 Found 587 hits with Last Name = 'bond' and Initial = 'br'
Found 587 hits with Last Name = 'bond' and Initial = 'br' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316640
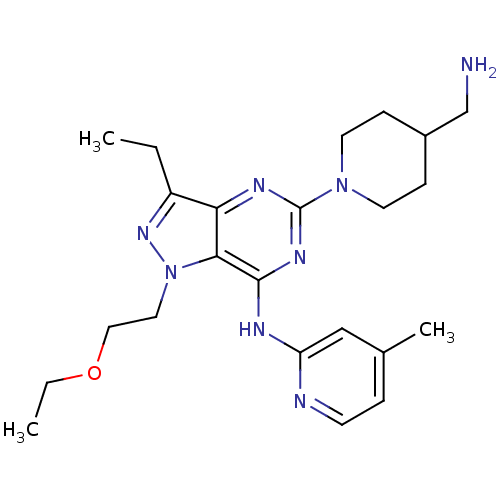
(5-(4-(aminomethyl)piperidin-1-yl)-1-(2-ethoxyethyl...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCC(CN)CC1 Show InChI InChI=1S/C23H34N8O/c1-4-18-20-21(31(29-18)12-13-32-5-2)22(26-19-14-16(3)6-9-25-19)28-23(27-20)30-10-7-17(15-24)8-11-30/h6,9,14,17H,4-5,7-8,10-13,15,24H2,1-3H3,(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357238
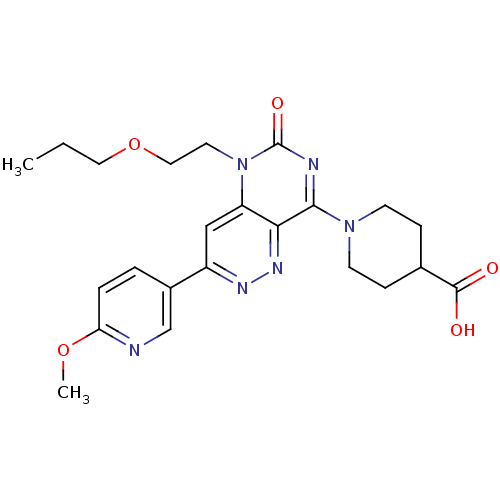
(CHEMBL1916488)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCC(CC1)C(O)=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H28N6O5/c1-3-11-34-12-10-29-18-13-17(16-4-5-19(33-2)24-14-16)26-27-20(18)21(25-23(29)32)28-8-6-15(7-9-28)22(30)31/h4-5,13-15H,3,6-12H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316638
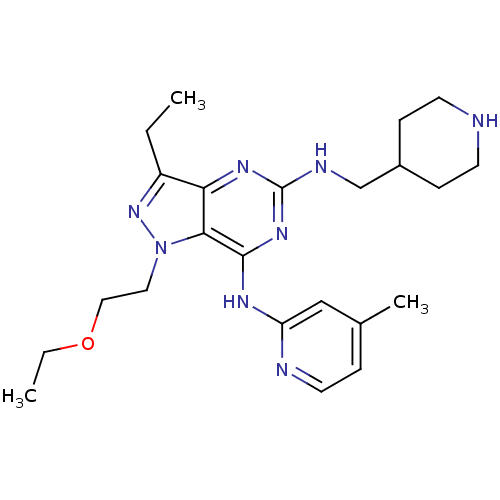
(1-(2-ethoxyethyl)-3-ethyl-N7-(4-methylpyridin-2-yl...)Show SMILES CCOCCn1nc(CC)c2nc(NCC3CCNCC3)nc(Nc3cc(C)ccn3)c12 Show InChI InChI=1S/C23H34N8O/c1-4-18-20-21(31(30-18)12-13-32-5-2)22(27-19-14-16(3)6-11-25-19)29-23(28-20)26-15-17-7-9-24-10-8-17/h6,11,14,17,24H,4-5,7-10,12-13,15H2,1-3H3,(H2,25,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357243
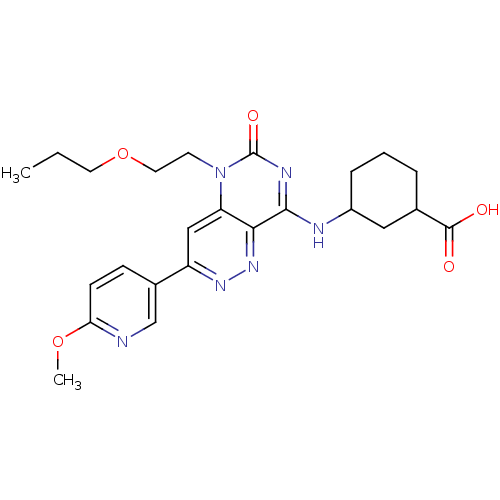
(CHEMBL1916483)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCCC(C2)C(O)=O)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O5/c1-3-10-35-11-9-30-19-13-18(16-7-8-20(34-2)25-14-16)28-29-21(19)22(27-24(30)33)26-17-6-4-5-15(12-17)23(31)32/h7-8,13-15,17H,3-6,9-12H2,1-2H3,(H,31,32)(H,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357241
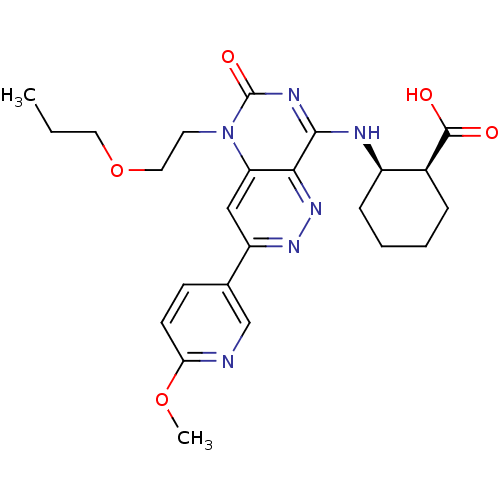
(CHEMBL1916485)Show SMILES CCCOCCn1c2cc(nnc2c(N[C@@H]2CCCC[C@@H]2C(O)=O)nc1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-13-18(15-8-9-20(34-2)25-14-15)28-29-21(19)22(27-24(30)33)26-17-7-5-4-6-16(17)23(31)32/h8-9,13-14,16-17H,3-7,10-12H2,1-2H3,(H,31,32)(H,26,27,33)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357234
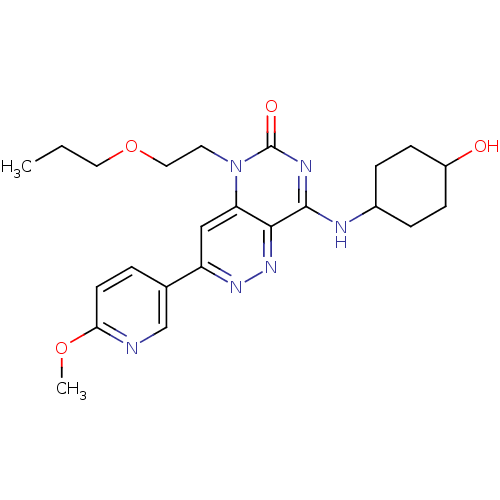
(CHEMBL1916475)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(22.74,-11.54,;21.41,-12.31,;20.07,-11.54,;18.74,-12.31,;18.74,-13.85,;17.41,-14.62,;17.41,-16.16,;18.74,-16.94,;20.06,-16.18,;21.39,-16.94,;21.39,-18.48,;20.06,-19.25,;18.73,-18.48,;17.41,-19.24,;17.41,-20.78,;16.08,-21.56,;14.75,-20.78,;13.42,-21.54,;13.41,-23.08,;12.08,-23.85,;14.75,-23.86,;16.09,-23.09,;16.08,-18.48,;16.08,-16.94,;14.74,-16.18,;22.71,-16.16,;22.71,-14.63,;24.03,-13.85,;25.38,-14.61,;26.71,-13.84,;28.04,-14.6,;25.38,-16.16,;24.05,-16.93,)| Show InChI InChI=1S/C23H30N6O4/c1-3-11-33-12-10-29-19-13-18(15-4-9-20(32-2)24-14-15)27-28-21(19)22(26-23(29)31)25-16-5-7-17(30)8-6-16/h4,9,13-14,16-17,30H,3,5-8,10-12H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357240
