 Found 146 hits with Last Name = 'budd' and Initial = 'c'
Found 146 hits with Last Name = 'budd' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50183182
(2''-benzyloxy-5''-chloro-[1,1';2',1'']terphenyl-3-...)Show SMILES OC(=O)c1cccc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H19ClO3/c27-21-13-14-25(30-17-18-7-2-1-3-8-18)24(16-21)23-12-5-4-11-22(23)19-9-6-10-20(15-19)26(28)29/h1-16H,17H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187694
(CHEMBL3828165)Show InChI InChI=1S/C21H37O3P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-20-15-17-21(18-16-20)19-25(22,23)24/h15-18H,2-14,19H2,1H3,(H2,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant ATX using FS3 as substrate assessed as enzyme-inhibitor complex incubated for 2 hrs by Michaelis-Menten eq... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187694

(CHEMBL3828165)Show InChI InChI=1S/C21H37O3P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-20-15-17-21(18-16-20)19-25(22,23)24/h15-18H,2-14,19H2,1H3,(H2,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant ATX using FS3 as substrate assessed as enzyme-substrate-inhibitor complex incubated for 2 hrs by Michaelis... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TP receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187695
(CHEMBL1632521)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ncc(C)c(OC)c2C)cc1)[C@@H](O)CP(O)(O)=O |r| Show InChI InChI=1S/C35H57N2O7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-34(39)37-31(33(38)26-45(40,41)42)23-29-19-21-30(22-20-29)44-25-32-28(3)35(43-4)27(2)24-36-32/h19-22,24,31,33,38H,5-18,23,25-26H2,1-4H3,(H,37,39)(H2,40,41,42)/t31-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal V5/His6-tagged ATX expressed in HEK293 cells assessed as choline release at 0.5 uM using oleoyl-lysophosph... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187695

