 Found 1266 hits with Last Name = 'nguyen' and Initial = 'b'
Found 1266 hits with Last Name = 'nguyen' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50106407
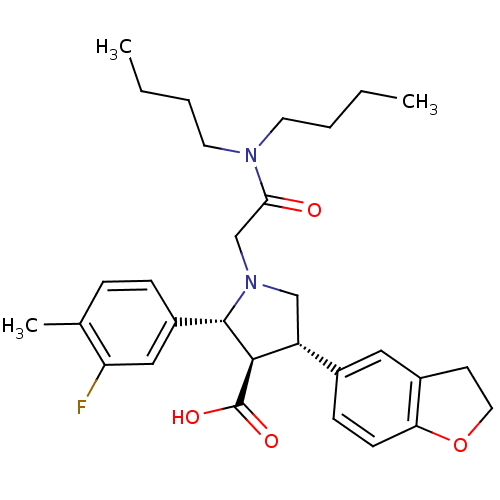
(1-Dibutylcarbamoylmethyl-4-(2,3-dihydro-benzofuran...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(C)c(F)c1)C(O)=O)c1ccc2OCCc2c1 Show InChI InChI=1S/C30H39FN2O4/c1-4-6-13-32(14-7-5-2)27(34)19-33-18-24(21-10-11-26-22(16-21)12-15-37-26)28(30(35)36)29(33)23-9-8-20(3)25(31)17-23/h8-11,16-17,24,28-29H,4-7,12-15,18-19H2,1-3H3,(H,35,36)/t24-,28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human Endothelin A receptor |
J Med Chem 44: 3978-84 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9PR2 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50051007
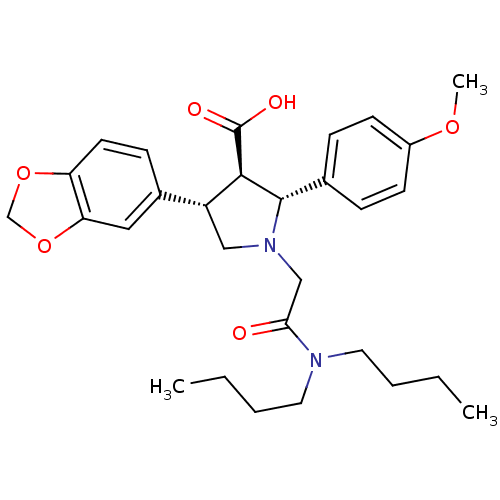
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
binding affinity against human Endothelin A receptor |
J Med Chem 44: 3978-84 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9PR2 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM86689
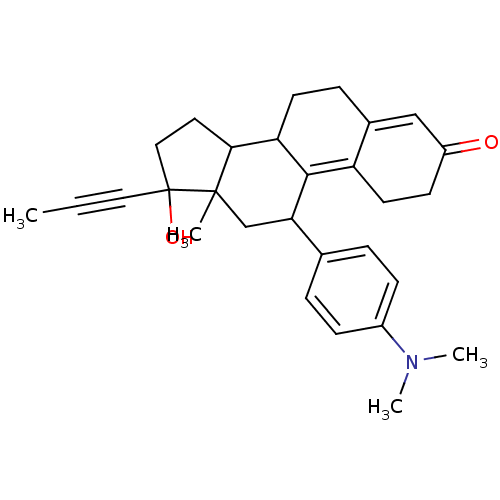
(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50066370
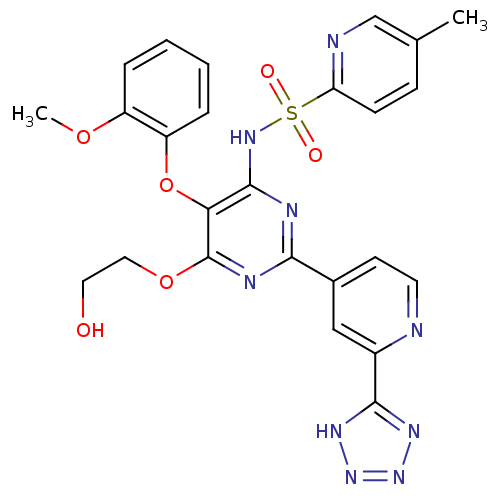
(5-Methyl-pyridine-2-sulfonic acid {6-(2-hydroxy-et...)Show SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(C)cn2)nc(nc1OCCO)-c1ccnc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C25H23N9O6S/c1-15-7-8-20(27-14-15)41(36,37)32-24-21(40-19-6-4-3-5-18(19)38-2)25(39-12-11-35)29-22(28-24)16-9-10-26-17(13-16)23-30-33-34-31-23/h3-10,13-14,35H,11-12H2,1-2H3,(H,28,29,32)(H,30,31,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50034263
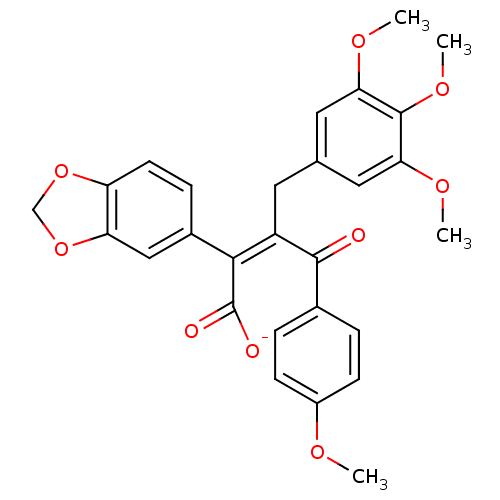
(CHEMBL25438 | PD-156707 | Sodium; (Z)-2-benzo[1,3]...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C28H26O9/c1-32-19-8-5-17(6-9-19)26(29)20(11-16-12-23(33-2)27(35-4)24(13-16)34-3)25(28(30)31)18-7-10-21-22(14-18)37-15-36-21/h5-10,12-14H,11,15H2,1-4H3,(H,30,31)/p-1/b25-20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50041617
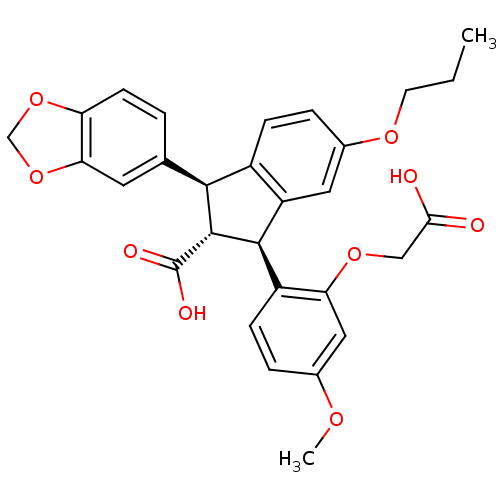
((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCC(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H28O9/c1-3-10-35-18-6-7-19-21(12-18)27(20-8-5-17(34-2)13-23(20)36-14-25(30)31)28(29(32)33)26(19)16-4-9-22-24(11-16)38-15-37-22/h4-9,11-13,26-28H,3,10,14-15H2,1-2H3,(H,30,31)(H,32,33)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50066398
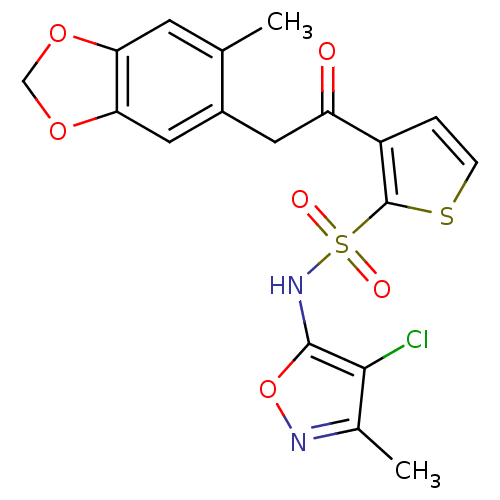
(3-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...)Show SMILES Cc1noc(NS(=O)(=O)c2sccc2C(=O)Cc2cc3OCOc3cc2C)c1Cl Show InChI InChI=1S/C18H15ClN2O6S2/c1-9-5-14-15(26-8-25-14)7-11(9)6-13(22)12-3-4-28-18(12)29(23,24)21-17-16(19)10(2)20-27-17/h3-5,7,21H,6,8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50066391
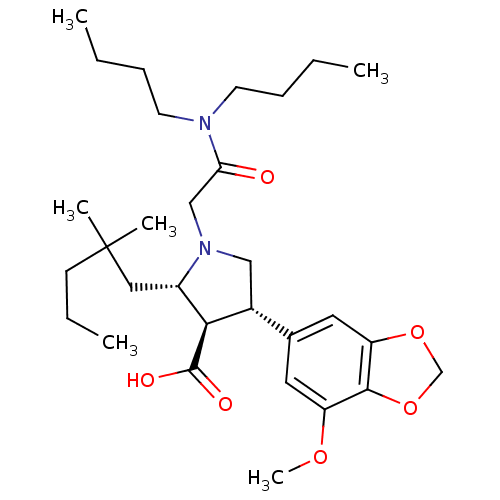
((2S,3R,4S)-1-Dibutylcarbamoylmethyl-2-(2,2-dimethy...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1CC(C)(C)CCC)C(O)=O)c1cc2OCOc2c(OC)c1 Show InChI InChI=1S/C30H48N2O6/c1-7-10-13-31(14-11-8-2)26(33)19-32-18-22(21-15-24(36-6)28-25(16-21)37-20-38-28)27(29(34)35)23(32)17-30(4,5)12-9-3/h15-16,22-23,27H,7-14,17-20H2,1-6H3,(H,34,35)/t22-,23+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against endothelin A receptor in MMQ cells in rat |
J Med Chem 44: 3978-84 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9PR2 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50066391