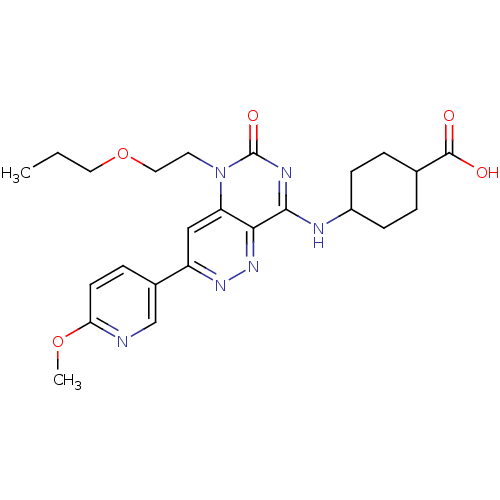
(CHEMBL1916486)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCC(CC2)C(O)=O)nc1=O)-c1ccc(OC)nc1 |(2.15,6.01,;.82,5.24,;-.51,6.01,;-1.85,5.24,;-1.85,3.69,;-3.18,2.92,;-3.18,1.38,;-1.85,.61,;-.53,1.37,;.8,.61,;.8,-.93,;-.53,-1.7,;-1.86,-.93,;-3.18,-1.7,;-3.18,-3.24,;-4.51,-4.01,;-5.85,-3.24,;-7.18,-4,;-7.18,-5.54,;-5.85,-6.32,;-4.51,-5.55,;-8.52,-6.31,;-9.85,-5.54,;-8.52,-7.85,;-4.51,-.93,;-4.51,.61,;-5.85,1.37,;2.12,1.39,;2.12,2.92,;3.44,3.7,;4.79,2.93,;6.12,3.71,;7.45,2.95,;4.79,1.39,;3.46,.62,)| Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-13-18(16-6-9-20(34-2)25-14-16)28-29-21(19)22(27-24(30)33)26-17-7-4-15(5-8-17)23(31)32/h6,9,13-15,17H,3-5,7-8,10-12H2,1-2H3,(H,31,32)(H,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357239
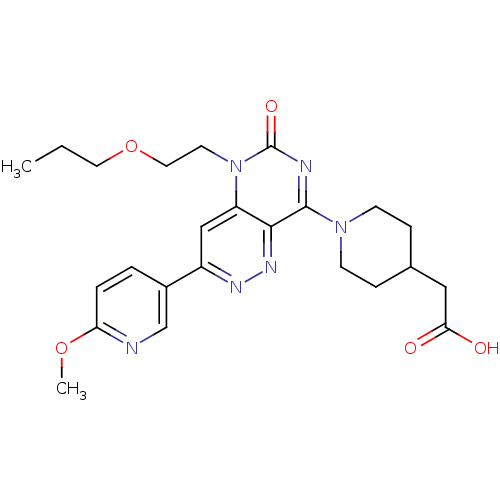
(CHEMBL1916487)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCC(CC(O)=O)CC1)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-14-18(17-4-5-20(34-2)25-15-17)27-28-22(19)23(26-24(30)33)29-8-6-16(7-9-29)13-21(31)32/h4-5,14-16H,3,6-13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300970
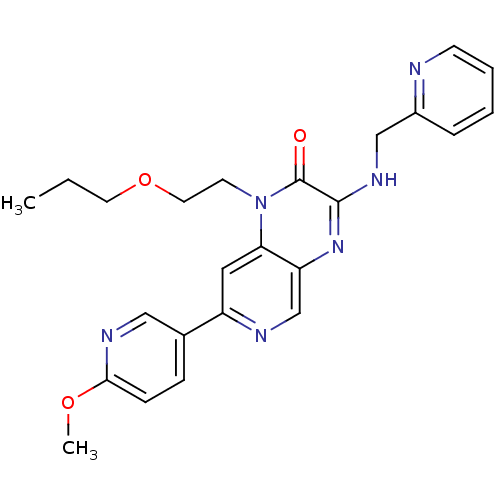
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCc2ccccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H26N6O3/c1-3-11-33-12-10-30-21-13-19(17-7-8-22(32-2)27-14-17)26-16-20(21)29-23(24(30)31)28-15-18-6-4-5-9-25-18/h4-9,13-14,16H,3,10-12,15H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316647
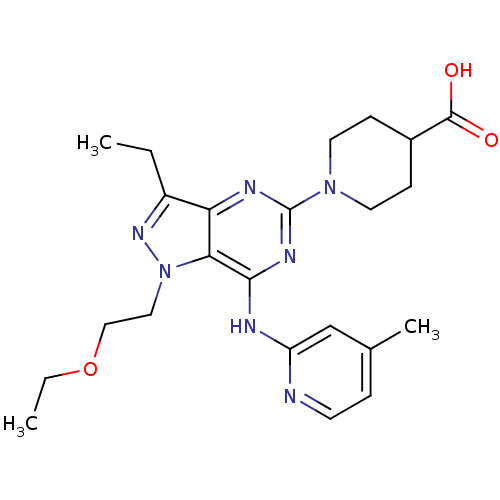
(1-(1-(2-ethoxyethyl)-3-ethyl-7-(4-methylpyridin-2-...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCC(CC1)C(O)=O Show InChI InChI=1S/C23H31N7O3/c1-4-17-19-20(30(28-17)12-13-33-5-2)21(25-18-14-15(3)6-9-24-18)27-23(26-19)29-10-7-16(8-11-29)22(31)32/h6,9,14,16H,4-5,7-8,10-13H2,1-3H3,(H,31,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357250
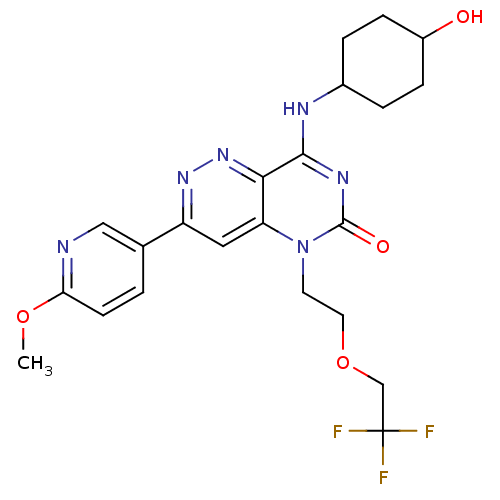
(CHEMBL1916476)Show SMILES COc1ccc(cn1)-c1cc2n(CCOCC(F)(F)F)c(=O)nc(NC3CCC(O)CC3)c2nn1 |(6.09,-33.21,;4.75,-32.45,;3.42,-33.22,;2.07,-32.46,;.75,-33.24,;.75,-34.77,;2.09,-35.54,;3.42,-34.77,;-.57,-35.55,;-1.9,-34.79,;-3.22,-35.55,;-4.55,-34.77,;-4.55,-33.23,;-3.22,-32.46,;-3.22,-30.92,;-1.88,-30.15,;-.55,-30.92,;-.55,-32.46,;.78,-30.15,;.78,-31.69,;-5.88,-35.55,;-7.22,-34.79,;-5.88,-37.09,;-4.55,-37.85,;-4.55,-39.4,;-5.88,-40.17,;-7.21,-39.39,;-8.54,-40.16,;-8.55,-41.7,;-9.88,-42.46,;-7.21,-42.47,;-5.87,-41.7,;-3.23,-37.09,;-1.9,-37.86,;-.57,-37.09,)| Show InChI InChI=1S/C22H25F3N6O4/c1-34-18-7-2-13(11-26-18)16-10-17-19(30-29-16)20(27-14-3-5-15(32)6-4-14)28-21(33)31(17)8-9-35-12-22(23,24)25/h2,7,10-11,14-15,32H,3-6,8-9,12H2,1H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357254
