

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3585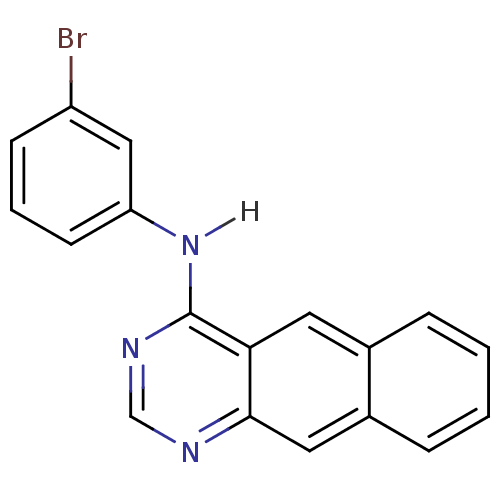 (4-[(3-Bromophenyl)amino]benzo[g]quinazoline | Benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556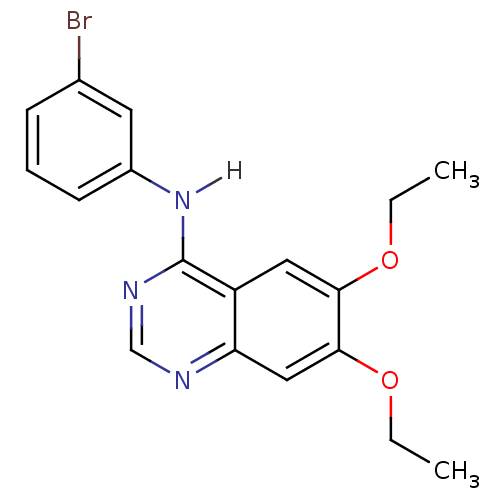 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3570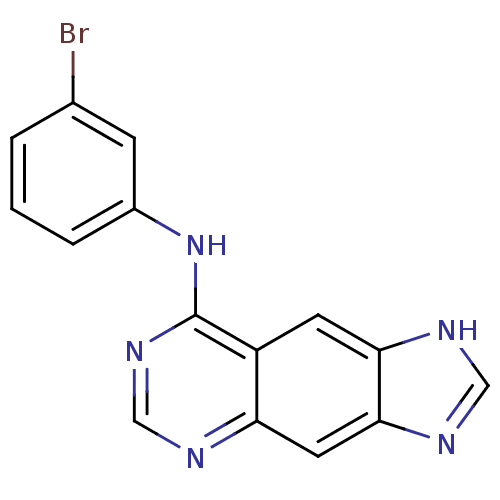 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3572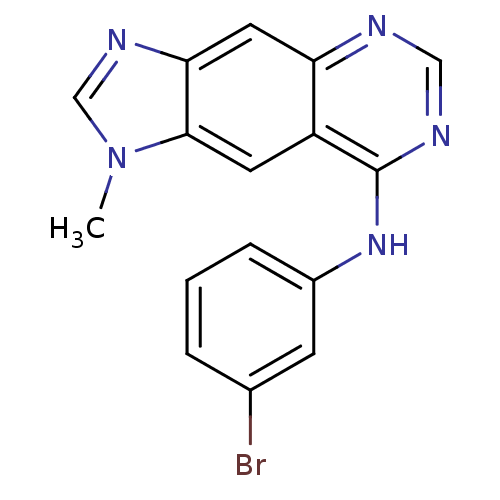 (8-[(3-Bromophenyl)amino]-1-methyl-1H-imidazo[4,5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3574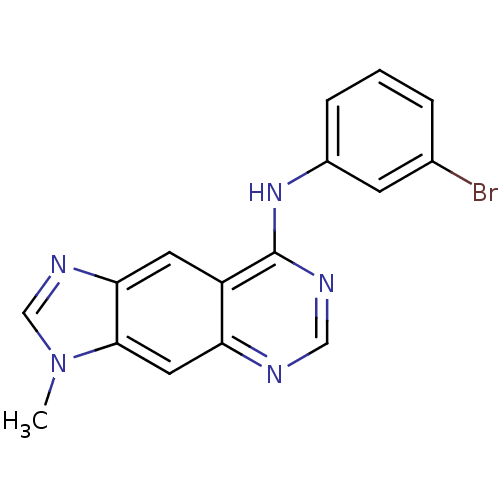 (8-[(3-Bromophenyl)amino]-3-methyl-3H-imidazo[4,5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032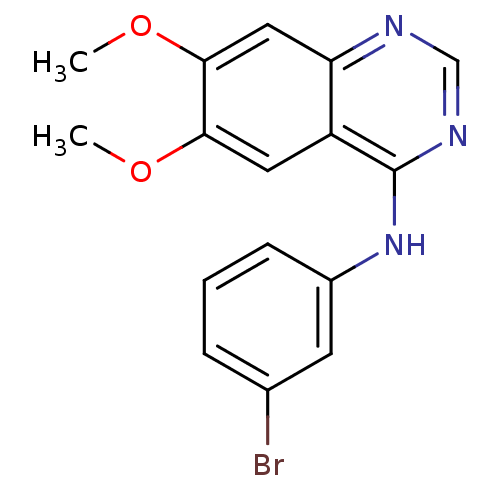 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3568 (CHEMBL327307 | N-(3,4-dibromophenyl)-6,7-dimethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3297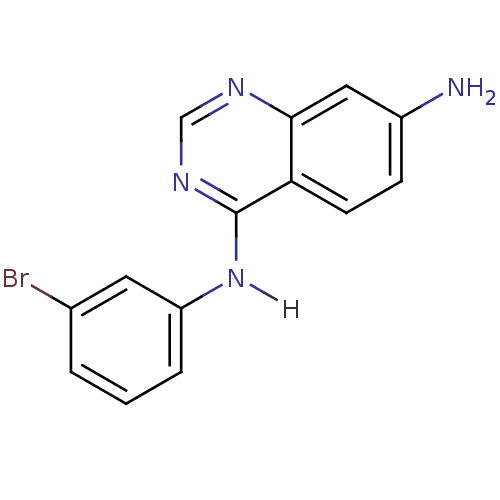 (4-Anilinoquinazoline deriv. 