

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50123504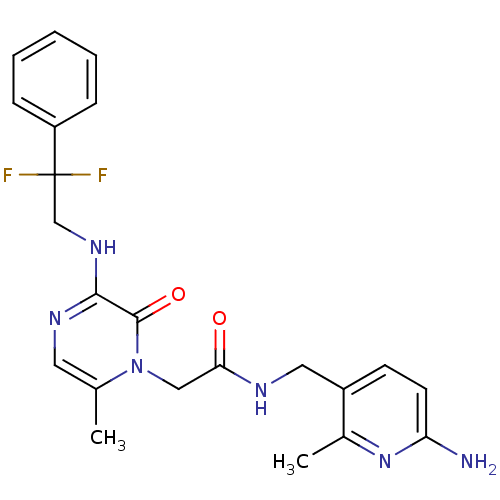 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273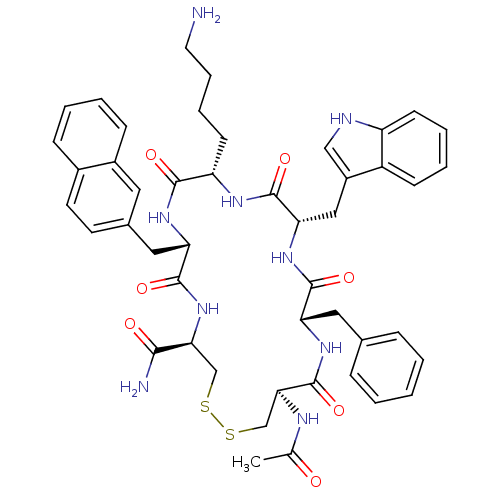 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377618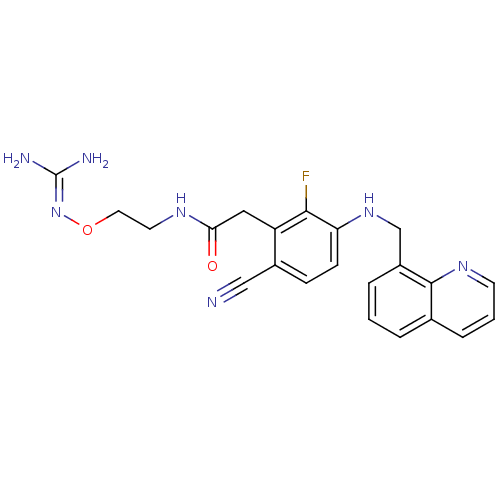 (CHEMBL254353) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377625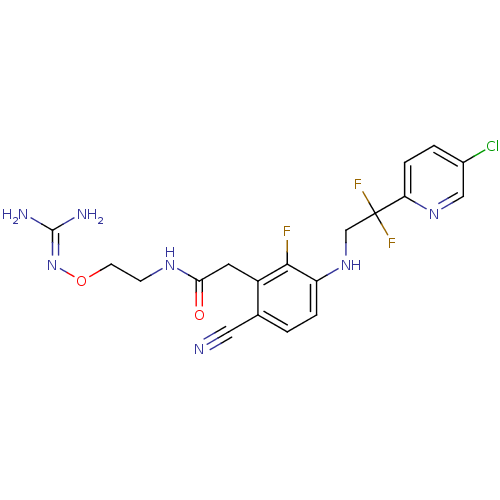 (CHEMBL254557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377615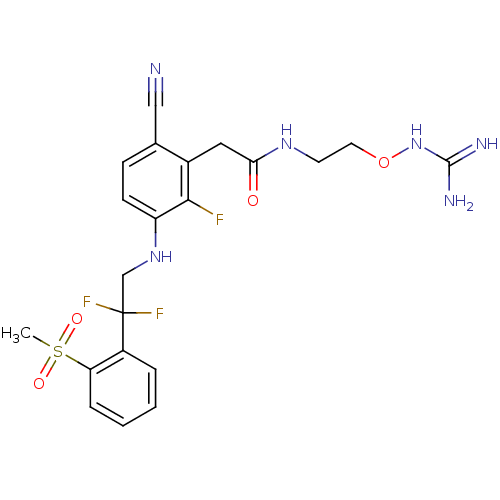 (CHEMBL254962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377623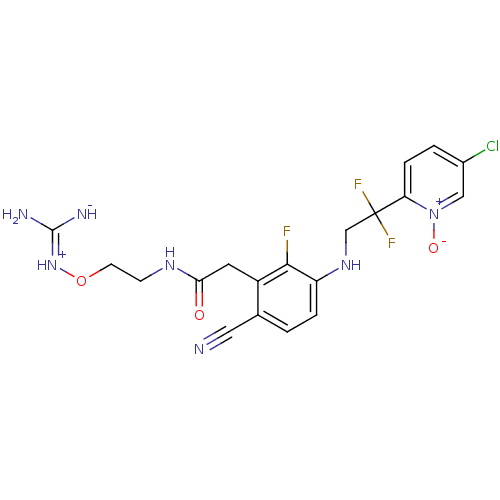 (CHEMBL254759) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377611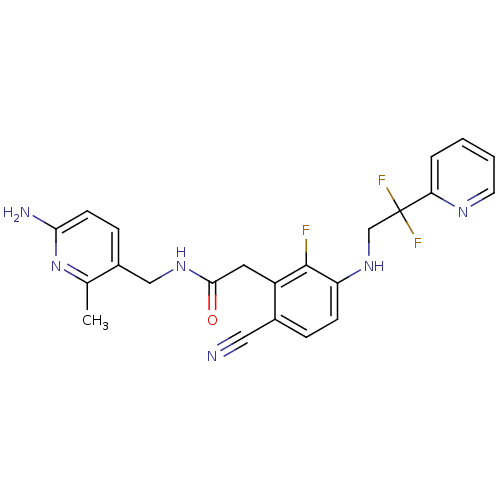 (CHEMBL258018) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377619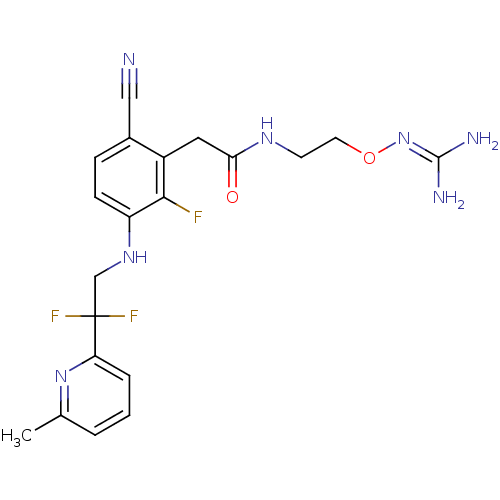 (CHEMBL402758) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377622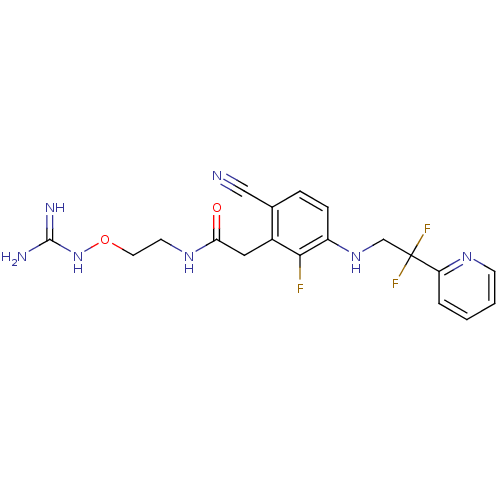 (CHEMBL257543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377620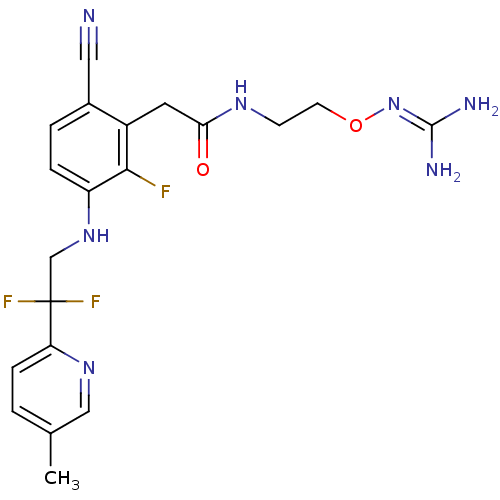 (CHEMBL254784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377614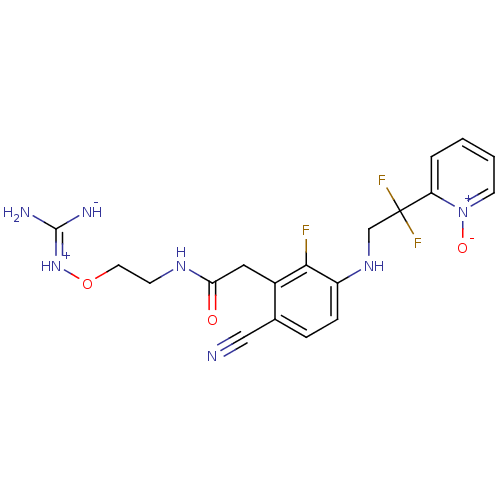 (CHEMBL401655) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312651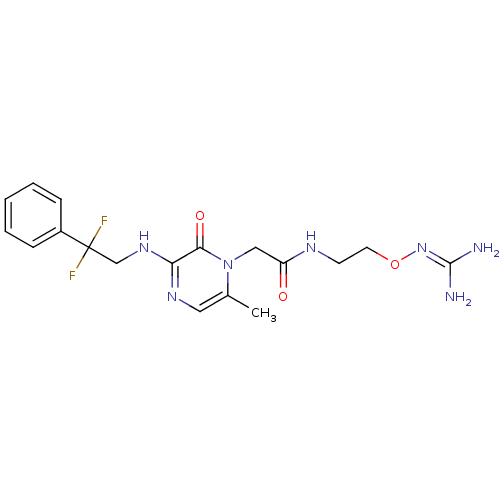 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231280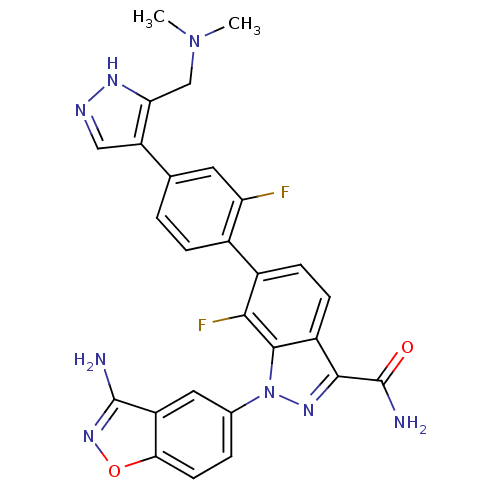 (1-(3-cyano-4-fluorophenyl)-6-[4-(3-dimethylaminome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377617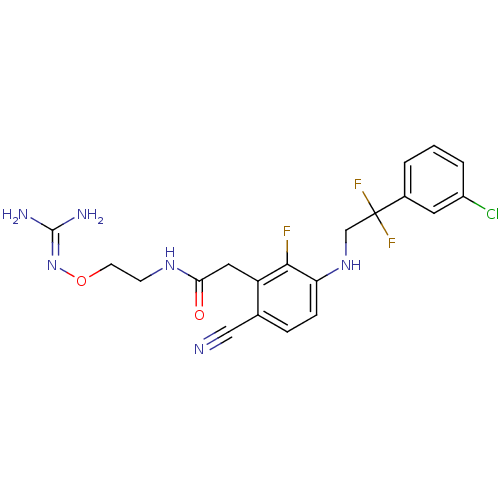 (CHEMBL403359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312653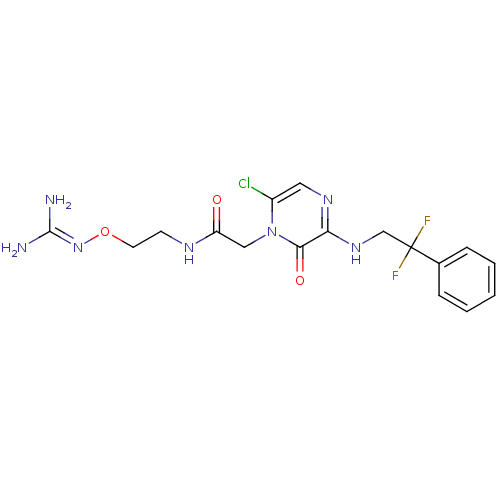 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377624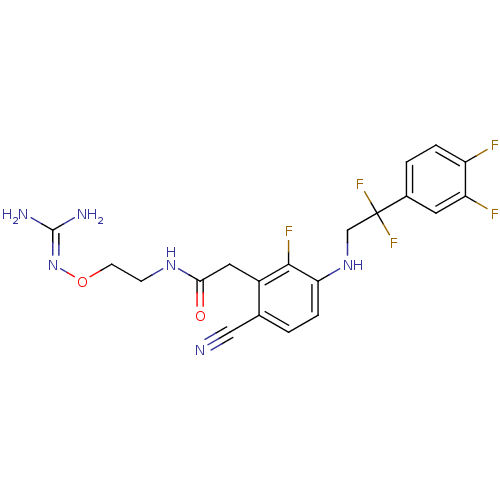 (CHEMBL403310) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312652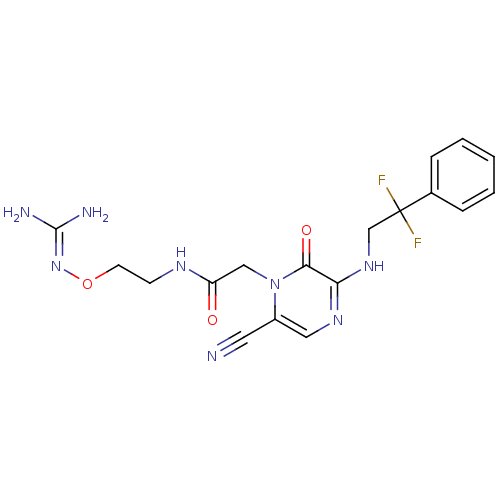 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208224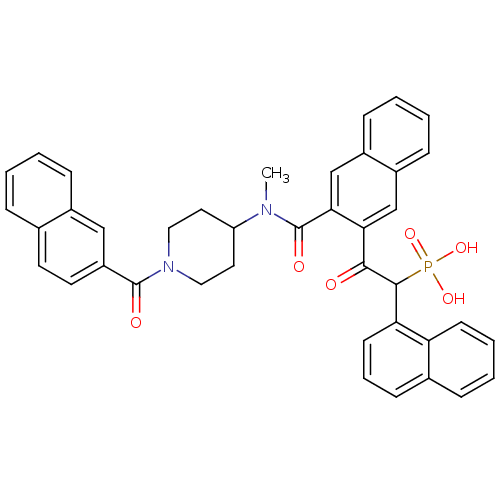 (2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377607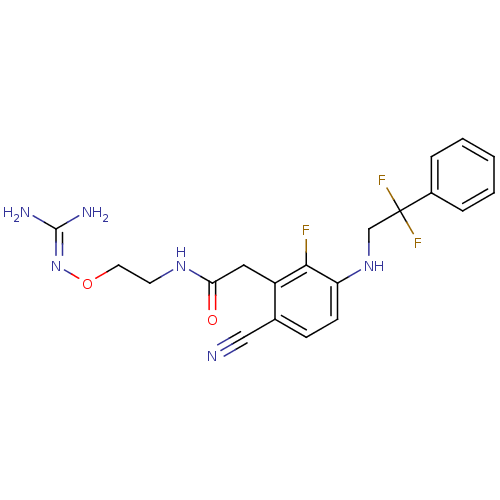 (CHEMBL404025) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377626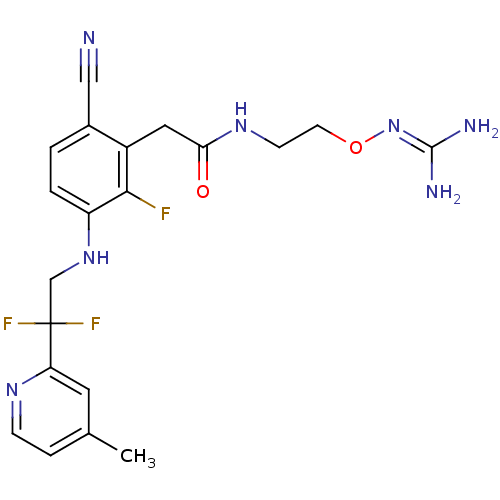 (CHEMBL254786) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231282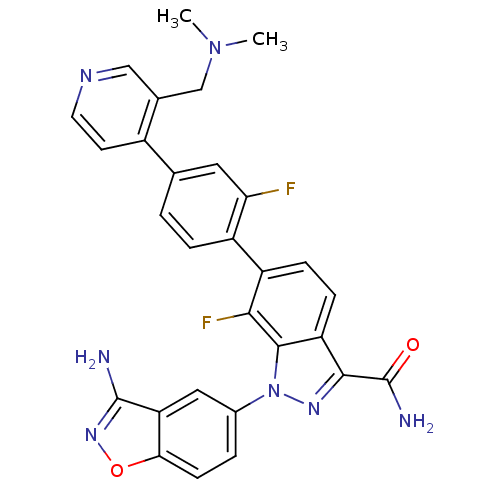 (1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377616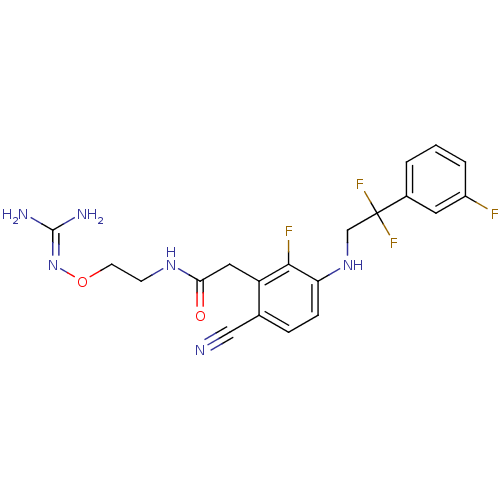 (CHEMBL258198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50149023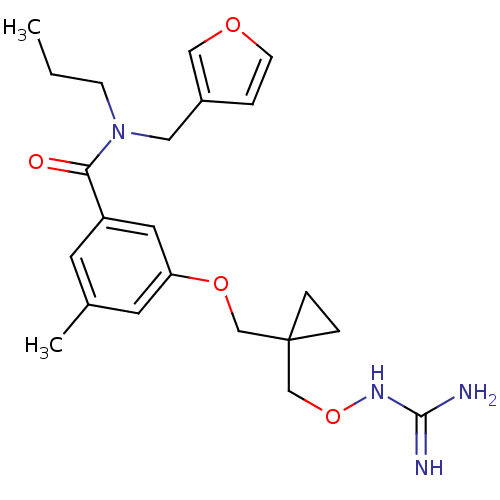 (3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302258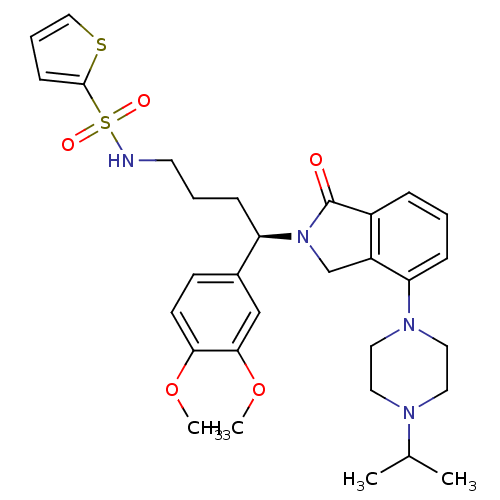 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50149023 (3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302257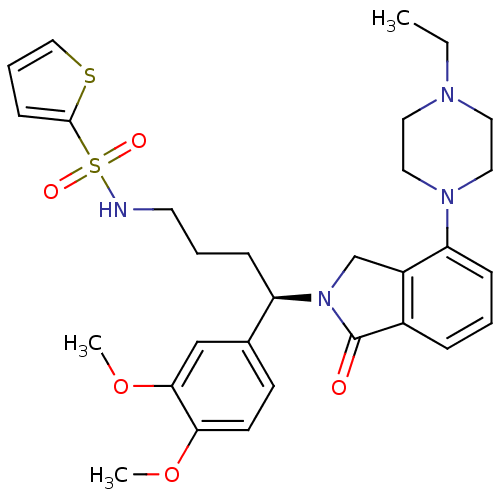 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377608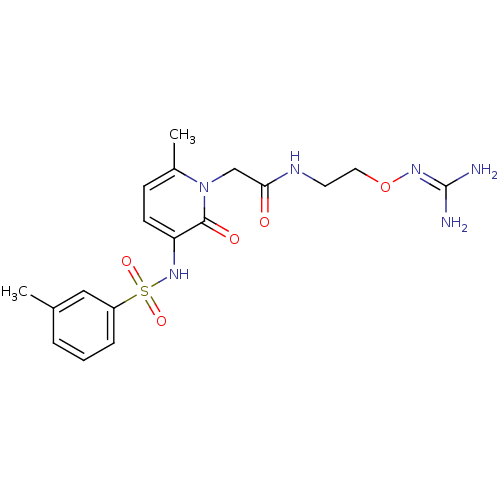 (CHEMBL256941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231275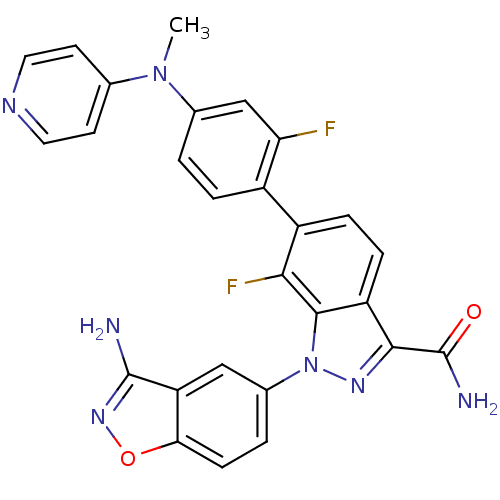 (1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231284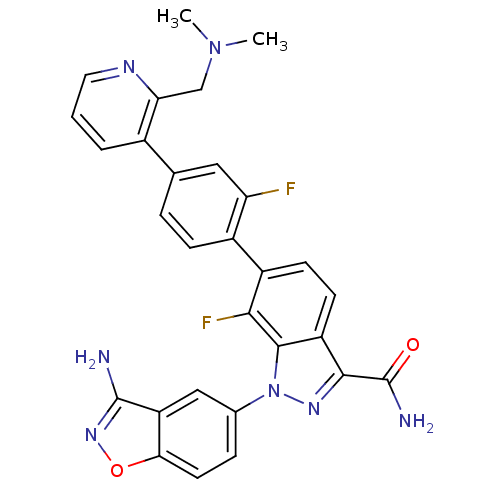 (1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(2-dimethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231271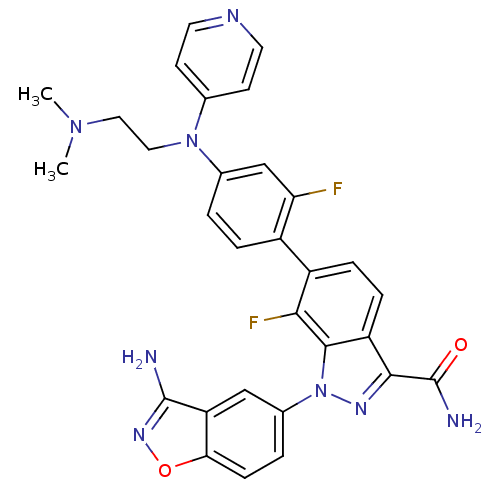 (1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231279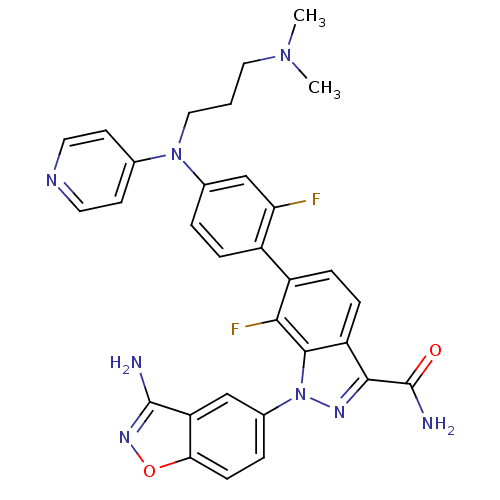 (1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231283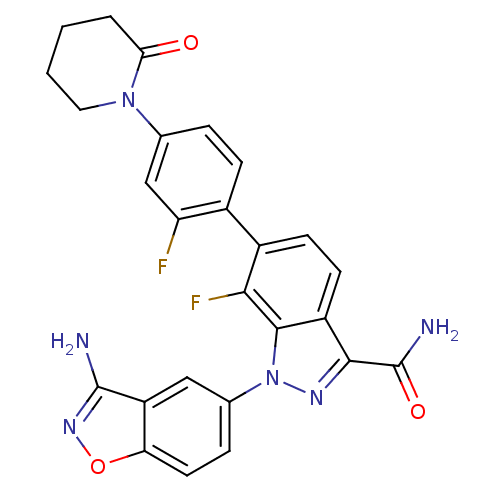 (1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312663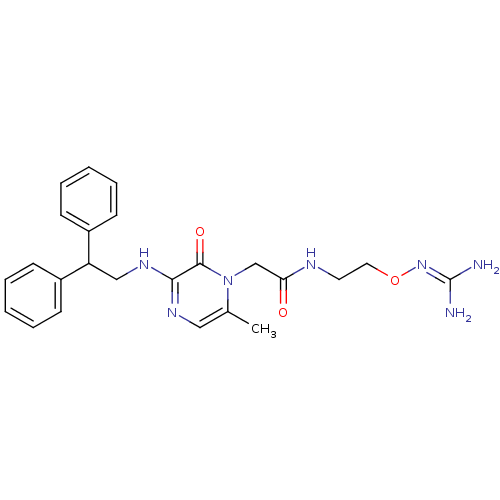 