((2S,3R,4S)-1-Dibutylcarbamoylmethyl-2-(2,2-dimethy...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1CC(C)(C)CCC)C(O)=O)c1cc2OCOc2c(OC)c1 Show InChI InChI=1S/C30H48N2O6/c1-7-10-13-31(14-11-8-2)26(33)19-32-18-22(21-15-24(36-6)28-25(16-21)37-20-38-28)27(29(34)35)23(32)17-30(4,5)12-9-3/h15-16,22-23,27H,7-14,17-20H2,1-6H3,(H,34,35)/t22-,23+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Membrane-associated progesterone receptor component 1
(RAT) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Membrane-associated progesterone receptor component 1
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061077
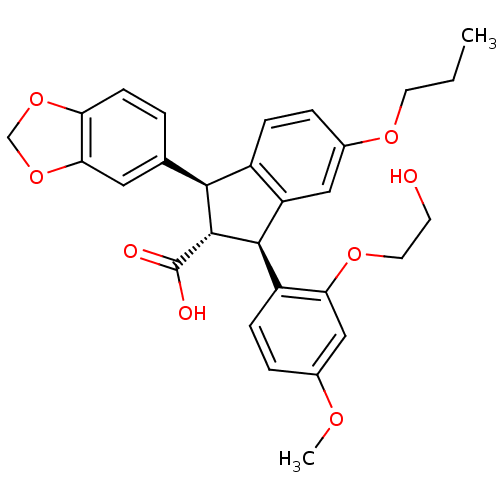
((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCCO)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H30O8/c1-3-11-34-19-6-7-20-22(14-19)27(21-8-5-18(33-2)15-24(21)35-12-10-30)28(29(31)32)26(20)17-4-9-23-25(13-17)37-16-36-23/h4-9,13-15,26-28,30H,3,10-12,16H2,1-2H3,(H,31,32)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061077