(CHEMBL1632521)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ncc(C)c(OC)c2C)cc1)[C@@H](O)CP(O)(O)=O |r| Show InChI InChI=1S/C35H57N2O7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-34(39)37-31(33(38)26-45(40,41)42)23-29-19-21-30(22-20-29)44-25-32-28(3)35(43-4)27(2)24-36-32/h19-22,24,31,33,38H,5-18,23,25-26H2,1-4H3,(H,37,39)(H2,40,41,42)/t31-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal V5/His6-tagged ATX expressed in HEK293 cells assessed as choline release at 0.5 uM using oleoyl-lysophosph... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Activity at EP3 receptor by FLIPR method |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187686
(CHEMBL3828074 | US10526329, Compound 2 | US1107261...)Show SMILES CCc1nc2c(C)cc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(O)C2)CC1 Show InChI InChI=1S/C30H33FN8O2S/c1-4-24-29(35(3)30-34-27(25(14-32)42-30)20-5-7-21(31)8-6-20)39-15-22(13-19(2)28(39)33-24)37-11-9-36(10-12-37)18-26(41)38-16-23(40)17-38/h5-8,13,15,23,40H,4,9-12,16-18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX using FS3 as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by fluorescence assay |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187688
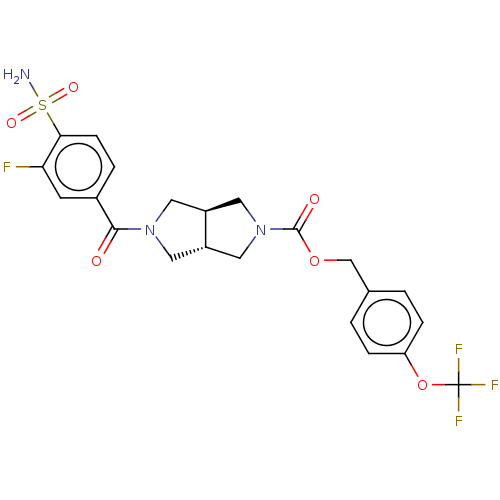
(CHEMBL3828650 | US10913745, Example 1.11)Show SMILES [H][C@]12CN(C[C@]1([H])CN(C2)C(=O)c1ccc(c(F)c1)S(N)(=O)=O)C(=O)OCc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C22H21F4N3O6S/c23-18-7-14(3-6-19(18)36(27,32)33)20(30)28-8-15-10-29(11-16(15)9-28)21(31)34-12-13-1-4-17(5-2-13)35-22(24,25)26/h1-7,15-16H,8-12H2,(H2,27,32,33)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged ATX expressed in HEK293E cells using lysophosphatidylcholine as substrate preincubated with enz... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187689
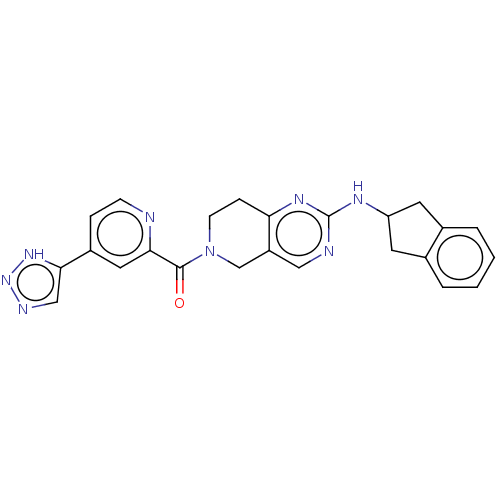
(CHEMBL3827513 | Example 9)Show SMILES O=C(N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1)c1cc(ccn1)-c1cnn[nH]1 Show InChI InChI=1S/C24H22N8O/c33-23(21-11-17(5-7-25-21)22-13-27-31-30-22)32-8-6-20-18(14-32)12-26-24(29-20)28-19-9-15-3-1-2-4-16(15)10-19/h1-5,7,11-13,19H,6,8-10,14H2,(H,26,28,29)(H,27,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged ATX expressed in HEK293E cells using lysophosphatidylcholine as substrate preincubated with enz... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241106
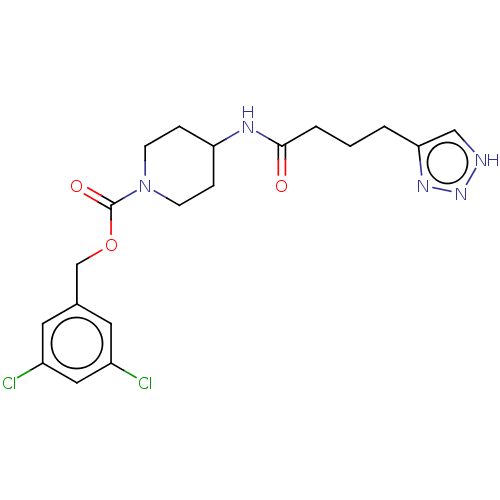
(US9409895, 17 | US9630945, 17)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c1 Show InChI InChI=1S/C19H23Cl2N5O3/c20-14-8-13(9-15(21)10-14)12-29-19(28)26-6-4-16(5-7-26)23-18(27)3-1-2-17-11-22-25-24-17/h8-11,16H,1-7,12H2,(H,23,27)(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ATX expressed in HEK cells using oleoyl-lysophosphatidylcholine as substrate preincubated for 20 mins followed by sub... |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413672