48 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3303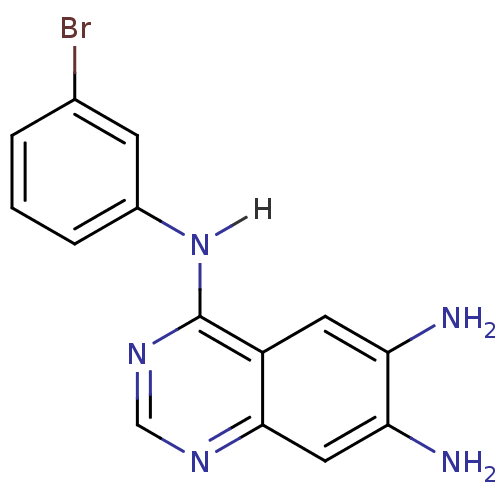 (4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3302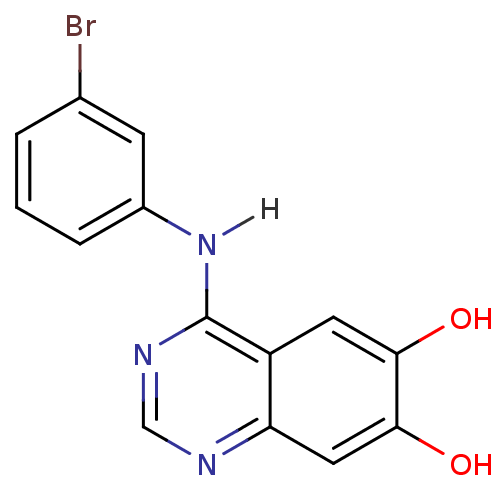 (4-Anilinoquinazoline deriv. 53 | 4-[(3-bromophenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3557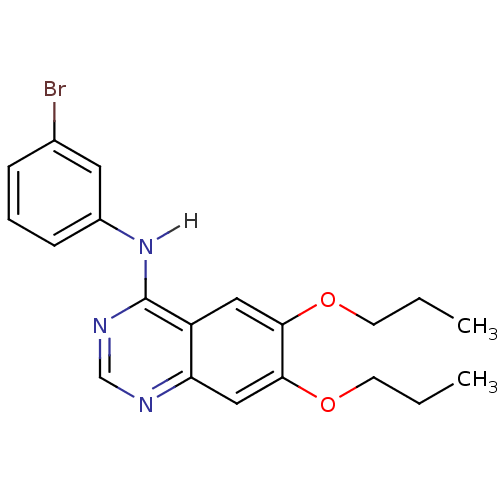 (CHEMBL418967 | N-(3-bromophenyl)-6,7-dipropoxyquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3534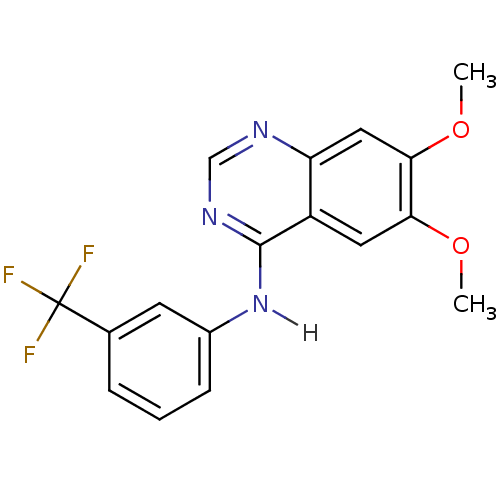 (6,7-dimethoxy-N-[3-(trifluoromethyl)phenyl]quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3520 (4-N-(3-chlorophenyl)quinazoline-4,7-diamine | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3571 (8-[(3-Bromophenyl)amino]-2-methyl-1H-imidazo[4,5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3532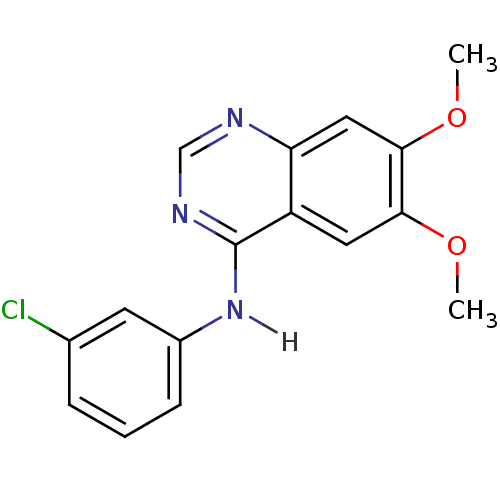 (CHEMBL540068 | CHEMBL7917 | N-(3-chlorophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3581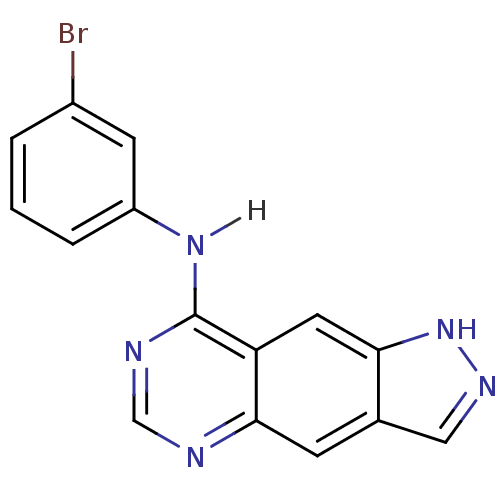 (8-[(3-Bromophenyl)amino]-1H-pyrazolo[3,4-g]quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3522 (4-N-(3-iodophenyl)quinazoline-4,7-diamine | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3582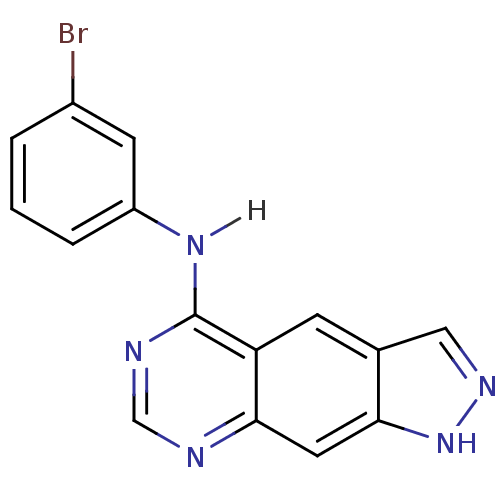 (5-[(3-Bromophenyl)amino]-1H-pyrazolo[4,3-g]quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583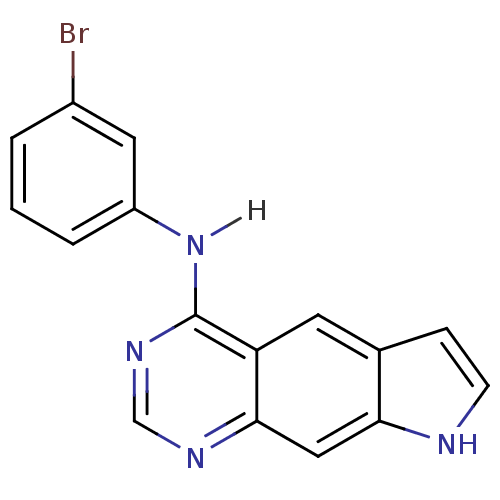 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3564 (4-[(3-Bromophenyl)amino]-5,6,7-trimethoxyquinazoli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3544 (4-N-(3-bromophenyl)-7-N-methylquinazoline-4,6,7-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3294 (4-Anilinoquinazoline deriv. 45 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3533 (CHEMBL1204305 | CHEMBL96065 | N-(3-iodophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3567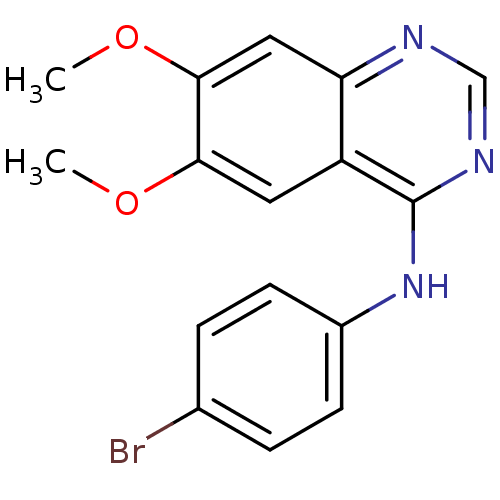 (CHEMBL328106 | N-(4-bromophenyl)-6,7-dimethoxyquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3584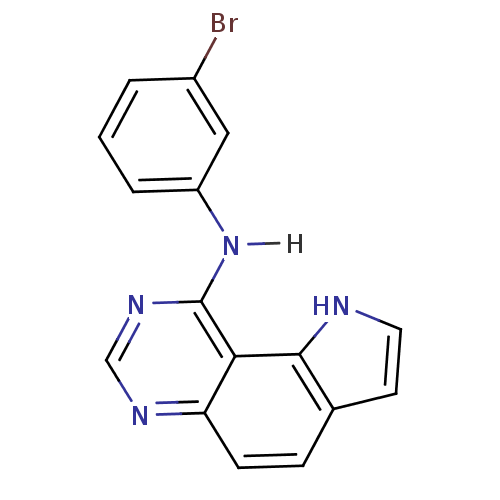 (9-[(3-Bromophenyl)amino]-1H-pyrrolo[2,3-f]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3573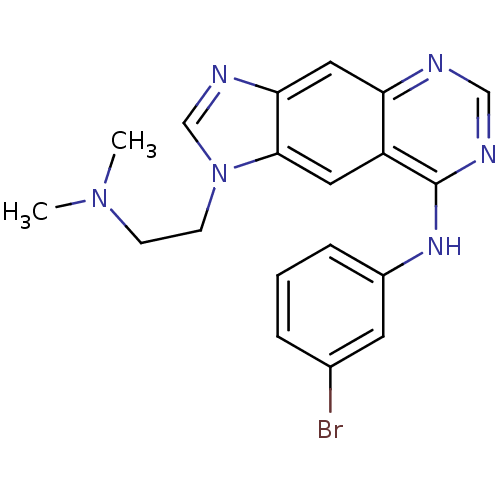 (8-[(3-Bromophenyl)amino]-1-[2-(dimethylamino)ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3586 (4-[(3-Bromophenyl)amino]pyrazino[2,3-g]quinazoline...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3519 (4-N-(3-fluorophenyl)quinazoline-4,7-diamine | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50245691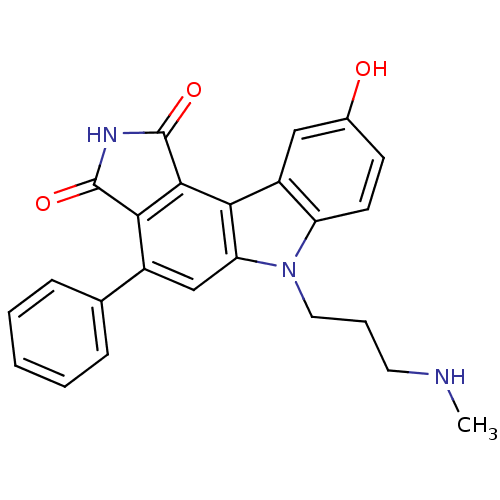 (9-hydroxy-6-(3-(methylamino)propyl)-4-phenylpyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of Chk1 kinase assessed as GST-Cdc25 phosphorylation by Western blot determination | Eur J Med Chem 43: 1276-96 (2008) Article DOI: 10.1016/j.ejmech.2007.07.016 BindingDB Entry DOI: 10.7270/Q26M36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50245342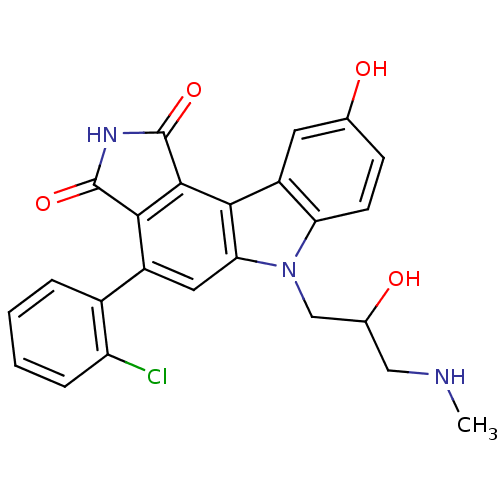 (4-(2-Chlorophenyl)-9-hydroxy-6-[2-hydroxy-3-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of Chk1 kinase assessed as GST-Cdc25 phosphorylation by Western blot determination | Eur J Med Chem 43: 1276-96 (2008) Article DOI: 10.1016/j.ejmech.2007.07.016 BindingDB Entry DOI: 10.7270/Q26M36MC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3523 (4-N-[3-(trifluoromethyl)phenyl]quinazoline-4,7-dia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3531 (CHEMBL541586 | CHEMBL94431 | N-(3-fluorophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3546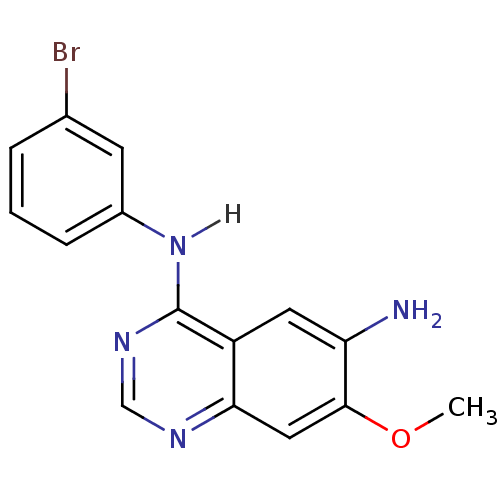 (4-N-(3-bromophenyl)-7-methoxyquinazoline-4,6-diami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3535 (4-N-(3-bromophenyl)-6-N-methylquinazoline-4,6-diam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3579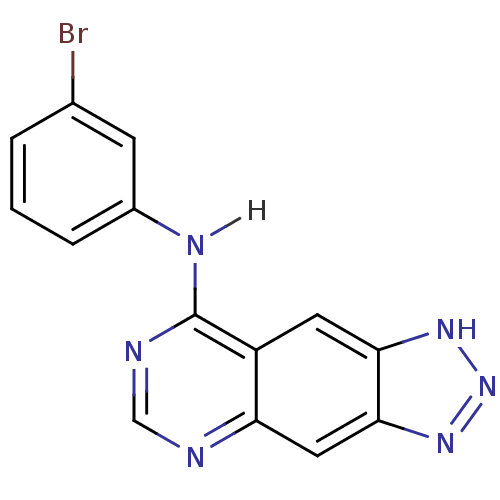 (8-[(3-Bromophenyl)amino]-1H-1,2,3-triazolo[4,5-g]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3538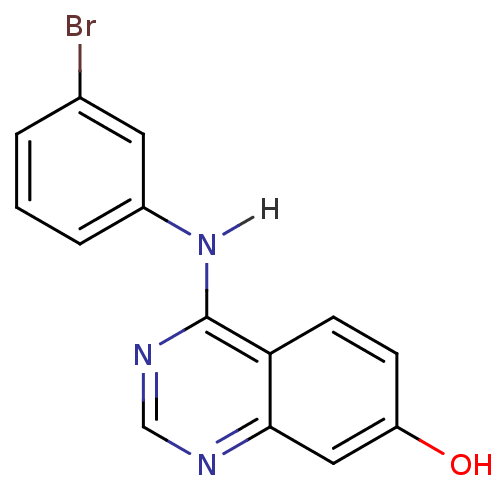 (4-[(3-bromophenyl)amino]quinazolin-7-ol | CHEMBL93...