((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCCO)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H30O8/c1-3-11-34-19-6-7-20-22(14-19)27(21-8-5-18(33-2)15-24(21)35-12-10-30)28(29(31)32)26(20)17-4-9-23-25(13-17)37-16-36-23/h4-9,13-15,26-28,30H,3,10-12,16H2,1-2H3,(H,31,32)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061101
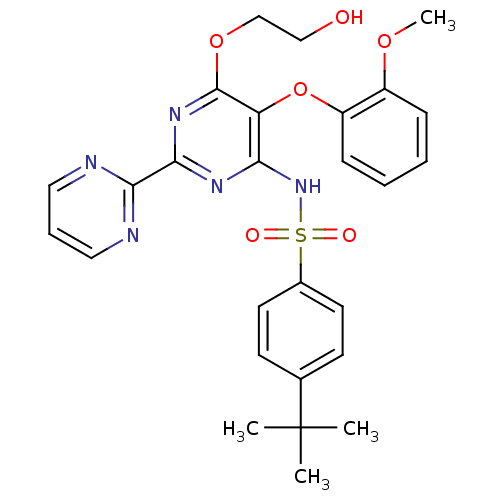
(4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...)Show SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCO)-c1ncccn1 Show InChI InChI=1S/C27H29N5O6S/c1-27(2,3)18-10-12-19(13-11-18)39(34,35)32-23-22(38-21-9-6-5-8-20(21)36-4)26(37-17-16-33)31-25(30-23)24-28-14-7-15-29-24/h5-15,33H,16-17H2,1-4H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50267297

(CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...)Show SMILES COC(=O)N[C@H](C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2ccccc2)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C47H60N6O6/c1-46(2,3)40(51-44(57)59-7)42(55)49-36(28-33-21-23-35(24-22-33)37-20-14-15-25-48-37)30-39(54)38(29-32-16-10-8-11-17-32)50-43(56)41(47(4,5)6)53-27-26-52(45(53)58)31-34-18-12-9-13-19-34/h8-25,36,38-41,54H,26-31H2,1-7H3,(H,49,55)(H,50,56)(H,51,57)/t36-,38-,39-,40+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50041617

((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCC(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H28O9/c1-3-10-35-18-6-7-19-21(12-18)27(20-8-5-17(34-2)13-23(20)36-14-25(30)31)28(29(32)33)26(19)16-4-9-22-24(11-16)38-15-37-22/h4-9,11-13,26-28H,3,10,14-15H2,1-2H3,(H,30,31)(H,32,33)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin B receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50034435
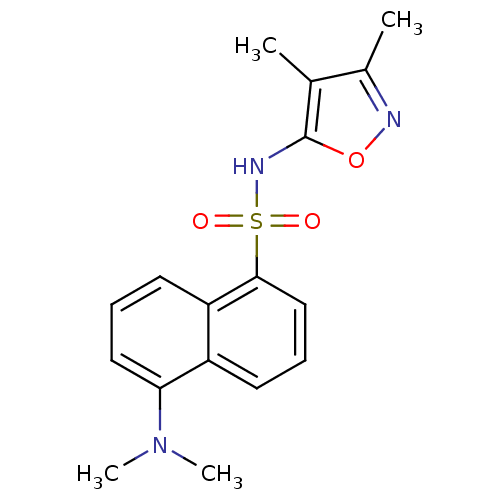
(5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-d...)Show InChI InChI=1S/C17H19N3O3S/c1-11-12(2)18-23-17(11)19-24(21,22)16-10-6-7-13-14(16)8-5-9-15(13)20(3)4/h5-10,19H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50267295

(CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2cccc(n2)C(C)(C)O)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C49H65N7O7/c1-47(2,3)41(54-45(60)63-9)43(58)52-36(28-33-21-23-34(24-22-33)37-19-13-14-25-50-37)30-39(57)38(29-32-16-11-10-12-17-32)53-44(59)42(48(4,5)6)56-27-26-55(46(56)61)31-35-18-15-20-40(51-35)49(7,8)62/h10-25,36,38-39,41-42,57,62H,26-31H2,1-9H3,(H,52,58)(H,53,59)(H,54,60)/t36-,38+,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50061077

((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCCO)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H30O8/c1-3-11-34-19-6-7-20-22(14-19)27(21-8-5-18(33-2)15-24(21)35-12-10-30)28(29(31)32)26(20)17-4-9-23-25(13-17)37-16-36-23/h4-9,13-15,26-28,30H,3,10-12,16H2,1-2H3,(H,31,32)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin B receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50061077

((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy...)Show SMILES CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCCO)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H30O8/c1-3-11-34-19-6-7-20-22(14-19)27(21-8-5-18(33-2)15-24(21)35-12-10-30)28(29(31)32)26(20)17-4-9-23-25(13-17)37-16-36-23/h4-9,13-15,26-28,30H,3,10-12,16H2,1-2H3,(H,31,32)/t26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin B receptor by measuring its ability to displace [125I]-ET-3 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50034263

(CHEMBL25438 | PD-156707 | Sodium; (Z)-2-benzo[1,3]...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C28H26O9/c1-32-19-8-5-17(6-9-19)26(29)20(11-16-12-23(33-2)27(35-4)24(13-16)34-3)25(28(30)31)18-7-10-21-22(14-18)37-15-36-21/h5-10,12-14H,11,15H2,1-4H3,(H,30,31)/p-1/b25-20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin B receptor by measuring its ability to displace [125I]-ET-3 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50066370