(CHEMBL516324)Show SMILES CCCC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C30H26ClNO4/c1-2-8-29(33)32-24-16-21(15-22(17-24)30(34)35)25-11-6-7-12-26(25)27-18-23(31)13-14-28(27)36-19-20-9-4-3-5-10-20/h3-7,9-18H,2,8,19H2,1H3,(H,32,33)(H,34,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187690
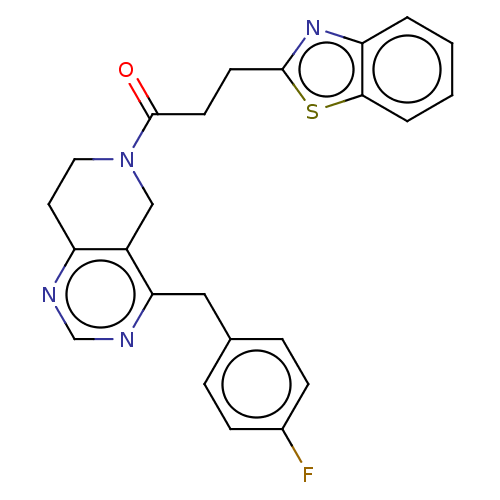
(CHEMBL3827088)Show SMILES Fc1ccc(Cc2ncnc3CCN(Cc23)C(=O)CCc2nc3ccccc3s2)cc1 Show InChI InChI=1S/C24H21FN4OS/c25-17-7-5-16(6-8-17)13-21-18-14-29(12-11-19(18)26-15-27-21)24(30)10-9-23-28-20-3-1-2-4-22(20)31-23/h1-8,15H,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ATX using FS3 as substrate incubated for 30 mins by fluorescence assay |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ATX using FS3 as substrate incubated for 30 mins by fluorescence assay |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413670
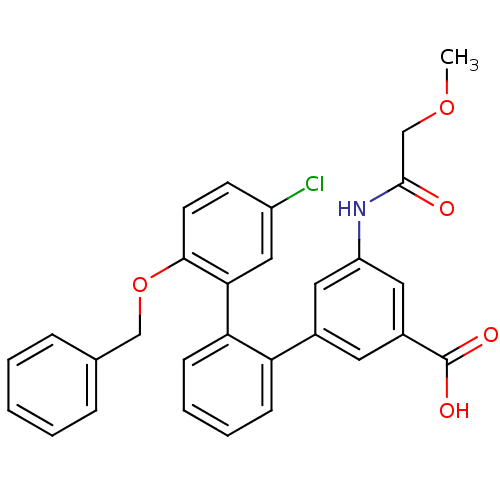
(CHEMBL458452)Show SMILES COCC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C29H24ClNO5/c1-35-18-28(32)31-23-14-20(13-21(15-23)29(33)34)24-9-5-6-10-25(24)26-16-22(30)11-12-27(26)36-17-19-7-3-2-4-8-19/h2-16H,17-18H2,1H3,(H,31,32)(H,33,34) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413667
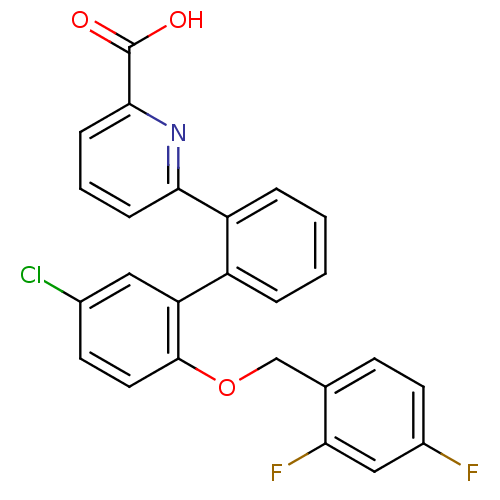
(CHEMBL514574)Show SMILES OC(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccc(F)cc1F Show InChI InChI=1S/C25H16ClF2NO3/c26-16-9-11-24(32-14-15-8-10-17(27)13-21(15)28)20(12-16)18-4-1-2-5-19(18)22-6-3-7-23(29-22)25(30)31/h1-13H,14H2,(H,30,31) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413668

(CHEMBL457142)Show SMILES OC(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccc(F)cc1 Show InChI InChI=1S/C25H17ClFNO3/c26-17-10-13-24(31-15-16-8-11-18(27)12-9-16)21(14-17)19-4-1-2-5-20(19)22-6-3-7-23(28-22)25(29)30/h1-14H,15H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413673

(CHEMBL456496)Show SMILES CCC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C29H24ClNO4/c1-2-28(32)31-23-15-20(14-21(16-23)29(33)34)24-10-6-7-11-25(24)26-17-22(30)12-13-27(26)35-18-19-8-4-3-5-9-19/h3-17H,2,18H2,1H3,(H,31,32)(H,33,34) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187689