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM3085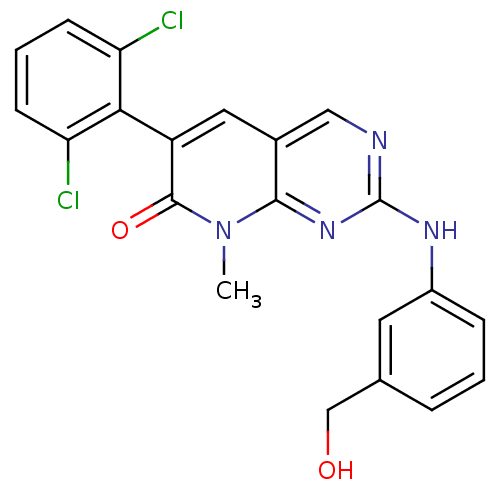 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM4213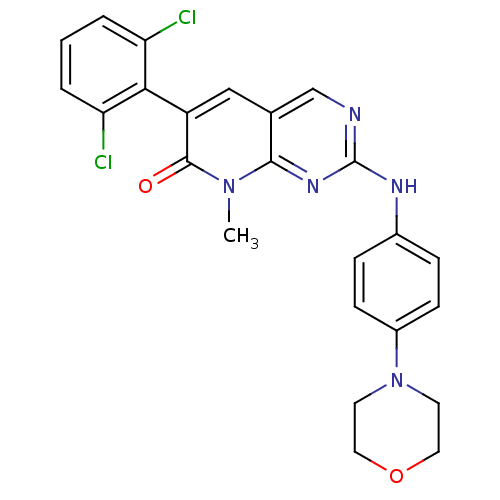 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6569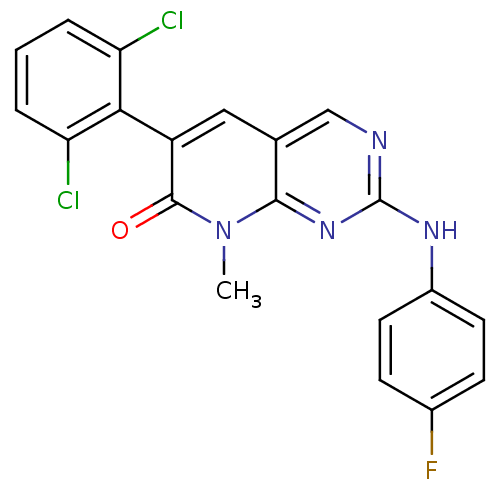 (6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6568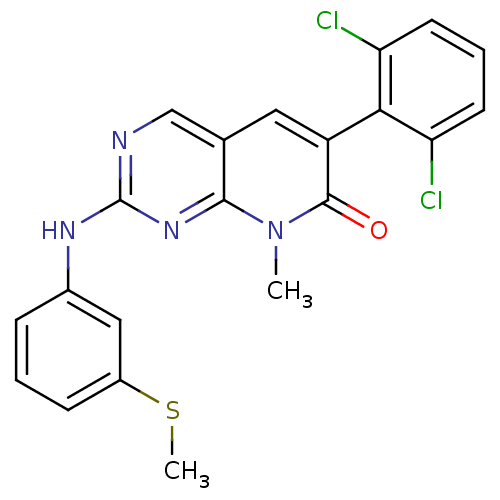 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6570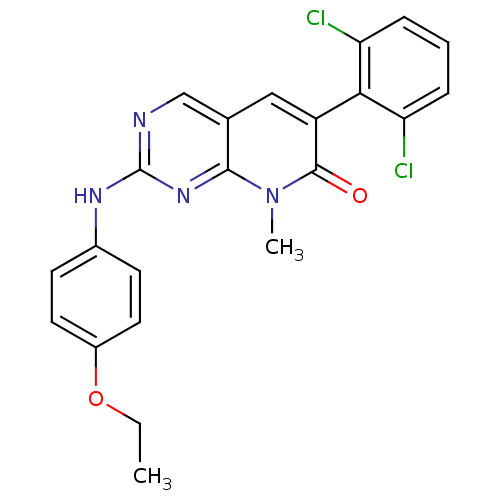 (6-(2,6-dichlorophenyl)-2-[(4-ethoxyphenyl)amino]-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6571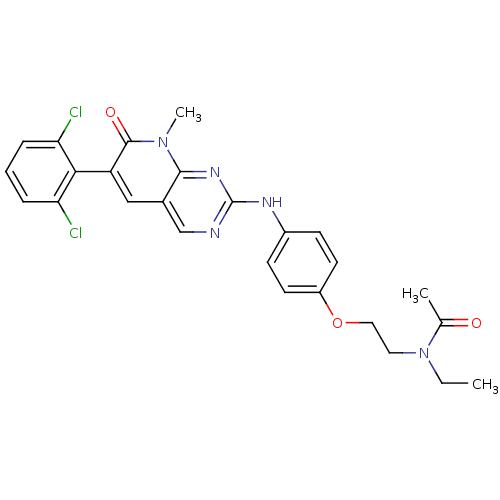 (N-[2-(4-{[6-(2,6-dichlorophenyl)-8-methyl-7-oxo-7H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6572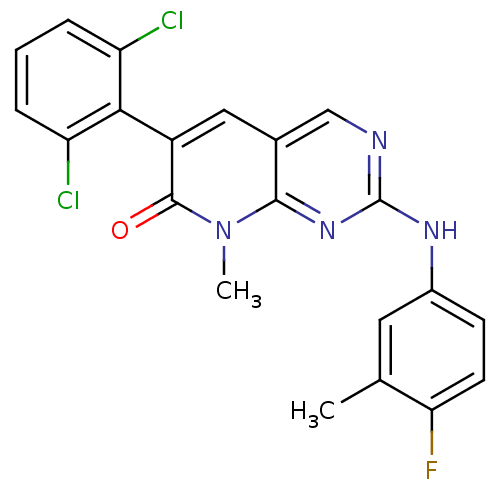 (6-(2,6-dichlorophenyl)-2-[(4-fluoro-3-methylphenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50245693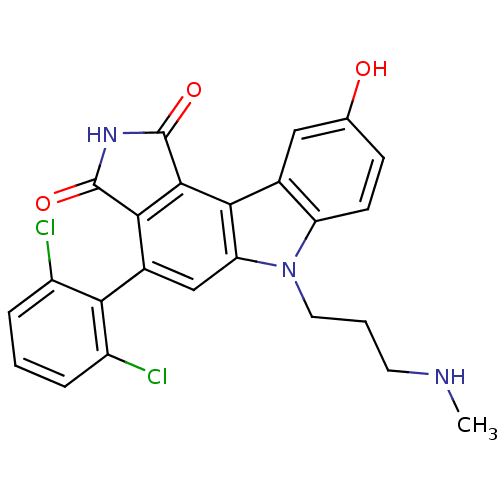 (4-(2,6-dichlorophenyl)-9-hydroxy-6-(3-(methylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of Chk1 kinase assessed as GST-Cdc25 phosphorylation by Western blot determination | Eur J Med Chem 43: 1276-96 (2008) Article DOI: 10.1016/j.ejmech.2007.07.016 BindingDB Entry DOI: 10.7270/Q26M36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3085 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.68 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50245523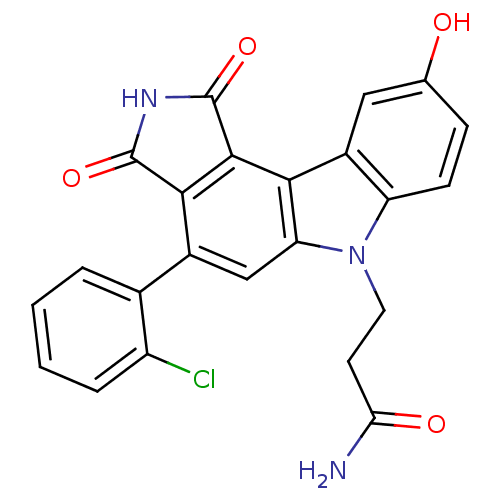 (3-(4-(2-chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human Wee1 assessed as polyornithine-tyrosine copolymer phosphorylation | Eur J Med Chem 43: 1276-96 (2008) Article DOI: 10.1016/j.ejmech.2007.07.016 BindingDB Entry DOI: 10.7270/Q26M36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3085 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 3276-92 (1998) Article DOI: 10.1021/jm9802259 BindingDB Entry DOI: 10.7270/Q27P8WK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3547 (4-N-(3-bromophenyl)-7-chloroquinazoline-4,6-diamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50245387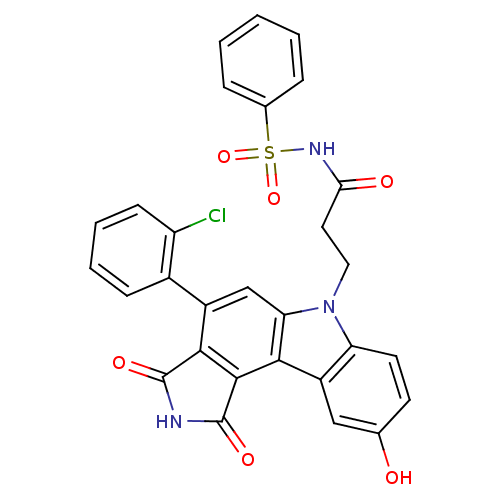 (3-(4-(2-chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human Wee1 assessed as polyornithine-tyrosine copolymer phosphorylation | Eur J Med Chem 43: 1276-96 (2008) Article DOI: 10.1016/j.ejmech.2007.07.016 BindingDB Entry DOI: 10.7270/Q26M36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50245496 (4-(2,6-Dichlorophenyl)-9-hydroxy-6-(3-hydroxypropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human Wee1 assessed as polyornithine-tyrosine copolymer phosphorylation | Eur J Med Chem 43: 1276-96 (2008) Article DOI: 10.1016/j.ejmech.2007.07.016 BindingDB Entry DOI: 10.7270/Q26M36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2009 total ) | Next | Last >> |