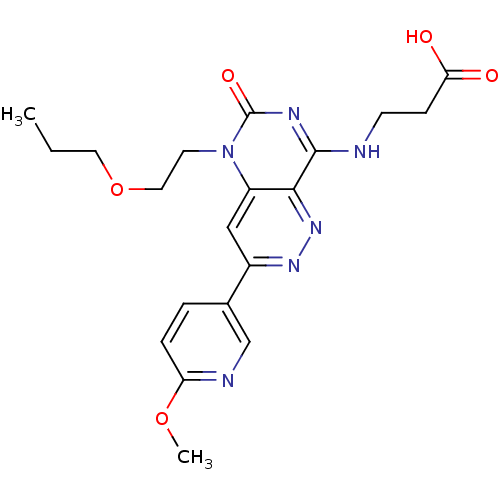
(CHEMBL1916303)Show SMILES CCCOCCn1c2cc(nnc2c(NCCC(O)=O)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H24N6O5/c1-3-9-31-10-8-26-15-11-14(13-4-5-16(30-2)22-12-13)24-25-18(15)19(23-20(26)29)21-7-6-17(27)28/h4-5,11-12H,3,6-10H2,1-2H3,(H,27,28)(H,21,23,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357244
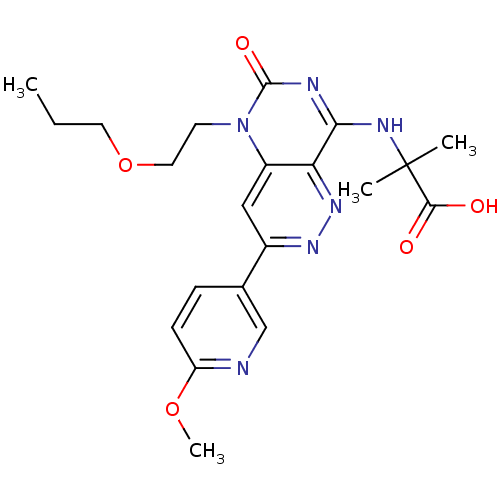
(CHEMBL1916482)Show SMILES CCCOCCn1c2cc(nnc2c(NC(C)(C)C(O)=O)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H26N6O5/c1-5-9-32-10-8-27-15-11-14(13-6-7-16(31-4)22-12-13)25-26-17(15)18(23-20(27)30)24-21(2,3)19(28)29/h6-7,11-12H,5,8-10H2,1-4H3,(H,28,29)(H,23,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300953
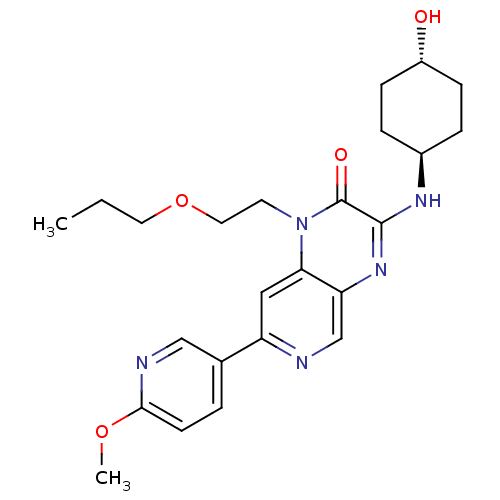
(3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxyp...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(24.97,-.51,;23.64,-1.28,;23.64,-2.82,;22.3,-3.59,;22.3,-5.13,;20.97,-5.9,;20.97,-7.44,;22.3,-8.21,;23.62,-7.45,;24.95,-8.21,;24.95,-9.75,;23.62,-10.52,;22.3,-9.75,;20.97,-10.52,;19.62,-9.75,;18.29,-10.51,;16.96,-9.74,;15.63,-10.51,;14.29,-9.73,;14.3,-8.19,;12.97,-7.41,;15.64,-7.43,;16.97,-8.2,;19.64,-8.21,;18.31,-7.44,;26.28,-7.43,;27.61,-8.2,;28.94,-7.43,;28.94,-5.89,;30.27,-5.11,;31.61,-5.87,;27.59,-5.12,;26.27,-5.9,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-19(16-4-9-22(32-2)26-14-16)25-15-20(21)28-23(24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300964
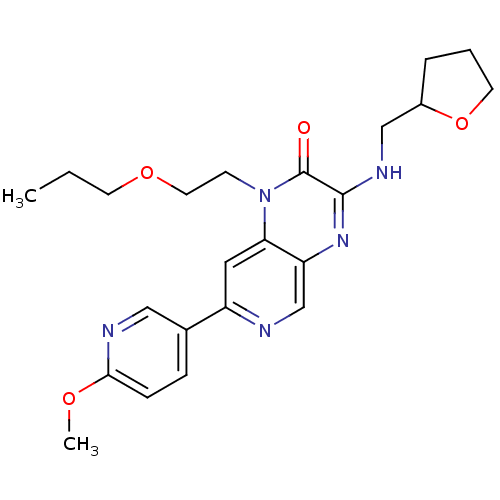
(CHEMBL584270 | rac-7-(6-methoxypyridin-3-yl)-1-(2-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC2CCCO2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H29N5O4/c1-3-9-31-11-8-28-20-12-18(16-6-7-21(30-2)25-13-16)24-15-19(20)27-22(23(28)29)26-14-17-5-4-10-32-17/h6-7,12-13,15,17H,3-5,8-11,14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316646
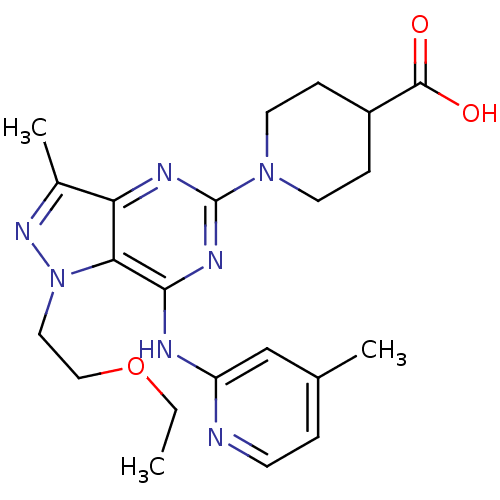
(1-(1-(2-ethoxyethyl)-3-methyl-7-(4-methylpyridin-2...)Show SMILES CCOCCn1nc(C)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCC(CC1)C(O)=O Show InChI InChI=1S/C22H29N7O3/c1-4-32-12-11-29-19-18(15(3)27-29)25-22(28-9-6-16(7-10-28)21(30)31)26-20(19)24-17-13-14(2)5-8-23-17/h5,8,13,16H,4,6-7,9-12H2,1-3H3,(H,30,31)(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300953