(CHEMBL3827513 | Example 9)Show SMILES O=C(N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1)c1cc(ccn1)-c1cnn[nH]1 Show InChI InChI=1S/C24H22N8O/c33-23(21-11-17(5-7-25-21)22-13-27-31-30-22)32-8-6-20-18(14-32)12-26-24(29-20)28-19-9-15-3-1-2-4-16(15)10-19/h1-5,7,11-13,19H,6,8-10,14H2,(H,26,28,29)(H,27,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of ATX-mediated LPA release in human plasma after 3 hrs by mass spectrometric analysis |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413671

(CHEMBL458448)Show SMILES CC(C)C(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C30H26ClNO4/c1-19(2)29(33)32-24-15-21(14-22(16-24)30(34)35)25-10-6-7-11-26(25)27-17-23(31)12-13-28(27)36-18-20-8-4-3-5-9-20/h3-17,19H,18H2,1-2H3,(H,32,33)(H,34,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413674

(CHEMBL456495)Show SMILES CC(=O)Nc1cc(cc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1)C(O)=O Show InChI InChI=1S/C28H22ClNO4/c1-18(31)30-23-14-20(13-21(15-23)28(32)33)24-9-5-6-10-25(24)26-16-22(29)11-12-27(26)34-17-19-7-3-2-4-8-19/h2-16H,17H2,1H3,(H,30,31)(H,32,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413669

(CHEMBL515981)Show SMILES OC(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C25H18ClNO3/c26-18-13-14-24(30-16-17-7-2-1-3-8-17)21(15-18)19-9-4-5-10-20(19)22-11-6-12-23(27-22)25(28)29/h1-15H,16H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413663

(CHEMBL466549)Show SMILES [O-]C(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1c(F)cc(F)cc1F Show InChI InChI=1S/C25H15ClF3NO3/c26-14-8-9-24(33-13-19-20(28)11-15(27)12-21(19)29)18(10-14)16-4-1-2-5-17(16)22-6-3-7-23(30-22)25(31)32/h1-12H,13H2,(H,31,32)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Rattus norvegicus (Rat)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to rat EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398093
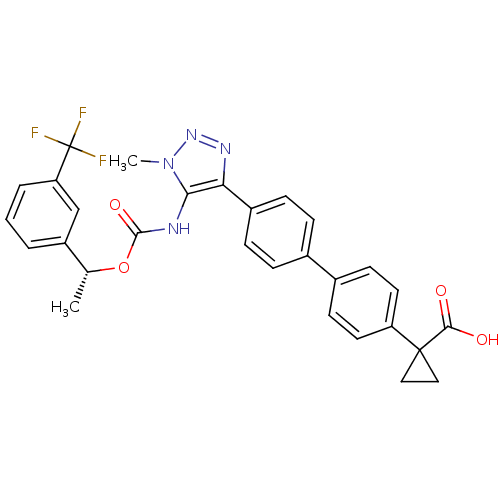
(CHEMBL2182046 | US9321738, 6)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C29H25F3N4O4/c1-17(21-4-3-5-23(16-21)29(30,31)32)40-27(39)33-25-24(34-35-36(25)2)20-8-6-18(7-9-20)19-10-12-22(13-11-19)28(14-15-28)26(37)38/h3-13,16-17H,14-15H2,1-2H3,(H,33,39)(H,37,38)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398094
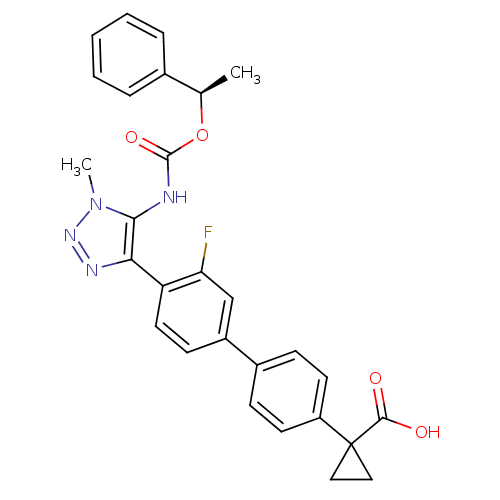
(CHEMBL2182044 | US9321738, 11)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1F)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C28H25FN4O4/c1-17(18-6-4-3-5-7-18)37-27(36)30-25-24(31-32-33(25)2)22-13-10-20(16-23(22)29)19-8-11-21(12-9-19)28(14-15-28)26(34)35/h3-13,16-17H,14-15H2,1-2H3,(H,30,36)(H,34,35)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398095

