H9NO6/c8-4(7(13)14)1-2(5(9)10)3(1)6(11)12/h1-4H,8H2,(H,9,10)(H,11,12)(H,13,14)/t2-,3-,4+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50004899
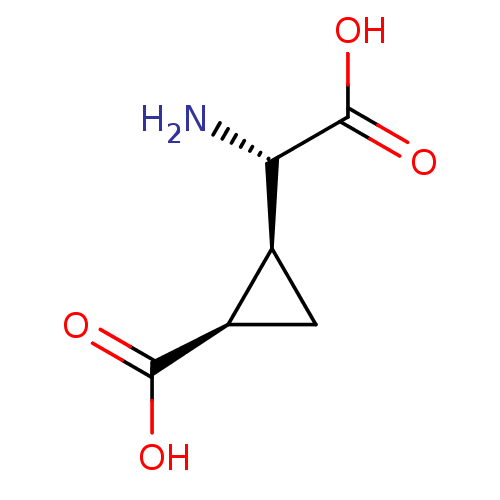
((1R,2S)-2-((S)-Amino-carboxy-methyl)-cyclopropanec...)Show InChI InChI=1S/C6H9NO4/c7-4(6(10)11)2-1-3(2)5(8)9/h2-4H,1,7H2,(H,8,9)(H,10,11)/t2-,3+,4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50080029

(4-((1R,2S)-3-(4-benzylpiperidin-1-yl)-1-hydroxy-2-...)Show SMILES C[C@@H](CN1CCC(Cc2ccccc2)CC1)[C@@H](O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C22H29NO2/c1-17(22(25)20-7-9-21(24)10-8-20)16-23-13-11-19(12-14-23)15-18-5-3-2-4-6-18/h2-10,17,19,22,24-25H,11-16H2,1H3/t17-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50032651
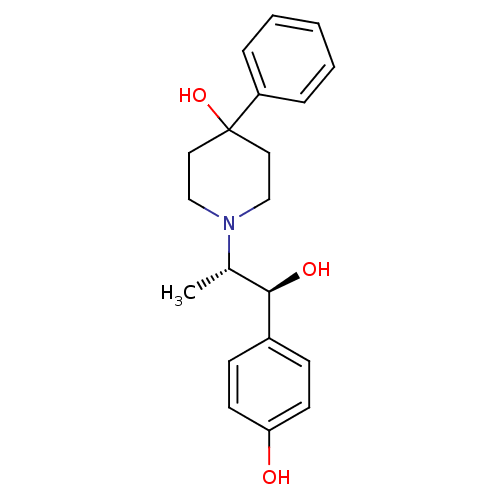
(1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...)Show SMILES C[C@@H]([C@@H](O)c1ccc(O)cc1)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C20H25NO3/c1-15(19(23)16-7-9-18(22)10-8-16)21-13-11-20(24,12-14-21)17-5-3-2-4-6-17/h2-10,15,19,22-24H,11-14H2,1H3/t15-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50004863
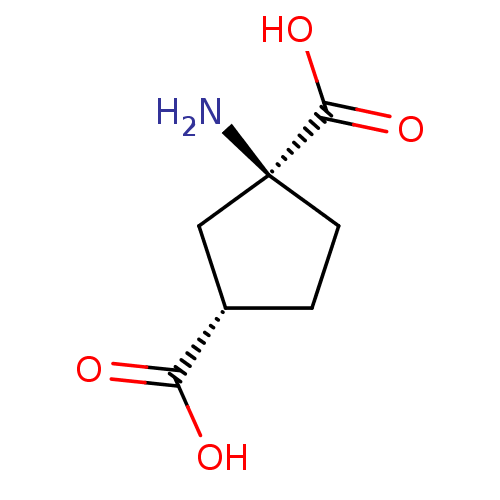
((+/-)-trans-ACPD | (1S,3S)-1-Amino-cyclopentane-1,...)Show InChI InChI=1S/C7H11NO4/c8-7(6(11)12)2-1-4(3-7)5(9)10/h4H,1-3,8H2,(H,9,10)(H,11,12)/t4-,7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM85212

(CAS_5311260 | LY341495 | NSC_5311260)Show SMILES NC(Cn1cnc2c1[nH]c(=O)[nH]c2=O)(C1CC1C(O)=O)C(O)=O Show InChI InChI=1S/C12H13N5O6/c13-12(10(21)22,5-1-4(5)9(19)20)2-17-3-14-6-7(17)15-11(23)16-8(6)18/h3-5H,1-2,13H2,(H,19,20)(H,21,22)(H2,15,16,18,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50007674