(5-Methyl-pyridine-2-sulfonic acid {6-(2-hydroxy-et...)Show SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(C)cn2)nc(nc1OCCO)-c1ccnc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C25H23N9O6S/c1-15-7-8-20(27-14-15)41(36,37)32-24-21(40-19-6-4-3-5-18(19)38-2)25(39-12-11-35)29-22(28-24)16-9-10-26-17(13-16)23-30-33-34-31-23/h3-10,13-14,35H,11-12H2,1-2H3,(H,28,29,32)(H,30,31,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin B receptor by measuring its ability to displace [125I]-ET-3 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(RAT) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50061101

(4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...)Show SMILES COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCO)-c1ncccn1 Show InChI InChI=1S/C27H29N5O6S/c1-27(2,3)18-10-12-19(13-11-18)39(34,35)32-23-22(38-21-9-6-5-8-20(21)36-4)26(37-17-16-33)31-25(30-23)24-28-14-7-15-29-24/h5-15,33H,16-17H2,1-4H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin B receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50267295

(CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2cccc(n2)C(C)(C)O)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C49H65N7O7/c1-47(2,3)41(54-45(60)63-9)43(58)52-36(28-33-21-23-34(24-22-33)37-19-13-14-25-50-37)30-39(57)38(29-32-16-11-10-12-17-32)53-44(59)42(48(4,5)6)56-27-26-55(46(56)61)31-35-18-15-20-40(51-35)49(7,8)62/h10-25,36,38-39,41-42,57,62H,26-31H2,1-9H3,(H,52,58)(H,53,59)(H,54,60)/t36-,38+,39+,41-,42-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D (unknown origin) |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(RAT) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50267297

(CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...)Show SMILES COC(=O)N[C@H](C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2ccccc2)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C47H60N6O6/c1-46(2,3)40(51-44(57)59-7)42(55)49-36(28-33-21-23-35(24-22-33)37-20-14-15-25-48-37)30-39(54)38(29-32-16-10-8-11-17-32)50-43(56)41(47(4,5)6)53-27-26-52(45(53)58)31-34-18-12-9-13-19-34/h8-25,36,38-41,54H,26-31H2,1-7H3,(H,49,55)(H,50,56)(H,51,57)/t36-,38-,39-,40+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50267297

(CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...)Show SMILES COC(=O)N[C@H](C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2ccccc2)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C47H60N6O6/c1-46(2,3)40(51-44(57)59-7)42(55)49-36(28-33-21-23-35(24-22-33)37-20-14-15-25-48-37)30-39(54)38(29-32-16-10-8-11-17-32)50-43(56)41(47(4,5)6)53-27-26-52(45(53)58)31-34-18-12-9-13-19-34/h8-25,36,38-41,54H,26-31H2,1-7H3,(H,49,55)(H,50,56)(H,51,57)/t36-,38-,39-,40+,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D (unknown origin) |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50066398

(3-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...)Show SMILES Cc1noc(NS(=O)(=O)c2sccc2C(=O)Cc2cc3OCOc3cc2C)c1Cl Show InChI InChI=1S/C18H15ClN2O6S2/c1-9-5-14-15(26-8-25-14)7-11(9)6-13(22)12-3-4-28-18(12)29(23,24)21-17-16(19)10(2)20-27-17/h3-5,7,21H,6,8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin B receptor by measuring its ability to displace [125I]-ET-3 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(RAT) | BDBM86689

(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50066391

((2S,3R,4S)-1-Dibutylcarbamoylmethyl-2-(2,2-dimethy...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1CC(C)(C)CCC)C(O)=O)c1cc2OCOc2c(OC)c1 Show InChI InChI=1S/C30H48N2O6/c1-7-10-13-31(14-11-8-2)26(33)19-32-18-22(21-15-24(36-6)28-25(16-21)37-20-38-28)27(29(34)35)23(32)17-30(4,5)12-9-3/h15-16,22-23,27H,7-14,17-20H2,1-6H3,(H,34,35)/t22-,23+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin B receptor by measuring its ability to displace [125I]-ET-3 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50267295

(CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2cccc(n2)C(C)(C)O)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C49H65N7O7/c1-47(2,3)41(54-45(60)63-9)43(58)52-36(28-33-21-23-34(24-22-33)37-19-13-14-25-50-37)30-39(57)38(29-32-16-11-10-12-17-32)53-44(59)42(48(4,5)6)56-27-26-55(46(56)61)31-35-18-15-20-40(51-35)49(7,8)62/h10-25,36,38-39,41-42,57,62H,26-31H2,1-9H3,(H,52,58)(H,53,59)(H,54,60)/t36-,38+,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50034435

(5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-d...)Show InChI InChI=1S/C17H19N3O3S/c1-11-12(2)18-23-17(11)19-24(21,22)16-10-6-7-13-14(16)8-5-9-15(13)20(3)4/h5-10,19H,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for binding affinity for human Endothelin B receptor by measuring its ability to displace [125I]-ET-3 from chinese hamster ovary cells(CHO) |
J Med Chem 41: 3261-75 (1998)
Article DOI: 10.1021/jm980217s
BindingDB Entry DOI: 10.7270/Q20Z72D4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50051007

((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptor |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50051007

((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding ability determined by the displacement of [125I]-ET-1 from the human endothelin A receptor |
J Med Chem 42: 3679-89 (1999)
Article DOI: 10.1021/jm990171i
BindingDB Entry DOI: 10.7270/Q2125RVT |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061096
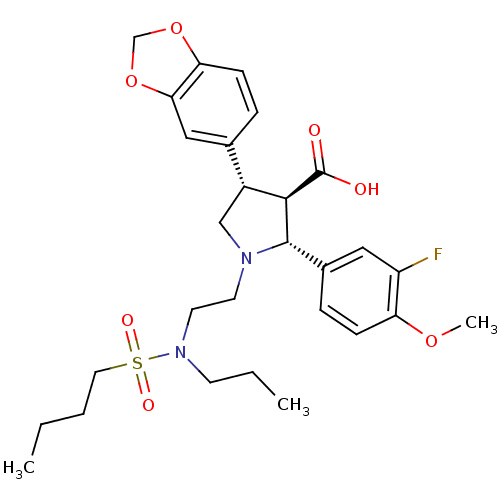
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...)Show SMILES CCCCS(=O)(=O)N(CCC)CCN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)c(F)c1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C28H37FN2O7S/c1-4-6-14-39(34,35)31(11-5-2)13-12-30-17-21(19-7-10-24-25(16-19)38-18-37-24)26(28(32)33)27(30)20-8-9-23(36-3)22(29)15-20/h7-10,15-16,21,26-27H,4-6,11-14,17-18H2,1-3H3,(H,32,33)/t21-,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061078
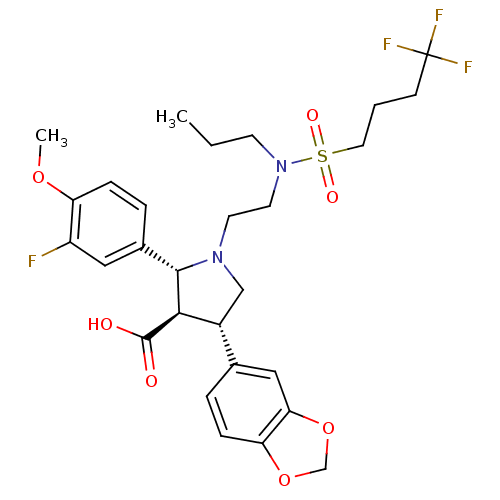
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-2-(3-fluoro-4-m...)Show SMILES CCCN(CCN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)c(F)c1)C(O)=O)c1ccc2OCOc2c1)S(=O)(=O)CCCC(F)(F)F Show InChI InChI=1S/C28H34F4N2O7S/c1-3-10-34(42(37,38)13-4-9-28(30,31)32)12-11-33-16-20(18-5-8-23-24(15-18)41-17-40-23)25(27(35)36)26(33)19-6-7-22(39-2)21(29)14-19/h5-8,14-15,20,25-26H,3-4,9-13,16-17H2,1-2H3,(H,35,36)/t20-,25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061086
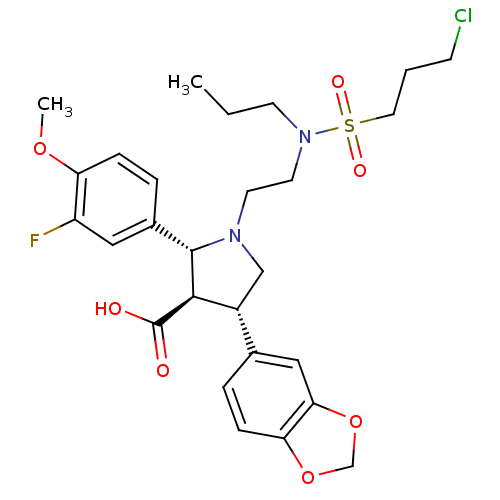
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(3-chloro...)Show SMILES CCCN(CCN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)c(F)c1)C(O)=O)c1ccc2OCOc2c1)S(=O)(=O)CCCCl Show InChI InChI=1S/C27H34ClFN2O7S/c1-3-10-31(39(34,35)13-4-9-28)12-11-30-16-20(18-5-8-23-24(15-18)38-17-37-23)25(27(32)33)26(30)19-6-7-22(36-2)21(29)14-19/h5-8,14-15,20,25-26H,3-4,9-13,16-17H2,1-2H3,(H,32,33)/t20-,25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50106408