(CHEMBL2182043 | US9321738, 2)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C28H26N4O4/c1-18(19-6-4-3-5-7-19)36-27(35)29-25-24(30-31-32(25)2)22-10-8-20(9-11-22)21-12-14-23(15-13-21)28(16-17-28)26(33)34/h3-15,18H,16-17H2,1-2H3,(H,29,35)(H,33,34)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398091
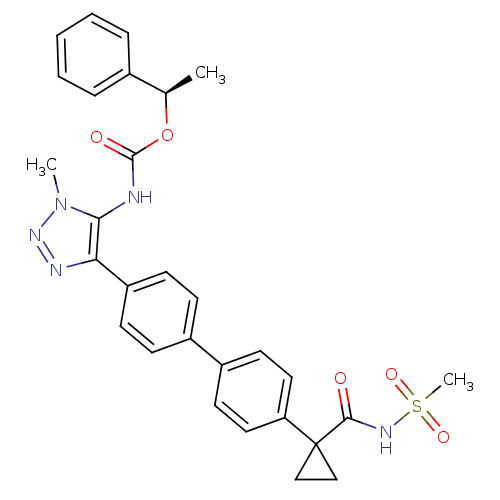
(CHEMBL2182049 | US9321738, 12)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(=O)NS(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C29H29N5O5S/c1-19(20-7-5-4-6-8-20)39-28(36)30-26-25(31-33-34(26)2)23-11-9-21(10-12-23)22-13-15-24(16-14-22)29(17-18-29)27(35)32-40(3,37)38/h4-16,19H,17-18H2,1-3H3,(H,30,36)(H,32,35)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413664

(CHEMBL467579)Show SMILES [O-]C(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccc(Cl)cc1F Show InChI InChI=1S/C25H16Cl2FNO3/c26-16-10-11-24(32-14-15-8-9-17(27)13-21(15)28)20(12-16)18-4-1-2-5-19(18)22-6-3-7-23(29-22)25(30)31/h1-13H,14H2,(H,30,31)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413685
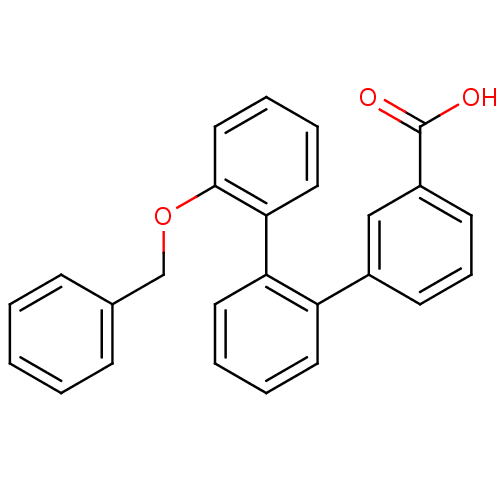