((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...)Show SMILES C[C@@H]([C@H](O)c1ccc(O)cc1)N1CCC(Cc2ccccc2)CC1 Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3/t16-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM85221
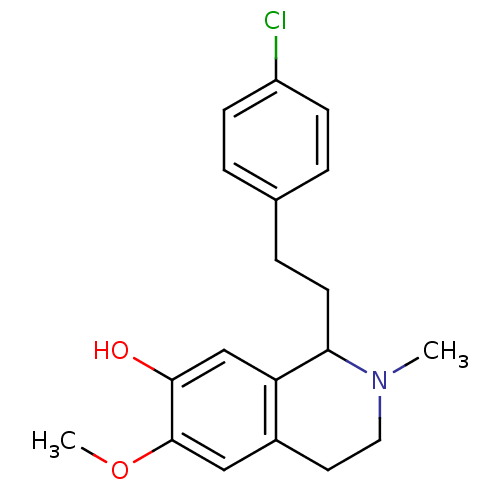
(Ro 04-5595)Show InChI InChI=1S/C19H22ClNO2/c1-21-10-9-14-11-19(23-2)18(22)12-16(14)17(21)8-5-13-3-6-15(20)7-4-13/h3-4,6-7,11-12,17,22H,5,8-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50034503

((1R,2R)-3-((S)-Amino-carboxy-methyl)-cyclopropane-...)Show SMILES N[C@@H](C1[C@H]([C@@H]1C(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C7H9NO6/c8-4(7(13)14)1-2(5(9)10)3(1)6(11)12/h1-4H,8H2,(H,9,10)(H,11,12)(H,13,14)/t2-,3-,4+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50062500
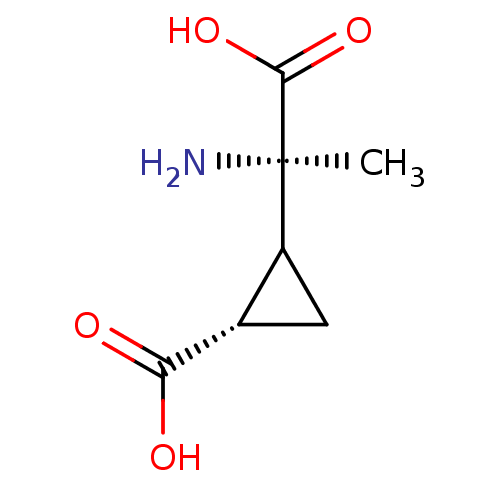
((S)-2-((R)-1-Amino-1-carboxy-ethyl)-cyclopropaneca...)Show InChI InChI=1S/C7H11NO4/c1-7(8,6(11)12)4-2-3(4)5(9)10/h3-4H,2,8H2,1H3,(H,9,10)(H,11,12)/t3-,4?,7+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50004899