(3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxyp...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(24.97,-.51,;23.64,-1.28,;23.64,-2.82,;22.3,-3.59,;22.3,-5.13,;20.97,-5.9,;20.97,-7.44,;22.3,-8.21,;23.62,-7.45,;24.95,-8.21,;24.95,-9.75,;23.62,-10.52,;22.3,-9.75,;20.97,-10.52,;19.62,-9.75,;18.29,-10.51,;16.96,-9.74,;15.63,-10.51,;14.29,-9.73,;14.3,-8.19,;12.97,-7.41,;15.64,-7.43,;16.97,-8.2,;19.64,-8.21,;18.31,-7.44,;26.28,-7.43,;27.61,-8.2,;28.94,-7.43,;28.94,-5.89,;30.27,-5.11,;31.61,-5.87,;27.59,-5.12,;26.27,-5.9,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-19(16-4-9-22(32-2)26-14-16)25-15-20(21)28-23(24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357263

(CHEMBL1916293)Show SMILES CCCOCCn1c2cc(ncc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(28.17,-7.71,;26.83,-8.48,;25.5,-7.71,;24.17,-8.48,;24.17,-10.02,;22.83,-10.79,;22.83,-12.33,;24.16,-13.11,;25.48,-12.34,;26.81,-13.1,;26.82,-14.65,;25.49,-15.41,;24.16,-14.65,;22.83,-15.41,;22.83,-16.95,;21.5,-17.72,;20.17,-16.95,;18.84,-17.71,;18.84,-19.25,;17.5,-20.02,;20.17,-20.02,;21.51,-19.26,;21.5,-14.65,;21.5,-13.11,;20.17,-12.34,;28.14,-12.33,;28.13,-10.79,;29.46,-10.01,;30.8,-10.78,;32.13,-10,;33.47,-10.77,;30.81,-12.32,;29.48,-13.1,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-20(16-4-9-22(32-2)26-14-16)25-15-19(21)23(28-24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300972
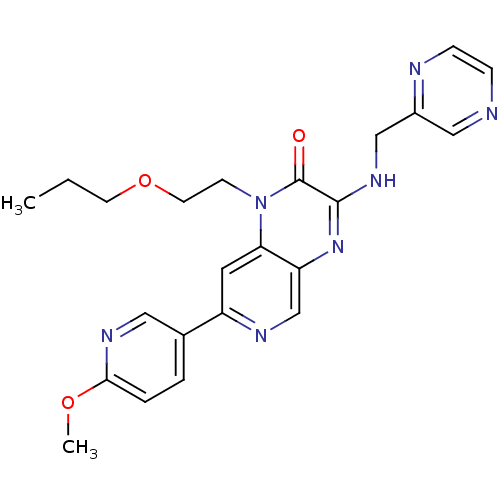
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCc2cnccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H25N7O3/c1-3-9-33-10-8-30-20-11-18(16-4-5-21(32-2)27-12-16)26-15-19(20)29-22(23(30)31)28-14-17-13-24-6-7-25-17/h4-7,11-13,15H,3,8-10,14H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357237
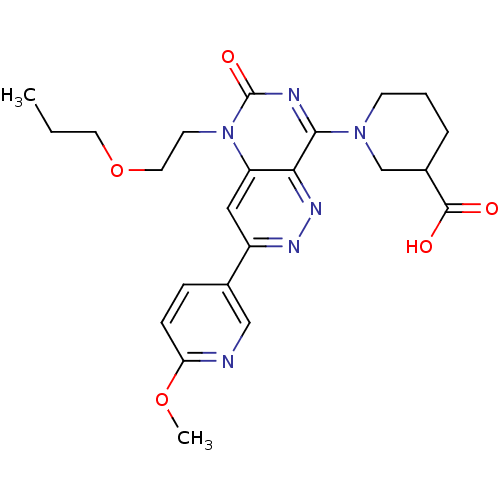
(CHEMBL1916489)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCCC(C1)C(O)=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H28N6O5/c1-3-10-34-11-9-29-18-12-17(15-6-7-19(33-2)24-13-15)26-27-20(18)21(25-23(29)32)28-8-4-5-16(14-28)22(30)31/h6-7,12-13,16H,3-5,8-11,14H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50318137
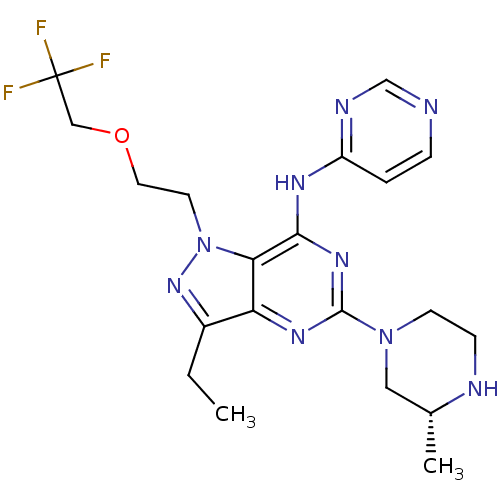
((R)-3-ethyl-5-(3-methylpiperazin-1-yl)-N-(pyrimidi...)Show SMILES CCc1nn(CCOCC(F)(F)F)c2c(Nc3ccncn3)nc(nc12)N1CCN[C@H](C)C1 |r| Show InChI InChI=1S/C20H26F3N9O/c1-3-14-16-17(32(30-14)8-9-33-11-20(21,22)23)18(27-15-4-5-24-12-26-15)29-19(28-16)31-7-6-25-13(2)10-31/h4-5,12-13,25H,3,6-11H2,1-2H3,(H,24,26,27,28,29)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 20: 3125-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.106