(CHEMBL516787)Show SMILES OC(=O)c1cccc(c1)-c1ccccc1-c1ccccc1OCc1ccccc1 Show InChI InChI=1S/C26H20O3/c27-26(28)21-12-8-11-20(17-21)22-13-4-5-14-23(22)24-15-6-7-16-25(24)29-18-19-9-2-1-3-10-19/h1-17H,18H2,(H,27,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413679
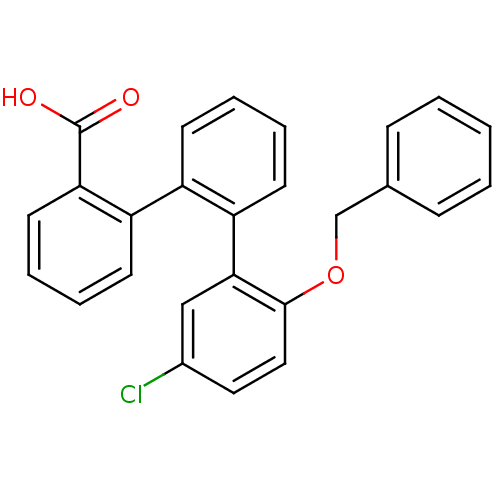
(CHEMBL456707)Show SMILES OC(=O)c1ccccc1-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H19ClO3/c27-19-14-15-25(30-17-18-8-2-1-3-9-18)24(16-19)22-12-5-4-10-20(22)21-11-6-7-13-23(21)26(28)29/h1-16H,17H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398101
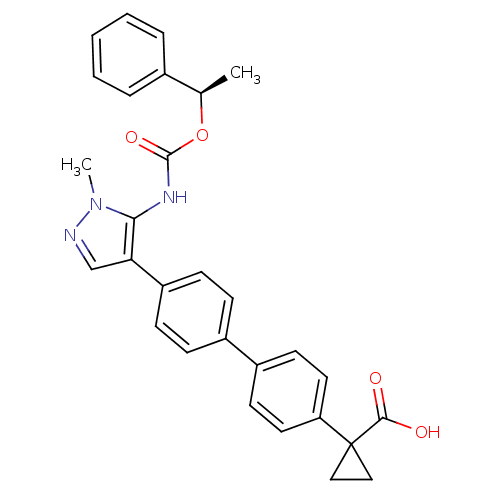
(CHEMBL2182063)Show SMILES C[C@@H](OC(=O)Nc1c(cnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C29H27N3O4/c1-19(20-6-4-3-5-7-20)36-28(35)31-26-25(18-30-32(26)2)23-10-8-21(9-11-23)22-12-14-24(15-13-22)29(16-17-29)27(33)34/h3-15,18-19H,16-17H2,1-2H3,(H,31,35)(H,33,34)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA1 in human lung fibroblasts assessed as inhibition of LPA-induced contraction after 18 hrs by 3D collagen gel contraction a... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398095