((1R,2S)-2-((S)-Amino-carboxy-methyl)-cyclopropanec...)Show InChI InChI=1S/C6H9NO4/c7-4(6(10)11)2-1-3(2)5(8)9/h2-4H,1,7H2,(H,8,9)(H,10,11)/t2-,3+,4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50013055
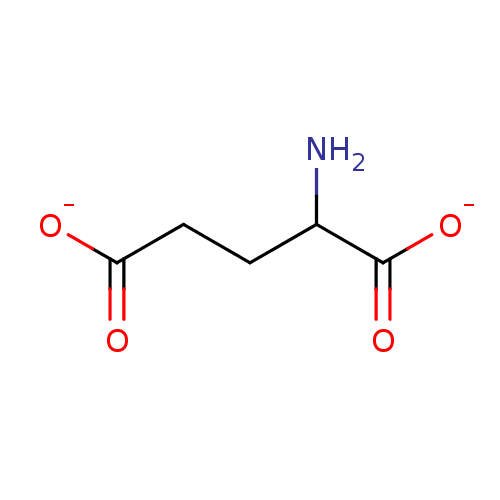
(2-aminopentanedioateglutamate | L-Glutamate | glut...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/p-2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM82355
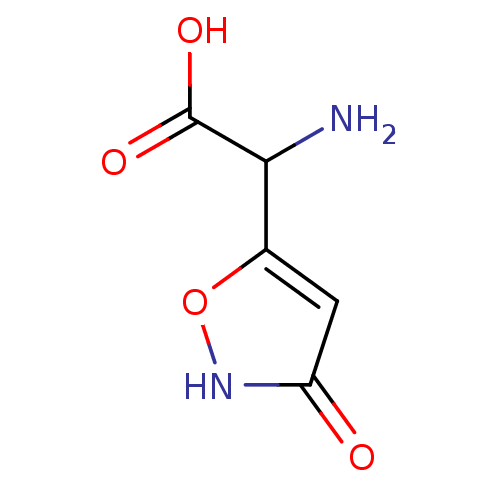
(Amino-(3-hydroxy-isoxazol-5-yl)-acetic acid(Iboten...)Show InChI InChI=1S/C5H6N2O4/c6-4(5(9)10)2-1-3(8)7-11-2/h1,4H,6H2,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM17660
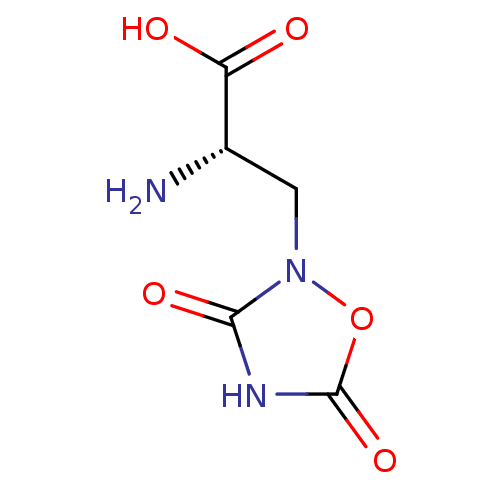
((2S)-2-amino-3-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl...)Show InChI InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50089910
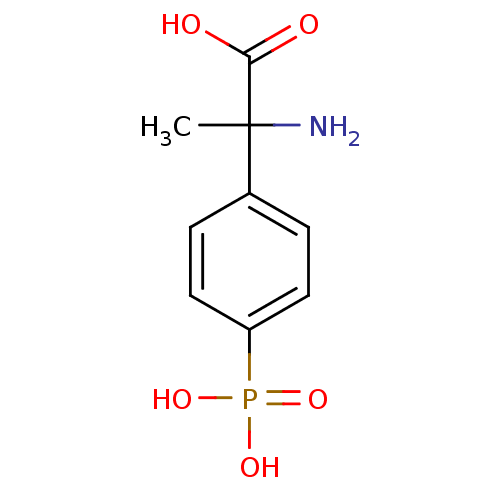
(2-Amino-2-(4-phosphono-phenyl)-propionic acid | 2-...)Show InChI InChI=1S/C9H12NO5P/c1-9(10,8(11)12)6-2-4-7(5-3-6)16(13,14)15/h2-5H,10H2,1H3,(H,11,12)(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM85077
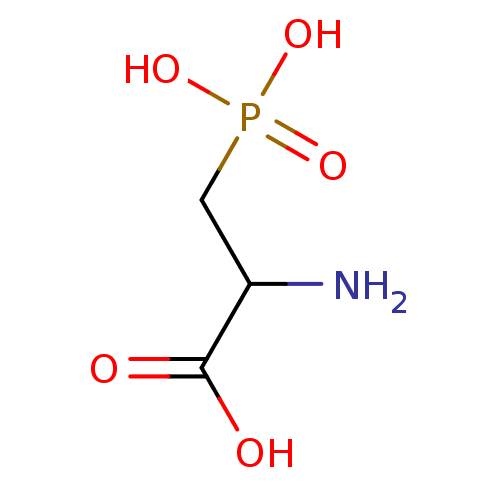
(3-Phosphono-D,L-2-aminopropionic acid, 7 | CAS_230...)Show InChI InChI=1S/C3H8NO5P/c4-2(3(5)6)1-10(7,8)9/h2H,1,4H2,(H,5,6)(H2,7,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50079387
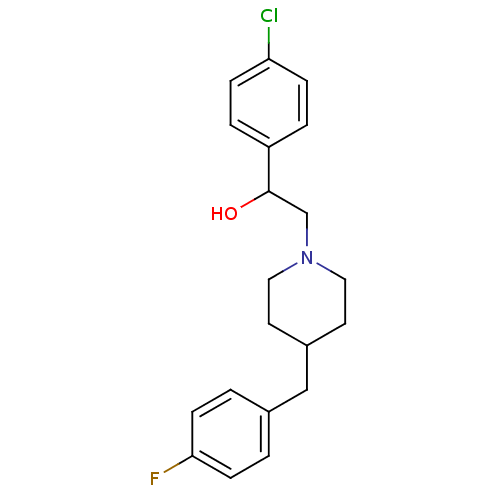
(1-(4-Chloro-phenyl)-2-[4-(4-fluoro-benzyl)-piperid...)Show InChI InChI=1S/C20H23ClFNO/c21-18-5-3-17(4-6-18)20(24)14-23-11-9-16(10-12-23)13-15-1-7-19(22)8-2-15/h1-8,16,20,24H,9-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50030627
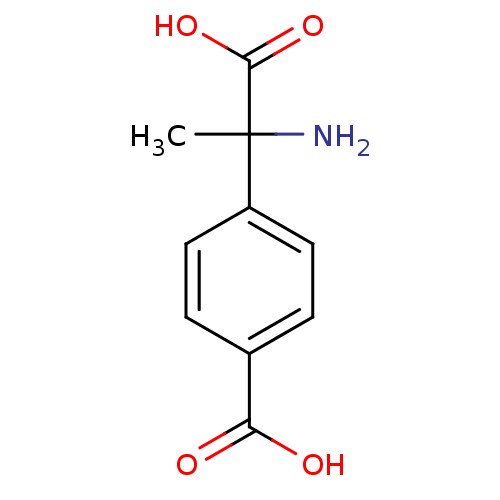
((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...)Show InChI InChI=1S/C10H11NO4/c1-10(11,9(14)15)7-4-2-6(3-5-7)8(12)13/h2-5H,11H2,1H3,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM85379
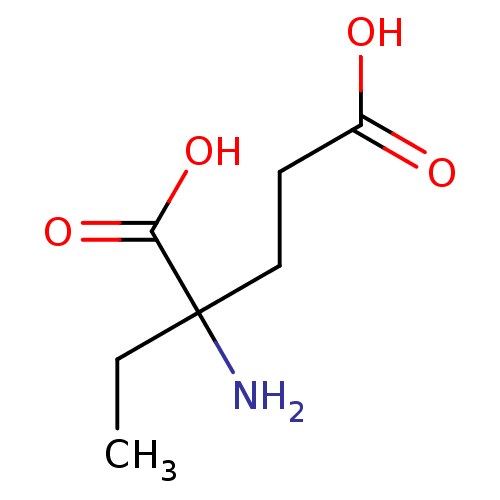
(CAS_5311079 | EGLU | NSC_5311079)Show InChI InChI=1S/C7H13NO4/c1-2-7(8,6(11)12)4-3-5(9)10/h2-4,8H2,1H3,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50004051
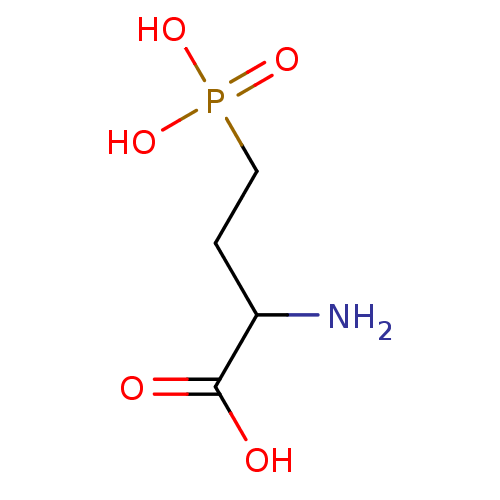
(2-Amino-4-phosphono-butyric acid | 2-Amino-4-phosp...)Show InChI InChI=1S/C4H10NO5P/c5-3(4(6)7)1-2-11(8,9)10/h3H,1-2,5H2,(H,6,7)(H2,8,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 71: 2558-64 (1998)
Article DOI: 10.1046/j.1471-4159.1998.71062558.x
BindingDB Entry DOI: 10.7270/Q25D8QCZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50004863