(4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)c(F)c1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H37FN2O6/c1-4-6-12-31(13-7-5-2)26(33)17-32-16-21(19-8-11-24-25(15-19)38-18-37-24)27(29(34)35)28(32)20-9-10-23(36-3)22(30)14-20/h8-11,14-15,21,27-28H,4-7,12-13,16-18H2,1-3H3,(H,34,35)/t21-,27-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against endothelin A receptor in MMQ cells in rat |
J Med Chem 44: 3978-84 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9PR2 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061085
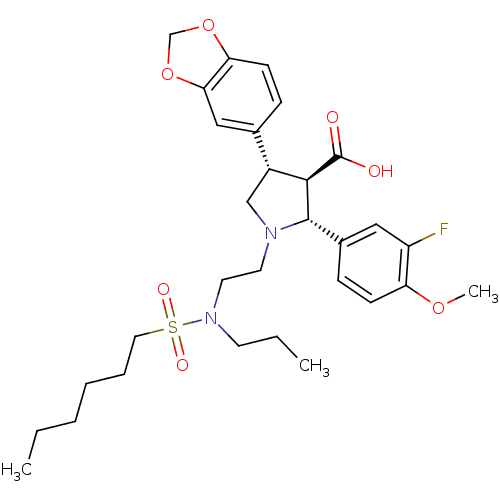
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-2-(3-fluoro-4-m...)Show SMILES CCCCCCS(=O)(=O)N(CCC)CCN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)c(F)c1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C30H41FN2O7S/c1-4-6-7-8-16-41(36,37)33(13-5-2)15-14-32-19-23(21-9-12-26-27(18-21)40-20-39-26)28(30(34)35)29(32)22-10-11-25(38-3)24(31)17-22/h9-12,17-18,23,28-29H,4-8,13-16,19-20H2,1-3H3,(H,34,35)/t23-,28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50061074
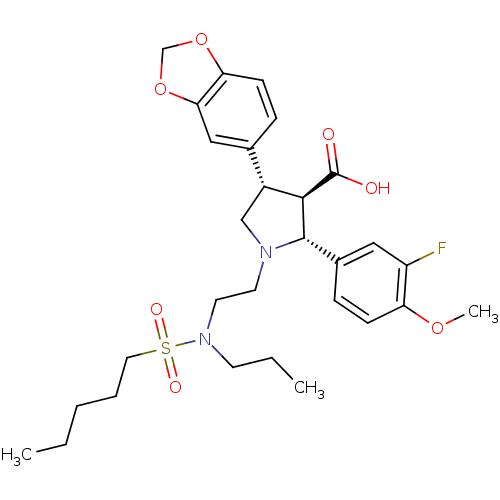
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-2-(3-fluoro-4-m...)Show SMILES CCCCCS(=O)(=O)N(CCC)CCN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)c(F)c1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H39FN2O7S/c1-4-6-7-15-40(35,36)32(12-5-2)14-13-31-18-22(20-8-11-25-26(17-20)39-19-38-25)27(29(33)34)28(31)21-9-10-24(37-3)23(30)16-21/h8-11,16-17,22,27-28H,4-7,12-15,18-19H2,1-3H3,(H,33,34)/t22-,27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). |
J Med Chem 40: 3217-27 (1997)
Article DOI: 10.1021/jm970101g
BindingDB Entry DOI: 10.7270/Q2C24VJR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data