(CHEMBL2182043 | US9321738, 2)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C28H26N4O4/c1-18(19-6-4-3-5-7-19)36-27(35)29-25-24(30-31-32(25)2)22-10-8-20(9-11-22)21-12-14-23(15-13-21)28(16-17-28)26(33)34/h3-15,18H,16-17H2,1-2H3,(H,29,35)(H,33,34)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA1 in human lung fibroblasts assessed as inhibition of LPA-induced contraction after 18 hrs by 3D collagen gel contraction a... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398094

(CHEMBL2182044 | US9321738, 11)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1F)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C28H25FN4O4/c1-17(18-6-4-3-5-7-18)37-27(36)30-25-24(31-32-33(25)2)22-13-10-20(16-23(22)29)19-8-11-21(12-9-19)28(14-15-28)26(34)35/h3-13,16-17H,14-15H2,1-2H3,(H,30,36)(H,34,35)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA1 in human lung fibroblasts assessed as inhibition of LPA-induced contraction after 18 hrs by 3D collagen gel contraction a... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398101

(CHEMBL2182063)Show SMILES C[C@@H](OC(=O)Nc1c(cnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C29H27N3O4/c1-19(20-6-4-3-5-7-20)36-28(35)31-26-25(18-30-32(26)2)23-10-8-21(9-11-23)22-12-14-24(15-13-22)29(16-17-29)27(33)34/h3-15,18-19H,16-17H2,1-2H3,(H,31,35)(H,33,34)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187696
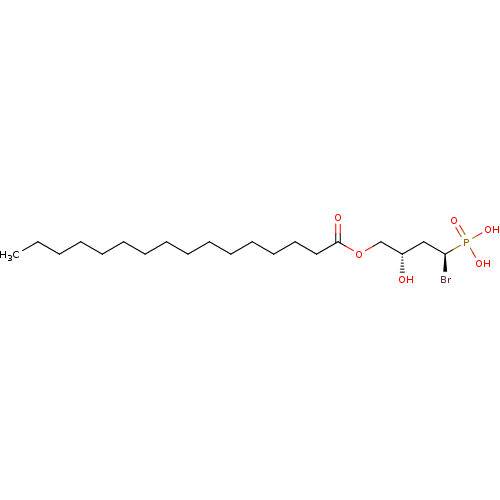
(CHEMBL3621356)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)C[C@H](Br)P(O)(O)=O |r| Show InChI InChI=1S/C20H40BrO6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-20(23)27-17-18(22)16-19(21)28(24,25)26/h18-19,22H,2-17H2,1H3,(H2,24,25,26)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant hemagglutinin-tagged ATX (unknown origin) using FS3 as substrate incubated for 2 hrs by fluorescence plate reader analysis |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398115
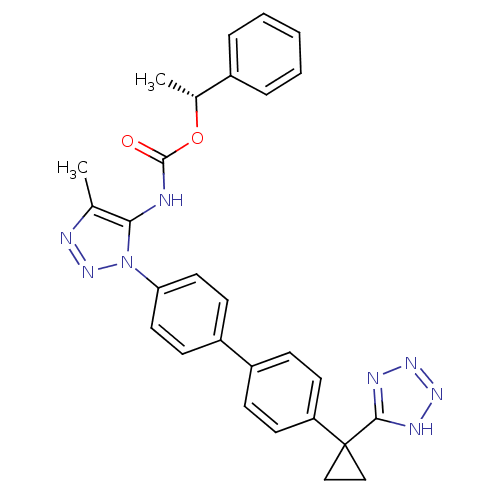
(CHEMBL2182038)Show SMILES C[C@@H](OC(=O)Nc1c(C)nnn1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)c1nnn[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C28H26N8O2/c1-18-25(29-27(37)38-19(2)20-6-4-3-5-7-20)36(35-30-18)24-14-10-22(11-15-24)21-8-12-23(13-9-21)28(16-17-28)26-31-33-34-32-26/h3-15,19H,16-17H2,1-2H3,(H,29,37)(H,31,32,33,34)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398090
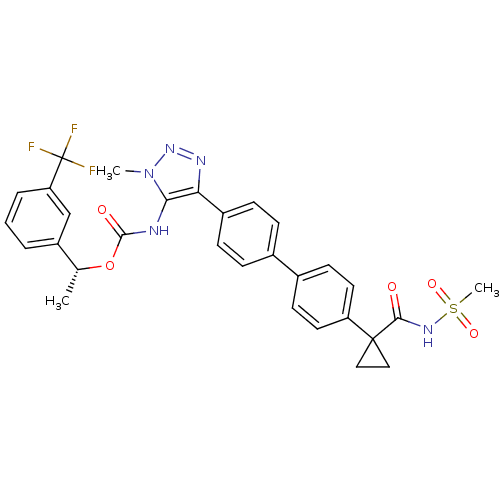
(CHEMBL2182050 | US9321738, 13)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(=O)NS(C)(=O)=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H28F3N5O5S/c1-18(22-5-4-6-24(17-22)30(31,32)33)43-28(40)34-26-25(35-37-38(26)2)21-9-7-19(8-10-21)20-11-13-23(14-12-20)29(15-16-29)27(39)36-44(3,41)42/h4-14,17-18H,15-16H2,1-3H3,(H,34,40)(H,36,39)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398100