((+/-)-trans-ACPD | (1S,3S)-1-Amino-cyclopentane-1,...)Show InChI InChI=1S/C7H11NO4/c8-7(6(11)12)2-1-4(3-7)5(9)10/h4H,1-3,8H2,(H,9,10)(H,11,12)/t4-,7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM79403
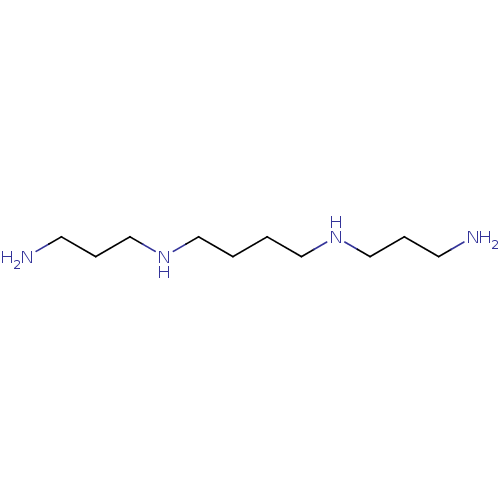
(3-aminopropyl-[4-(3-aminopropylamino)butyl]amine;h...)Show InChI InChI=1S/C10H26N4/c11-5-3-9-13-7-1-2-8-14-10-4-6-12/h13-14H,1-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM82355

(Amino-(3-hydroxy-isoxazol-5-yl)-acetic acid(Iboten...)Show InChI InChI=1S/C5H6N2O4/c6-4(5(9)10)2-1-3(8)7-11-2/h1,4H,6H2,(H,7,8)(H,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50030627

((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...)Show InChI InChI=1S/C10H11NO4/c1-10(11,9(14)15)7-4-2-6(3-5-7)8(12)13/h2-5H,11H2,1H3,(H,12,13)(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50062500

((S)-2-((R)-1-Amino-1-carboxy-ethyl)-cyclopropaneca...)Show InChI InChI=1S/C7H11NO4/c1-7(8,6(11)12)4-2-3(4)5(9)10/h3-4H,2,8H2,1H3,(H,9,10)(H,11,12)/t3-,4?,7+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM85077

(3-Phosphono-D,L-2-aminopropionic acid, 7 | CAS_230...)Show InChI InChI=1S/C3H8NO5P/c4-2(3(5)6)1-10(7,8)9/h2H,1,4H2,(H,5,6)(H2,7,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50009353
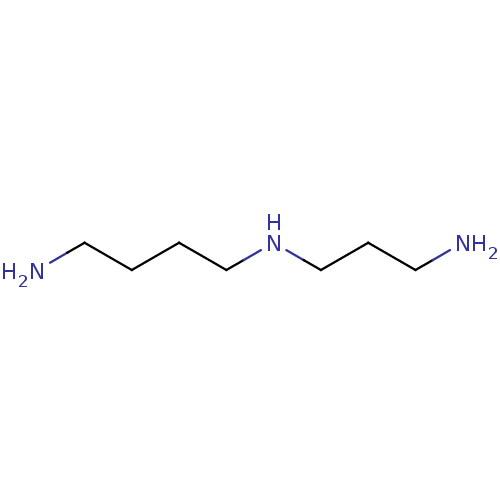
(1,5,10-triazadecane | 4-azaoctamethylenediamine | ...)Show InChI InChI=1S/C7H19N3/c8-4-1-2-6-10-7-3-5-9/h10H,1-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Neurochem 70: 2147-55 (1998)
Article DOI: 10.1046/j.1471-4159.1998.70052147.x
BindingDB Entry DOI: 10.7270/Q2KP80Q7 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM17660