BindingDB Entry DOI: 10.7270/Q2WH2QXD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296256
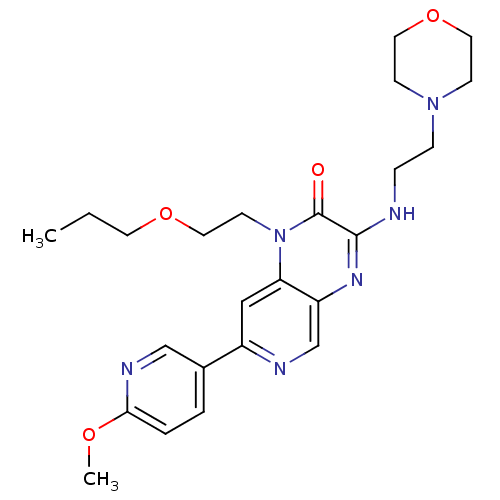
(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4092-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.004
BindingDB Entry DOI: 10.7270/Q2ZG6S9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316642
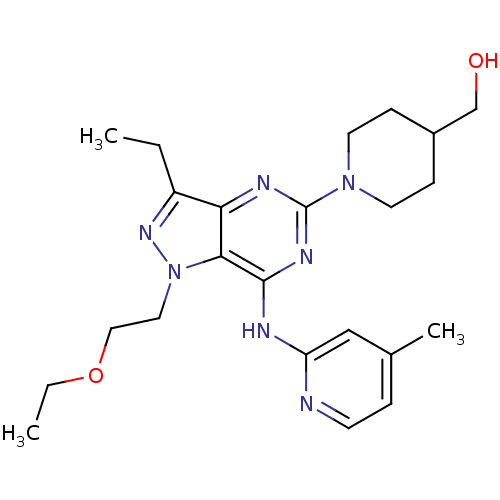
((1-(1-(2-ethoxyethyl)-3-ethyl-7-(4-methylpyridin-2...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCC(CO)CC1 Show InChI InChI=1S/C23H33N7O2/c1-4-18-20-21(30(28-18)12-13-32-5-2)22(25-19-14-16(3)6-9-24-19)27-23(26-20)29-10-7-17(15-31)8-11-29/h6,9,14,17,31H,4-5,7-8,10-13,15H2,1-3H3,(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300976
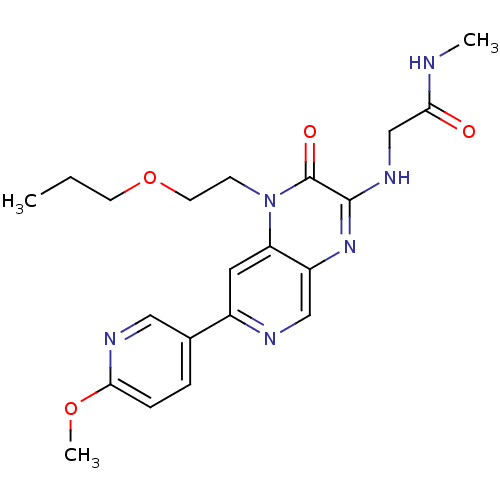
(2-(7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)NC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H26N6O4/c1-4-8-31-9-7-27-17-10-15(14-5-6-19(30-3)24-11-14)23-12-16(17)26-20(21(27)29)25-13-18(28)22-2/h5-6,10-12H,4,7-9,13H2,1-3H3,(H,22,28)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316639
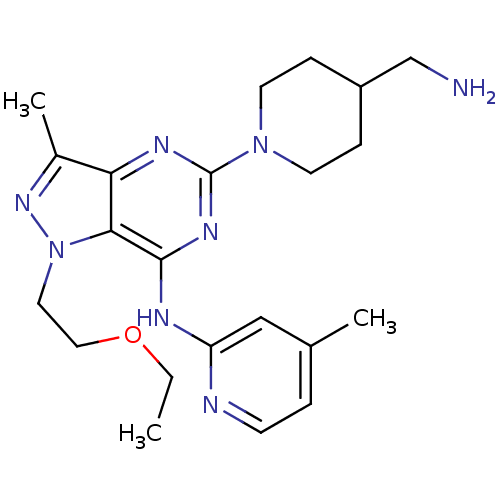
(5-(4-(aminomethyl)piperidin-1-yl)-1-(2-ethoxyethyl...)Show SMILES CCOCCn1nc(C)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCC(CN)CC1 Show InChI InChI=1S/C22H32N8O/c1-4-31-12-11-30-20-19(16(3)28-30)26-22(29-9-6-17(14-23)7-10-29)27-21(20)25-18-13-15(2)5-8-24-18/h5,8,13,17H,4,6-7,9-12,14,23H2,1-3H3,(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300983
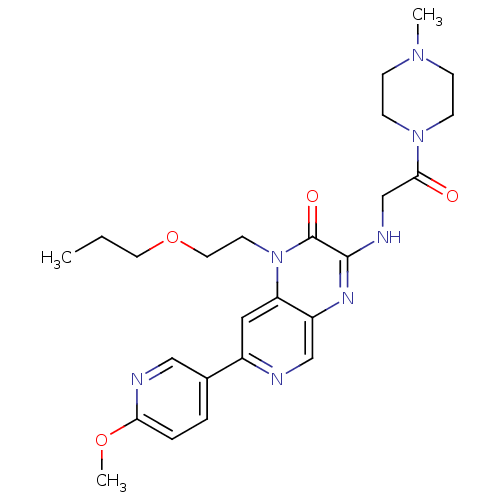
(7-(6-methoxypyridin-3-yl)-3-(2-(4-methylpiperazin-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCN(C)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H33N7O4/c1-4-12-36-13-11-32-21-14-19(18-5-6-22(35-3)27-15-18)26-16-20(21)29-24(25(32)34)28-17-23(33)31-9-7-30(2)8-10-31/h5-6,14-16H,4,7-13,17H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296256

(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316651
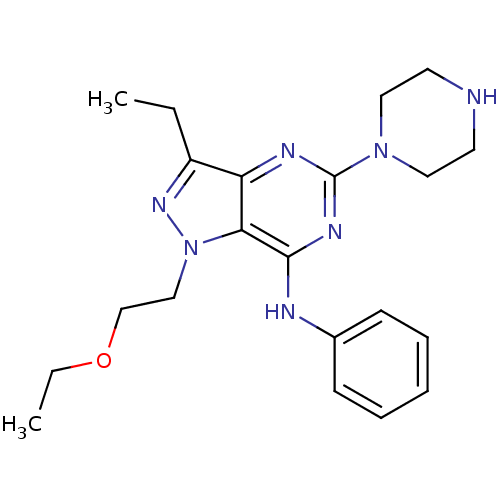
(1-(2-ethoxyethyl)-3-ethyl-N-phenyl-5-(piperazin-1-...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3ccccc3)c12)N1CCNCC1 Show InChI InChI=1S/C21H29N7O/c1-3-17-18-19(28(26-17)14-15-29-4-2)20(23-16-8-6-5-7-9-16)25-21(24-18)27-12-10-22-11-13-27/h5-9,22H,3-4,10-15H2,1-2H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357264
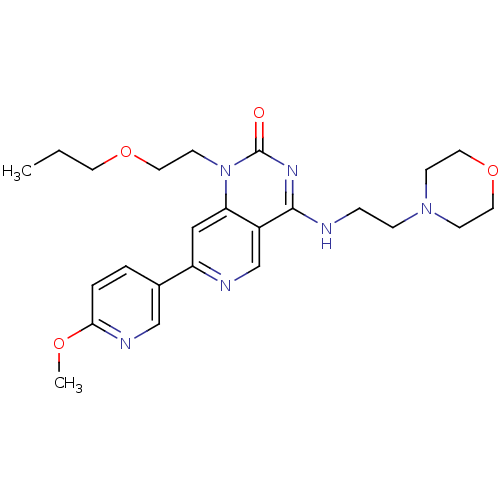
(CHEMBL1916291)Show SMILES CCCOCCn1c2cc(ncc2c(NCCN2CCOCC2)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-20(18-4-5-22(32-2)27-16-18)26-17-19(21)23(28-24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300977