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302271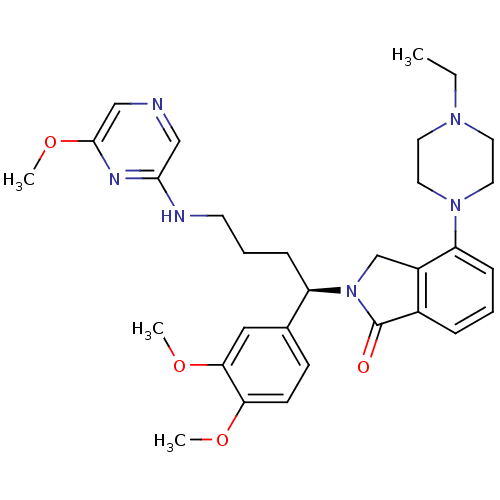 ((R)-2-(1-(3,4-dimethoxyphenyl)-4-(6-methoxypyrazin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878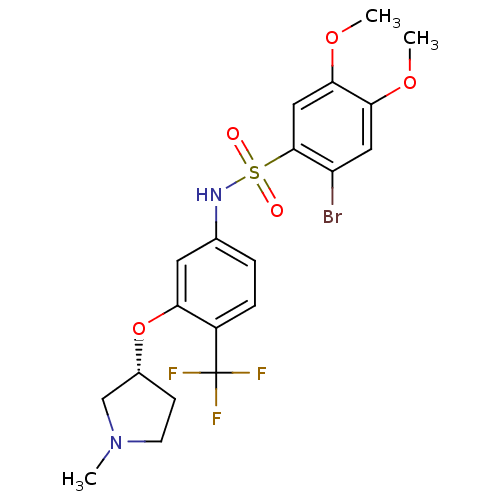 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231270 (1-(3-aminobenzo[d]isoxazol-5-yl)-6-(2'-dimethylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 51: 282-97 (2008) Article DOI: 10.1021/jm701217r BindingDB Entry DOI: 10.7270/Q2K64HTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377613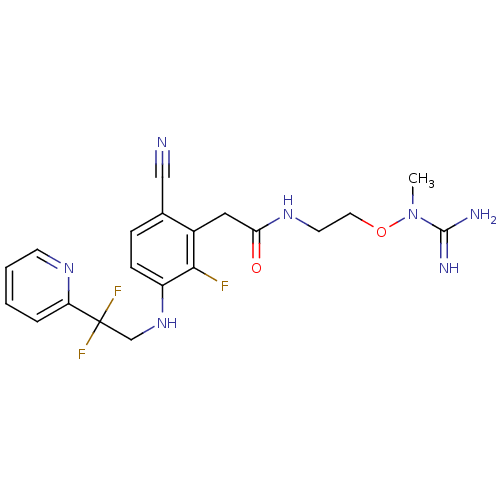 (CHEMBL258225) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377621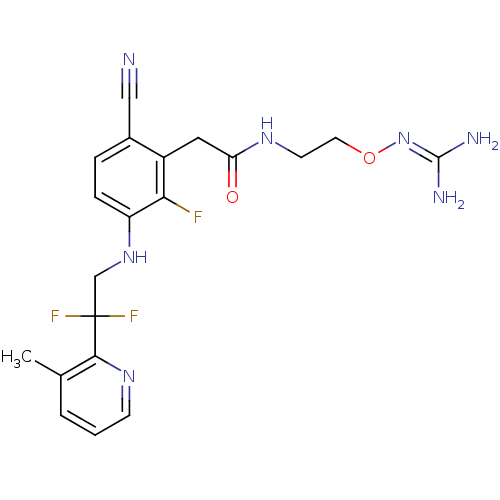 (CHEMBL254785) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312658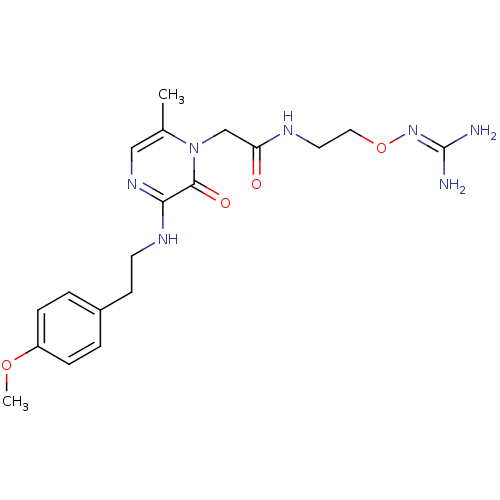 