((2S)-2-amino-3-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl...)Show InChI InChI=1S/C5H7N3O5/c6-2(3(9)10)1-8-4(11)7-5(12)13-8/h2H,1,6H2,(H,9,10)(H,7,11,12)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50004051

(2-Amino-4-phosphono-butyric acid | 2-Amino-4-phosp...)Show InChI InChI=1S/C4H10NO5P/c5-3(4(6)7)1-2-11(8,9)10/h3H,1-2,5H2,(H,6,7)(H2,8,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 228-33 (1998)
BindingDB Entry DOI: 10.7270/Q2MP51T5 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM172038
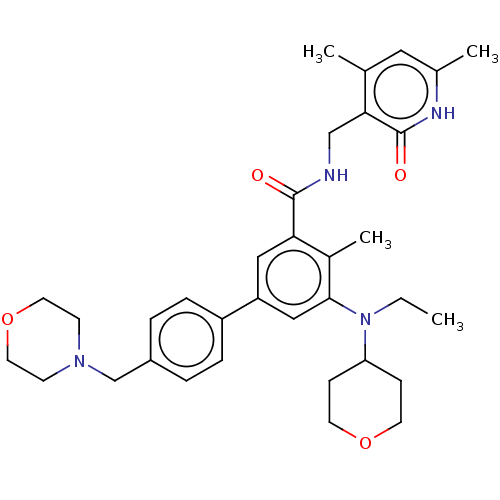
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Agonist activity was determined against hPR (human progesterone receptor) compared to that of progesterone (100%) |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM608937
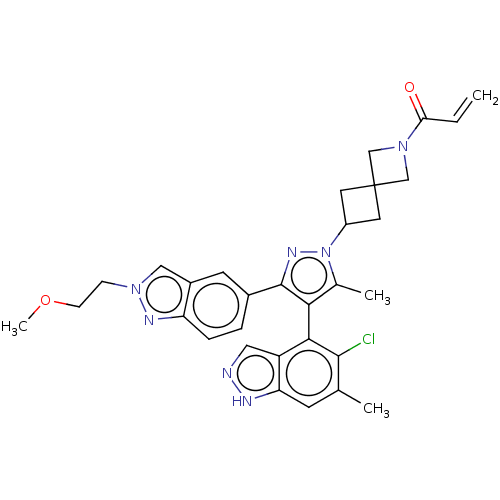
(1-(6-{(4M)-4-(5-Chloro-6- methyl-1H-indazol-4-yl)-...)Show SMILES COCCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(9.82,-.8,;9.42,-2.28,;7.94,-2.68,;6.85,-1.59,;5.36,-1.99,;4.21,-.96,;2.88,-1.73,;1.42,-1.26,;.27,-2.29,;.59,-3.79,;2.06,-4.27,;3.2,-3.24,;4.73,-3.4,;-1.19,-1.81,;-1.67,-.35,;-3.21,-.35,;-3.98,.99,;-5.47,1.39,;-5.07,2.87,;-3.58,2.48,;-6.55,3.27,;-6.16,4.76,;-4.67,4.36,;-7.25,5.85,;-6.85,7.34,;-8.73,5.45,;-9.82,6.54,;-3.68,-1.81,;-5.15,-2.29,;-2.44,-2.72,;-2.44,-4.26,;-3.77,-5.03,;-5.11,-4.26,;-3.77,-6.57,;-5.11,-7.34,;-2.44,-7.34,;-1.1,-6.57,;.36,-7.04,;1.27,-5.8,;.36,-4.55,;-1.1,-5.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50236010

(CHEMBL4103319)Show SMILES C(Nc1ncc(-c2ccc(CN3CCCC3)cc2)c2nncn12)c1ccco1 Show InChI InChI=1S/C21H22N6O/c1-2-10-26(9-1)14-16-5-7-17(8-6-16)19-13-23-21(27-15-24-25-20(19)27)22-12-18-4-3-11-28-18/h3-8,11,13,15H,1-2,9-10,12,14H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50609524
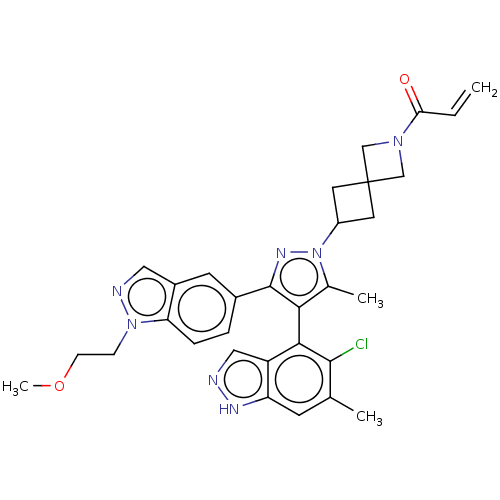
(CHEMBL5281254)Show SMILES COCCn1ncc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(6.51,-8.61,;5.19,-9.32,;3.92,-8.53,;2.6,-9.24,;1.33,-8.44,;-.1,-9.02,;-1.09,-7.85,;-.28,-6.54,;-.71,-5.06,;.35,-3.95,;1.85,-4.31,;2.28,-5.79,;1.22,-6.91,;.03,-2.44,;1.07,-1.29,;.29,.05,;.92,1.46,;2.28,2.07,;1.62,3.49,;.22,2.79,;3.03,4.13,;2.4,5.53,;.99,4.9,;2.94,6.97,;4.17,7.17,;1.97,8.17,;2.4,9.32,;-1.21,-.28,;-2.14,.54,;-1.35,-1.8,;-2.7,-2.58,;-2.7,-4.12,;-1.62,-4.74,;-4.03,-4.89,;-4.03,-6.12,;-5.37,-4.12,;-5.37,-2.58,;-6.51,-1.54,;-5.88,-.13,;-4.35,-.29,;-4.03,-1.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235990