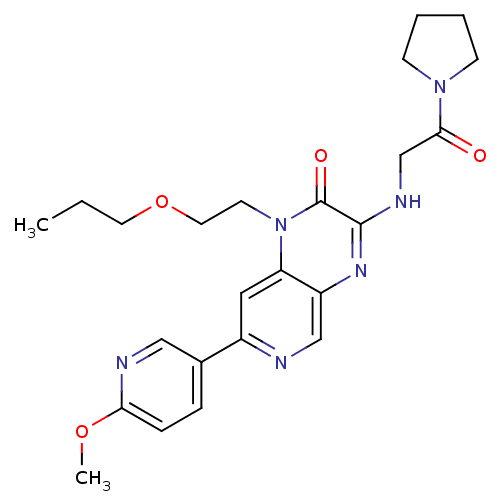
(7-(6-methoxypyridin-3-yl)-3-(2-oxo-2-(pyrrolidin-1...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O4/c1-3-11-34-12-10-30-20-13-18(17-6-7-21(33-2)26-14-17)25-15-19(20)28-23(24(30)32)27-16-22(31)29-8-4-5-9-29/h6-7,13-15H,3-5,8-12,16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316645
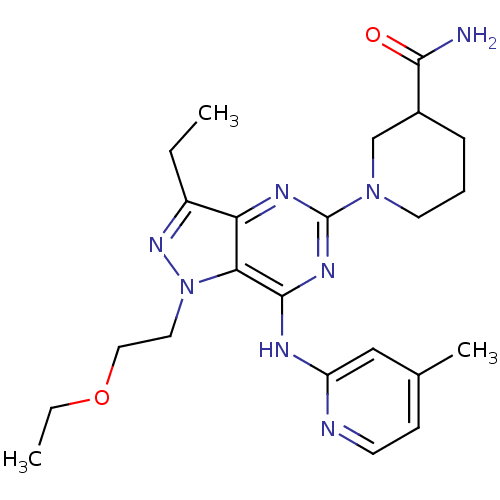
(1-(1-(2-ethoxyethyl)-3-ethyl-7-(4-methylpyridin-2-...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCCC(C1)C(N)=O Show InChI InChI=1S/C23H32N8O2/c1-4-17-19-20(31(29-17)11-12-33-5-2)22(26-18-13-15(3)8-9-25-18)28-23(27-19)30-10-6-7-16(14-30)21(24)32/h8-9,13,16H,4-7,10-12,14H2,1-3H3,(H2,24,32)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316644
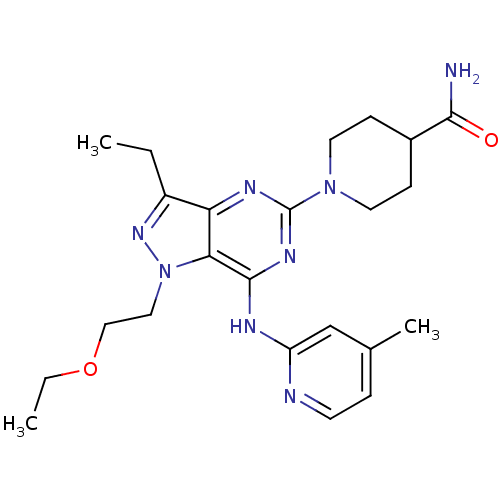
(1-(1-(2-ethoxyethyl)-3-ethyl-7-(4-methylpyridin-2-...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3cc(C)ccn3)c12)N1CCC(CC1)C(N)=O Show InChI InChI=1S/C23H32N8O2/c1-4-17-19-20(31(29-17)12-13-33-5-2)22(26-18-14-15(3)6-9-25-18)28-23(27-19)30-10-7-16(8-11-30)21(24)32/h6,9,14,16H,4-5,7-8,10-13H2,1-3H3,(H2,24,32)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357258

(CHEMBL1916299)Show SMILES CCCOCCn1c2cc(nnc2c(NCCN2CCOCC2)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H31N7O4/c1-3-11-33-14-10-30-19-15-18(17-4-5-20(32-2)25-16-17)27-28-21(19)22(26-23(30)31)24-6-7-29-8-12-34-13-9-29/h4-5,15-16H,3,6-14H2,1-2H3,(H,24,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300989
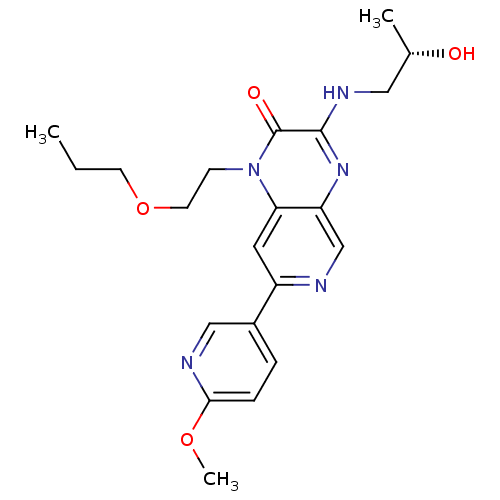
((S)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(ncc2nc(NC[C@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-12-15)22-13-17(18)25-20(21(26)28)24-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50316666

(1-(2-ethoxyethyl)-3-ethyl-N5-methyl-N5-(1-methylpi...)Show SMILES CCOCCn1nc(CC)c2nc(nc(Nc3cc(C)ccn3)c12)N(C)C1CCN(C)CC1 Show InChI InChI=1S/C24H36N8O/c1-6-19-21-22(32(29-19)14-15-33-7-2)23(26-20-16-17(3)8-11-25-20)28-24(27-21)31(5)18-9-12-30(4)13-10-18/h8,11,16,18H,6-7,9-10,12-15H2,1-5H3,(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 in human platelet by assessed as hydrolysis of [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 20: 3120-4 (2010)
BindingDB Entry DOI: 10.7270/Q2K64K0H |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50318144

(3-ethyl-N-(4-fluorophenyl)-5-(piperazin-1-yl)-1-(2...)Show SMILES CCc1nn(CCOCC(F)(F)F)c2c(Nc3ccc(F)cc3)nc(nc12)N1CCNCC1 Show InChI InChI=1S/C21H25F4N7O/c1-2-16-17-18(32(30-16)11-12-33-13-21(23,24)25)19(27-15-5-3-14(22)4-6-15)29-20(28-17)31-9-7-26-8-10-31/h3-6,26H,2,7-13H2,1H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 20: 3125-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.106