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377612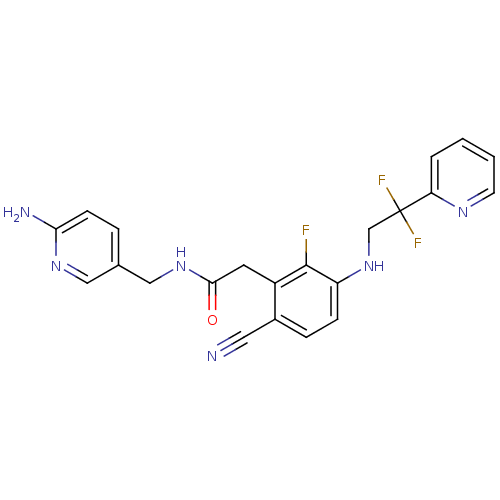 (CHEMBL255916) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208222 ((E)-2-(3-chloro-5-fluorostyrylamino)-1-(5-chlorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302267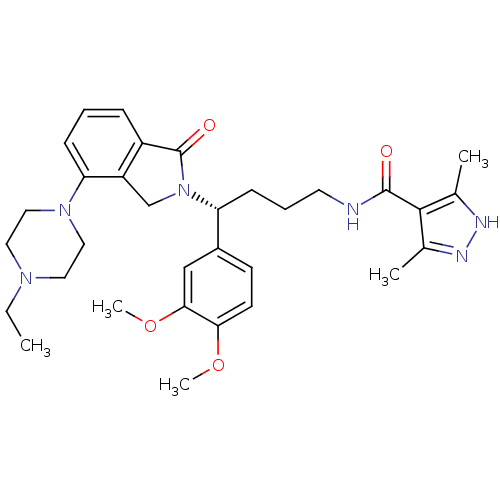 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50162995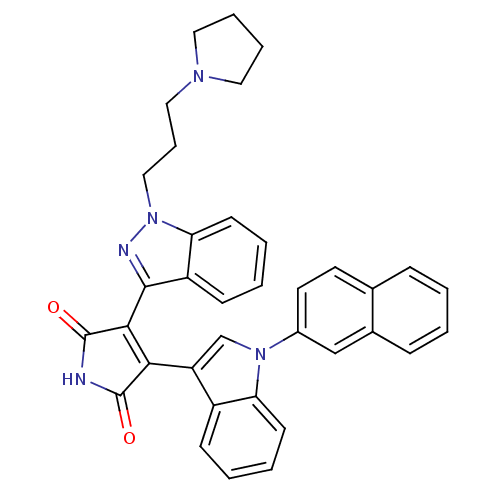 (3-[1-(1,2-Dihydro-pyridin-2-yl)-1H-indazol-3-yl]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of Potassium channel HERG | J Med Chem 48: 1725-8 (2005) Article DOI: 10.1021/jm049478u BindingDB Entry DOI: 