(CHEMBL4088683)Show SMILES CN(C)Cc1ccc(cc1)-c1cc(C#N)c(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C21H20N6O/c1-26(2)13-15-5-7-16(8-6-15)19-10-17(11-22)20(27-14-24-25-21(19)27)23-12-18-4-3-9-28-18/h3-10,14,23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISA |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235996
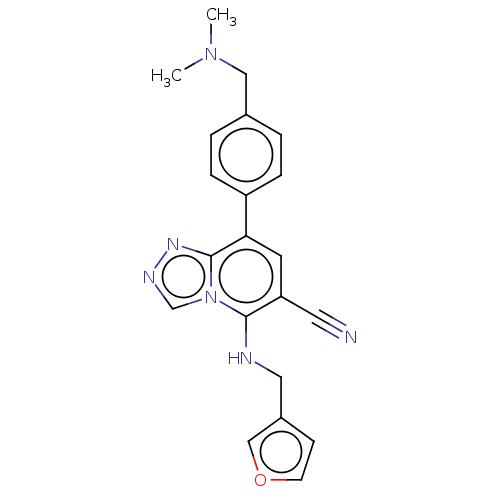
(CHEMBL4079806)Show SMILES CN(C)Cc1ccc(cc1)-c1cc(C#N)c(NCc2ccoc2)n2cnnc12 Show InChI InChI=1S/C21H20N6O/c1-26(2)12-15-3-5-17(6-4-15)19-9-18(10-22)20(27-14-24-25-21(19)27)23-11-16-7-8-28-13-16/h3-9,13-14,23H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISA |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50609523
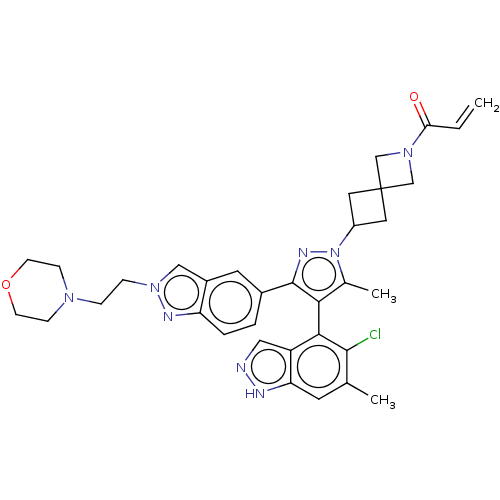
(CHEMBL5271997)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2nn(CCN3CCOCC3)cc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-1.82,5.46,;-1.41,4.3,;-2.27,3.04,;-1.37,1.81,;.09,2.31,;.06,3.85,;1.29,4.77,;2.79,4.65,;2.9,6.21,;1.34,6.28,;4.43,6.08,;4.56,7.61,;3.03,7.74,;5.73,8.61,;6.89,8.19,;5.46,10.12,;6.4,10.92,;-1.83,.34,;-3.29,-.12,;-3.64,-1.62,;-2.51,-2.66,;-2.53,-4.2,;-1.07,-4.7,;-.61,-6.17,;.89,-6.51,;1.35,-7.98,;2.85,-8.32,;3.31,-9.79,;2.26,-10.92,;.76,-10.58,;.3,-9.11,;-.15,-3.47,;-1.04,-2.21,;-.69,-.71,;-3.81,3.01,;-4.56,1.67,;-3.93,.61,;-6.1,1.65,;-6.7,.57,;-6.89,2.97,;-6.14,4.31,;-6.64,5.77,;-5.41,6.69,;-4.15,5.81,;-4.6,4.34,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50236013