BindingDB Entry DOI: 10.7270/Q2WH2QXD |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348676
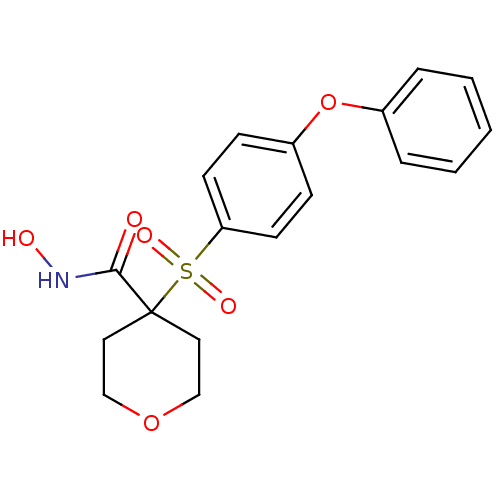
(CHEMBL1801044)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H19NO6S/c20-17(19-21)18(10-12-24-13-11-18)26(22,23)16-8-6-15(7-9-16)25-14-4-2-1-3-5-14/h1-9,21H,10-13H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300991
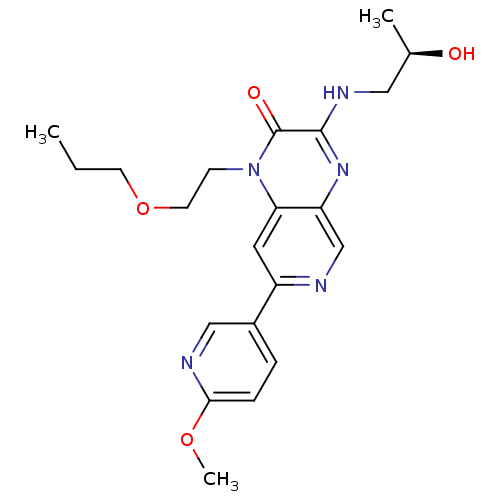
((R)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(ncc2nc(NC[C@@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-12-15)22-13-17(18)25-20(21(26)28)24-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50348652
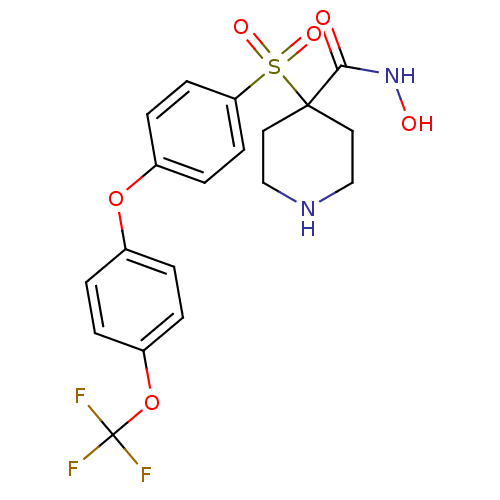
(CHEMBL1801429)Show SMILES ONC(=O)C1(CCNCC1)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C19H19F3N2O6S/c20-19(21,22)30-15-3-1-13(2-4-15)29-14-5-7-16(8-6-14)31(27,28)18(17(25)24-26)9-11-23-12-10-18/h1-8,23,26H,9-12H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50348664
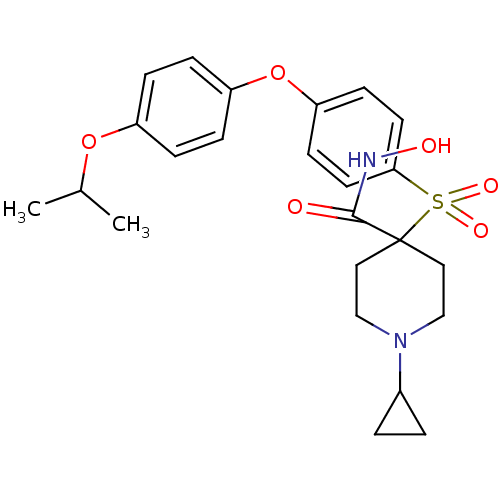
(CHEMBL1801417)Show SMILES CC(C)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC2)C2CC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H30N2O6S/c1-17(2)31-19-5-7-20(8-6-19)32-21-9-11-22(12-10-21)33(29,30)24(23(27)25-28)13-15-26(16-14-24)18-3-4-18/h5-12,17-18,28H,3-4,13-16H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50348687
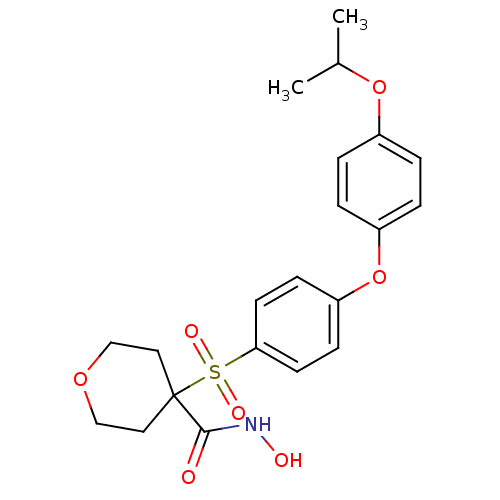
(CHEMBL1801049)Show SMILES CC(C)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C21H25NO7S/c1-15(2)28-16-3-5-17(6-4-16)29-18-7-9-19(10-8-18)30(25,26)21(20(23)22-24)11-13-27-14-12-21/h3-10,15,24H,11-14H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50348676

(CHEMBL1801044)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H19NO6S/c20-17(19-21)18(10-12-24-13-11-18)26(22,23)16-8-6-15(7-9-16)25-14-4-2-1-3-5-14/h1-9,21H,10-13H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357262
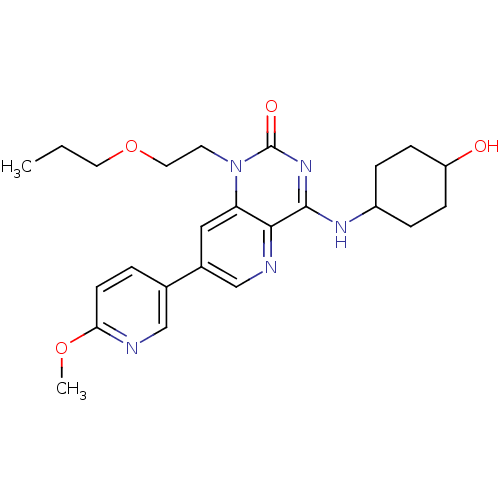
(CHEMBL1916295)Show SMILES CCCOCCn1c2cc(cnc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(25.6,-22.57,;24.26,-23.34,;22.93,-22.57,;21.6,-23.34,;21.6,-24.88,;20.26,-25.65,;20.26,-27.19,;21.59,-27.97,;22.91,-27.21,;24.24,-27.97,;24.25,-29.51,;22.92,-30.28,;21.59,-29.51,;20.26,-30.27,;20.26,-31.81,;18.93,-32.59,;17.6,-31.81,;16.27,-32.57,;16.27,-34.11,;14.93,-34.88,;17.6,-34.89,;18.94,-34.12,;18.93,-29.51,;18.93,-27.97,;17.6,-27.2,;25.57,-27.19,;25.56,-25.65,;26.89,-24.88,;28.23,-25.64,;29.56,-24.87,;30.9,-25.63,;28.23,-27.18,;26.9,-27.96,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-20-13-17(16-4-9-21(32-2)25-14-16)15-26-22(20)23(28-24(29)31)27-18-5-7-19(30)8-6-18/h4,9,13-15,18-19,30H,3,5-8,10-12H2,1-2H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357235