10.7270/Q2765DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302264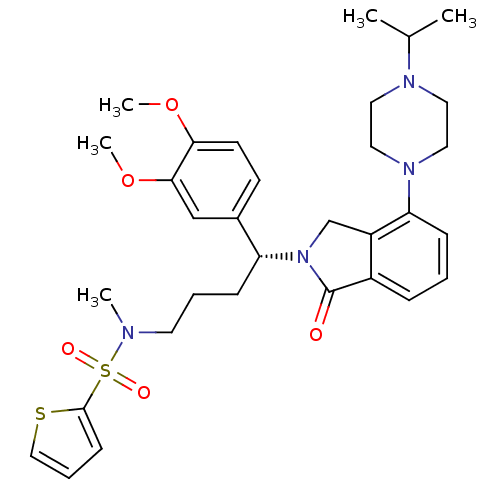 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312662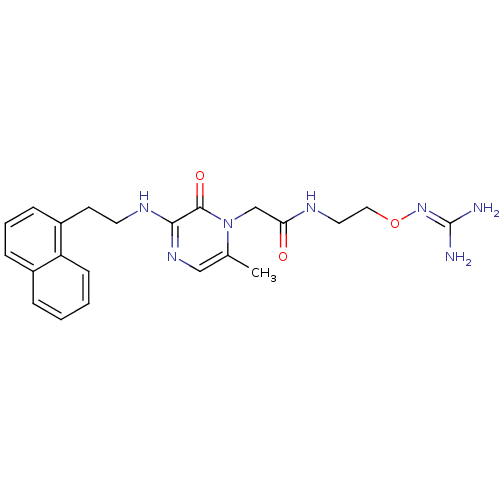 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312654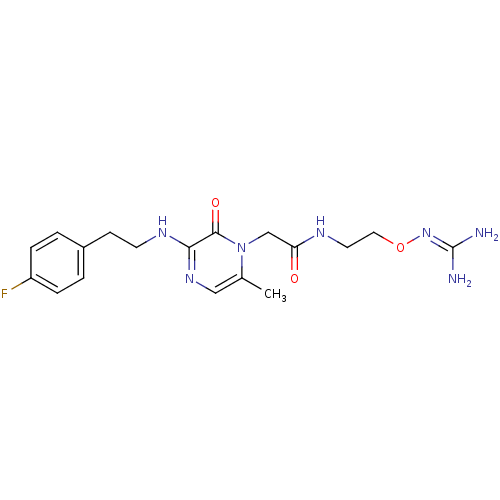 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302253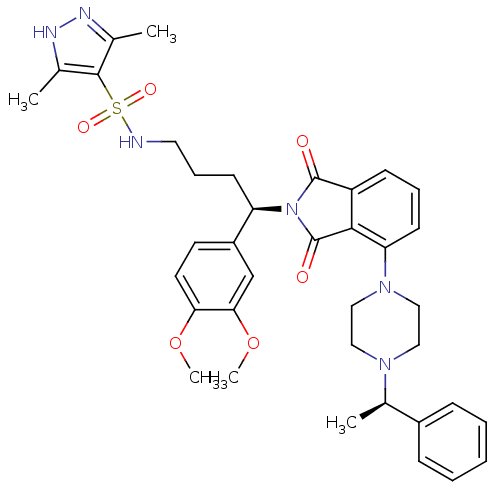 (CHEMBL567759 | N-((R)-4-(3,4-dimethoxyphenyl)-4-(1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50312656 (1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | J Med Chem 53: 1843-56 (2010) Article DOI: 10.1021/jm901802n BindingDB Entry DOI: 10.7270/Q22807Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1009 total ) | Next | Last >> |