(CHEMBL4081748)Show InChI InChI=1S/C19H20N6O/c1-24(2)12-14-5-7-15(8-6-14)17-11-21-19(25-13-22-23-18(17)25)20-10-16-4-3-9-26-16/h3-9,11,13H,10,12H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged EED (76 to 441 residues) (unknown origin) expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL asses... |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM291729
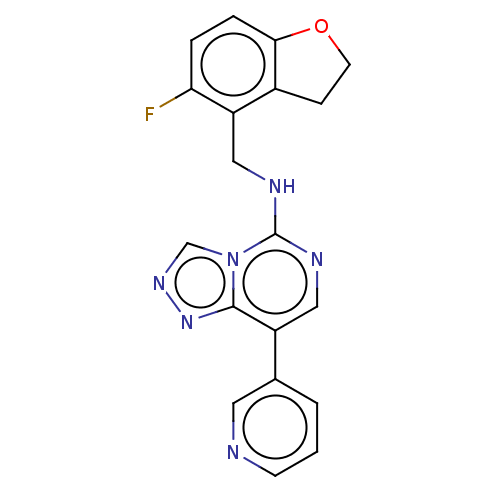
(N-((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-8-...)Show InChI InChI=1S/C19H15FN6O/c20-16-3-4-17-13(5-7-27-17)15(16)10-23-19-22-9-14(12-2-1-6-21-8-12)18-25-24-11-26(18)19/h1-4,6,8-9,11H,5,7,10H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02148
BindingDB Entry DOI: 10.7270/Q2Z323QR |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235997

(CHEMBL4105572)Show InChI InChI=1S/C19H20N6O/c1-24(2)11-14-3-5-16(6-4-14)17-10-21-19(25-13-22-23-18(17)25)20-9-15-7-8-26-12-15/h3-8,10,12-13H,9,11H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISA |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50579985

(JDQ-443 | JDQ443 | Jdq 443 | Jdq-443 | Nvp-jdq-443...)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2n(C)ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(8.62,-12.01,;10.09,-11.52,;11.34,-12.43,;12.58,-11.52,;12.1,-10.05,;10.56,-10.06,;9.66,-8.81,;9.89,-7.29,;8.37,-7.05,;8.13,-8.58,;8.77,-5.56,;7.27,-5.16,;6.88,-6.65,;6.5,-3.83,;7.27,-2.49,;4.96,-3.83,;4.19,-2.5,;14.04,-11.99,;15.18,-10.95,;16.64,-11.42,;16.97,-12.93,;18.31,-13.69,;19.71,-13.06,;18,-15.2,;16.47,-15.37,;15.84,-13.97,;14.37,-13.49,;11.35,-13.97,;10.02,-14.74,;8.69,-13.97,;10.02,-16.28,;8.69,-17.05,;11.35,-17.05,;12.69,-16.27,;14.15,-16.75,;15.06,-15.5,;14.15,-14.25,;12.69,-14.73,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM225230

(EED226 | US11013745, Compound EED226)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C17H15N5O3S/c1-26(23,24)14-6-4-12(5-7-14)15-10-19-17(22-11-20-21-16(15)22)18-9-13-3-2-8-25-13/h2-8,10-11H,9H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
| Assay Description
All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383]. |
Nat Chem Biol 13: 381-388 (2017)
Article DOI: 10.1038/nchembio.2304
BindingDB Entry DOI: 10.7270/Q2S75F64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50236006

(CHEMBL4099766)Show SMILES CN(C)Cc1ccc(cc1)-c1cc(C#N)c(NCc2cccs2)n2cnnc12 Show InChI InChI=1S/C21H20N6S/c1-26(2)13-15-5-7-16(8-6-15)19-10-17(11-22)20(27-14-24-25-21(19)27)23-12-18-4-3-9-28-18/h3-10,14,23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Agonist activity was determined against hPR (human progesterone receptor) compared to that of progesterone (100%) |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM608886
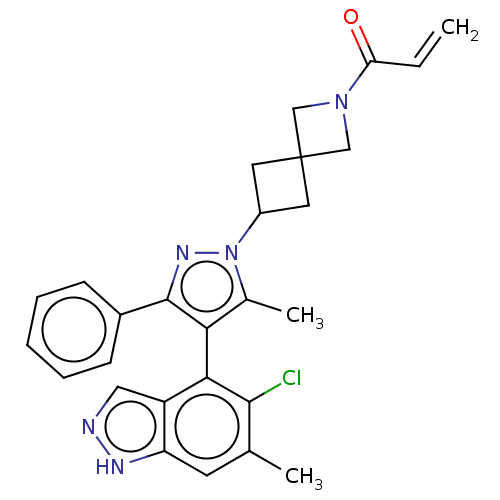
(1-{6-[(4M)-4-(5-Chloro-6- methyl-1H-indazol-4-yl)-...)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccccc1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-2.38,-2.83,;-.92,-2.36,;.33,-3.26,;1.57,-2.36,;1.1,-.89,;-.44,-.89,;-1.21,.44,;-2.7,.84,;-2.3,2.33,;-.81,1.93,;-3.79,2.73,;-3.39,4.22,;-1.9,3.82,;-4.48,5.3,;-5.97,4.91,;-4.08,6.79,;-5.17,7.88,;3.04,-2.83,;3.36,-4.34,;4.82,-4.81,;5.97,-3.78,;5.65,-2.28,;4.18,-1.8,;.33,-4.8,;-1.01,-5.57,;-2.34,-4.8,;-1.01,-7.11,;-2.34,-7.88,;.33,-7.88,;1.66,-7.11,;3.13,-7.59,;4.03,-6.34,;3.13,-5.09,;1.66,-5.57,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM608850
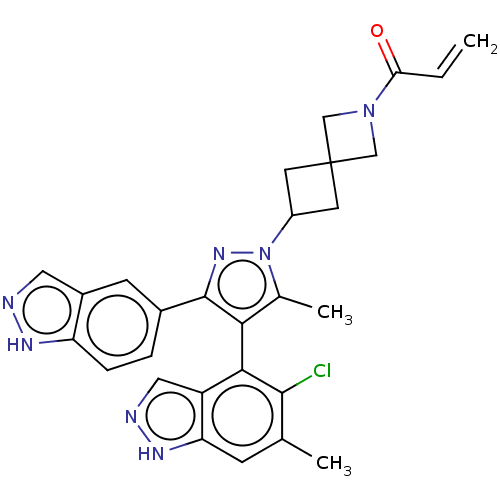
(US11702409, Example 12a | US11702409, Example 12b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2[nH]ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-2.64,-2.29,;-1.17,-1.81,;.07,-2.72,;1.32,-1.81,;.84,-.35,;-.7,-.35,;-1.47,.99,;-2.95,1.39,;-2.56,2.87,;-1.07,2.48,;-4.04,3.27,;-3.65,4.76,;-2.16,4.36,;-4.73,5.85,;-4.34,7.34,;-6.22,5.45,;-7.31,6.54,;2.78,-2.29,;3.69,-1.04,;5.22,-1.2,;5.85,-2.61,;7.31,-3.08,;7.31,-4.62,;5.85,-5.1,;4.94,-3.85,;3.41,-3.69,;.07,-4.26,;-1.26,-5.03,;-2.59,-4.26,;-1.26,-6.57,;-2.59,-7.34,;.07,-7.34,;1.41,-6.57,;2.87,-7.04,;3.78,-5.8,;2.87,-4.55,;1.41,-5.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM225230