(CHEMBL2182064)Show SMILES C[C@@H](OC(=O)Nc1c(cnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1F |r| Show InChI InChI=1S/C29H26FN3O4/c1-18(23-5-3-4-6-25(23)30)37-28(36)32-26-24(17-31-33(26)2)21-9-7-19(8-10-21)20-11-13-22(14-12-20)29(15-16-29)27(34)35/h3-14,17-18H,15-16H2,1-2H3,(H,32,36)(H,34,35)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398123
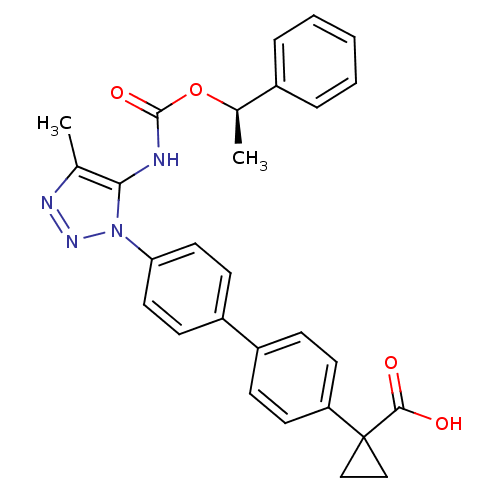
(CHEMBL2182029)Show SMILES C[C@@H](OC(=O)Nc1c(C)nnn1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C28H26N4O4/c1-18-25(29-27(35)36-19(2)20-6-4-3-5-7-20)32(31-30-18)24-14-10-22(11-15-24)21-8-12-23(13-9-21)28(16-17-28)26(33)34/h3-15,19H,16-17H2,1-2H3,(H,29,35)(H,33,34)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187687
(CHEMBL3828733 | US11124490, Compound 918013)Show SMILES Fc1cccc(NC(=O)c2cc(c(Cl)cc2Cl)S(=O)(=O)N2CCOCC2)c1 Show InChI InChI=1S/C17H15Cl2FN2O4S/c18-14-10-15(19)16(27(24,25)22-4-6-26-7-5-22)9-13(14)17(23)21-12-3-1-2-11(20)8-12/h1-3,8-10H,4-7H2,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using FS3 as substrate measured after 3 hrs by fluorescence assay |
J Med Chem 59: 5604-21 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01599
BindingDB Entry DOI: 10.7270/Q2BZ681D |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398116
(CHEMBL2182037)Show SMILES C[C@@H](OC(=O)Nc1c(C)nnn1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(=O)NS(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C29H29N5O5S/c1-19-26(30-28(36)39-20(2)21-7-5-4-6-8-21)34(33-31-19)25-15-11-23(12-16-25)22-9-13-24(14-10-22)29(17-18-29)27(35)32-40(3,37)38/h4-16,20H,17-18H2,1-3H3,(H,30,36)(H,32,35)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398103

(CHEMBL2182061)Show SMILES C[C@@H](OC(=O)Nc1c(cnn1C)-c1ccc(cc1)-c1ccc(CC(O)=O)cc1)c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O4/c1-18(20-6-4-3-5-7-20)34-27(33)29-26-24(17-28-30(26)2)23-14-12-22(13-15-23)21-10-8-19(9-11-21)16-25(31)32/h3-15,17-18H,16H2,1-2H3,(H,29,33)(H,31,32)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398122

(CHEMBL2182030)Show SMILES C[C@@H](OC(=O)Nc1c(C)nnn1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1F |r| Show InChI InChI=1S/C28H25FN4O4/c1-17-25(30-27(36)37-18(2)23-5-3-4-6-24(23)29)33(32-31-17)22-13-9-20(10-14-22)19-7-11-21(12-8-19)28(15-16-28)26(34)35/h3-14,18H,15-16H2,1-2H3,(H,30,36)(H,34,35)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398113

(CHEMBL2182040)Show SMILES C[C@@H](OC(=O)Nc1cnnn1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C27H24N4O4/c1-18(19-5-3-2-4-6-19)35-26(34)29-24-17-28-30-31(24)23-13-9-21(10-14-23)20-7-11-22(12-8-20)27(15-16-27)25(32)33/h2-14,17-18H,15-16H2,1H3,(H,29,34)(H,32,33)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398126

(CHEMBL2182024)Show SMILES CC[C@@H](C)OC(=O)Nc1c(cnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O |r| Show InChI InChI=1S/C25H27N3O4/c1-4-16(2)32-24(31)27-22-21(15-26-28(22)3)19-7-5-17(6-8-19)18-9-11-20(12-10-18)25(13-14-25)23(29)30/h5-12,15-16H,4,13-14H2,1-3H3,(H,27,31)(H,29,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50413662

(CHEMBL459537)Show SMILES [O-]C(=O)c1cccc(n1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1F Show InChI InChI=1S/C25H17ClFNO3/c26-17-12-13-24(31-15-16-6-1-4-9-21(16)27)20(14-17)18-7-2-3-8-19(18)22-10-5-11-23(28-22)25(29)30/h1-14H,15H2,(H,29,30)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data