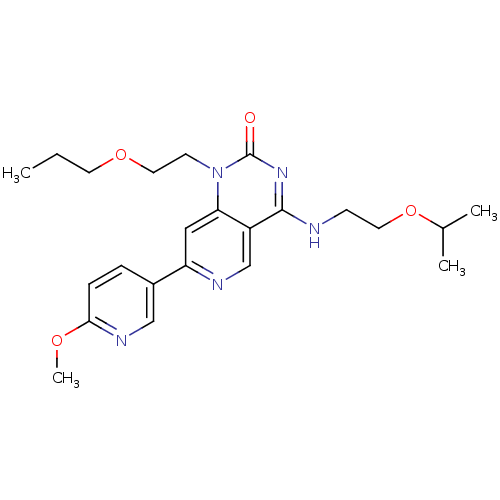
(CHEMBL1916290)Show SMILES CCCOCCn1c2cc(ncc2c(NCCOC(C)C)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H31N5O4/c1-5-10-31-12-9-28-20-13-19(17-6-7-21(30-4)26-14-17)25-15-18(20)22(27-23(28)29)24-8-11-32-16(2)3/h6-7,13-16H,5,8-12H2,1-4H3,(H,24,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348647
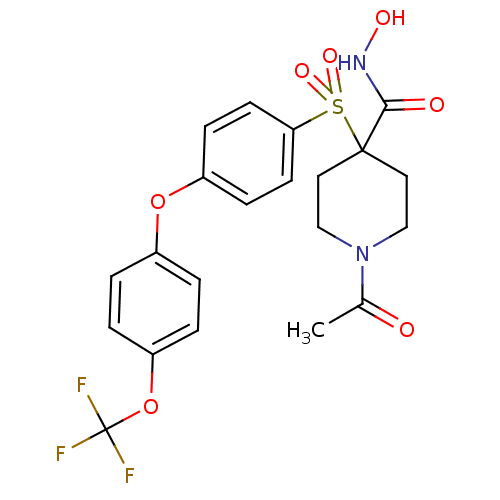
(CHEMBL1801396)Show SMILES CC(=O)N1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C21H21F3N2O7S/c1-14(27)26-12-10-20(11-13-26,19(28)25-29)34(30,31)18-8-6-16(7-9-18)32-15-2-4-17(5-3-15)33-21(22,23)24/h2-9,29H,10-13H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348658
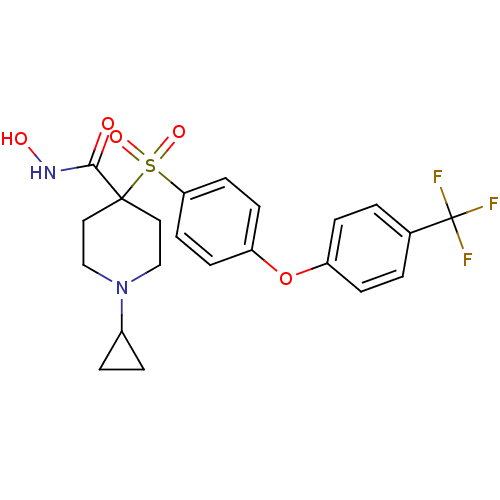
(CHEMBL1801424)Show SMILES ONC(=O)C1(CCN(CC1)C1CC1)S(=O)(=O)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H23F3N2O5S/c23-22(24,25)15-1-5-17(6-2-15)32-18-7-9-19(10-8-18)33(30,31)21(20(28)26-29)11-13-27(14-12-21)16-3-4-16/h1-2,5-10,16,29H,3-4,11-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348664

(CHEMBL1801417)Show SMILES CC(C)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC2)C2CC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H30N2O6S/c1-17(2)31-19-5-7-20(8-6-19)32-21-9-11-22(12-10-21)33(29,30)24(23(27)25-28)13-15-26(16-14-24)18-3-4-18/h5-12,17-18,28H,3-4,13-16H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348680

(CHEMBL1801414)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(cc2)C(C)C)cc1 Show InChI InChI=1S/C24H32N2O6S/c1-18(2)19-4-6-20(7-5-19)32-21-8-10-22(11-9-21)33(29,30)24(23(27)25-28)12-14-26(15-13-24)16-17-31-3/h4-11,18,28H,12-17H2,1-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348666
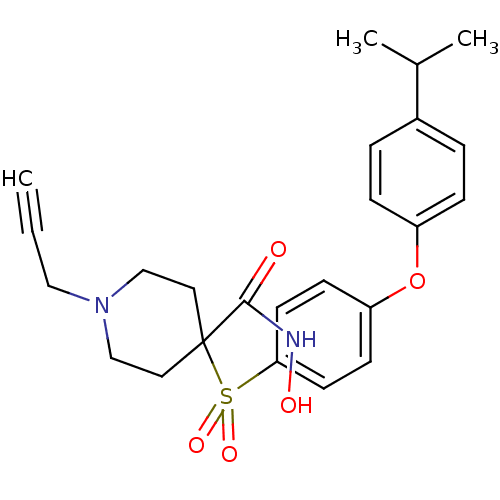
(CHEMBL1801413)Show SMILES CC(C)c1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC#C)CC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H28N2O5S/c1-4-15-26-16-13-24(14-17-26,23(27)25-28)32(29,30)22-11-9-21(10-12-22)31-20-7-5-19(6-8-20)18(2)3/h1,5-12,18,28H,13-17H2,2-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348681
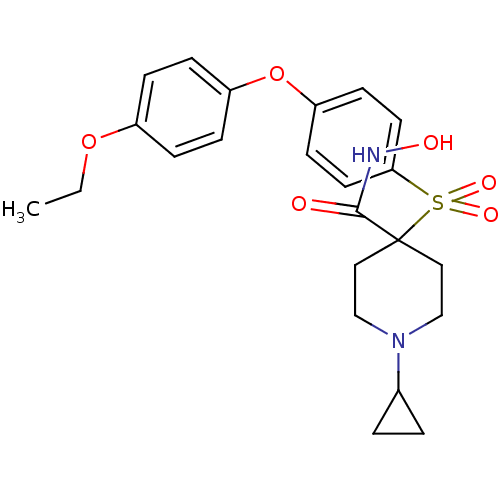
(CHEMBL1801412)Show SMILES CCOc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC2)C2CC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O6S/c1-2-30-18-5-7-19(8-6-18)31-20-9-11-21(12-10-20)32(28,29)23(22(26)24-27)13-15-25(16-14-23)17-3-4-17/h5-12,17,27H,2-4,13-16H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data