(EED226 | US11013745, Compound EED226)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C17H15N5O3S/c1-26(23,24)14-6-4-12(5-7-14)15-10-19-17(22-11-20-21-16(15)22)18-9-13-3-2-8-25-13/h2-8,10-11H,9H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 23.4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis Institutes for BioMedical Research
| Assay Description
To assess the compounds potency in the H3K27me0 peptide (21–44)-based PRC2 enzymatic assay, compounds were serially diluted three-fold in DMSO t... |
Nat Chem Biol 13: 381-388 (2017)
Article DOI: 10.1038/nchembio.2304
BindingDB Entry DOI: 10.7270/Q2S75F64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM608857
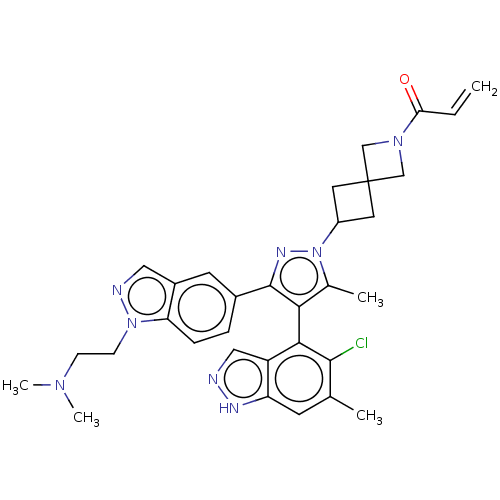
(US11702409, Example 15a | US11702409, Example 15b)Show SMILES CN(C)CCn1ncc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(6.12,-8.04,;6.89,-6.7,;8.43,-6.7,;6.12,-5.37,;6.89,-4.03,;6.12,-2.7,;6.75,-1.29,;5.6,-.26,;4.27,-1.03,;2.81,-.56,;1.66,-1.59,;1.98,-3.09,;3.45,-3.57,;4.59,-2.54,;.2,-1.11,;-.28,.35,;-1.82,.35,;-2.59,1.69,;-4.08,2.09,;-3.68,3.57,;-2.19,3.17,;-5.17,3.97,;-4.77,5.46,;-3.28,5.06,;-5.86,6.55,;-5.46,8.04,;-7.34,6.15,;-8.43,7.24,;-2.29,-1.11,;-3.76,-1.59,;-1.05,-2.02,;-1.05,-3.56,;-2.38,-4.33,;-3.72,-3.56,;-2.38,-5.87,;-3.72,-6.64,;-1.05,-6.64,;.28,-5.87,;1.75,-6.34,;2.65,-5.1,;1.75,-3.85,;.28,-4.33,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50236010

(CHEMBL4103319)Show SMILES C(Nc1ncc(-c2ccc(CN3CCCC3)cc2)c2nncn12)c1ccco1 Show InChI InChI=1S/C21H22N6O/c1-2-10-26(9-1)14-16-5-7-17(8-6-16)19-13-23-21(27-15-24-25-20(19)27)22-12-18-4-3-11-28-18/h3-8,11,13,15H,1-2,9-10,12,14H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISA |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data