

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25771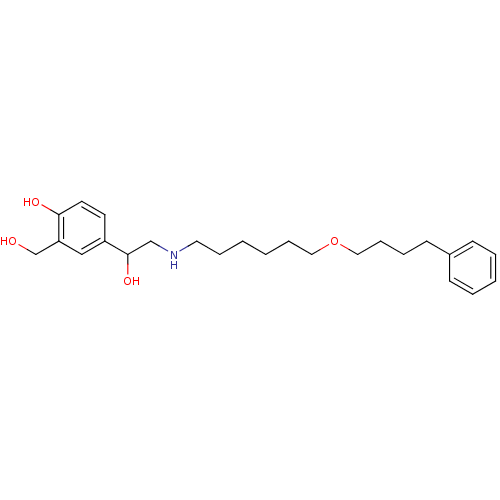 (1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318156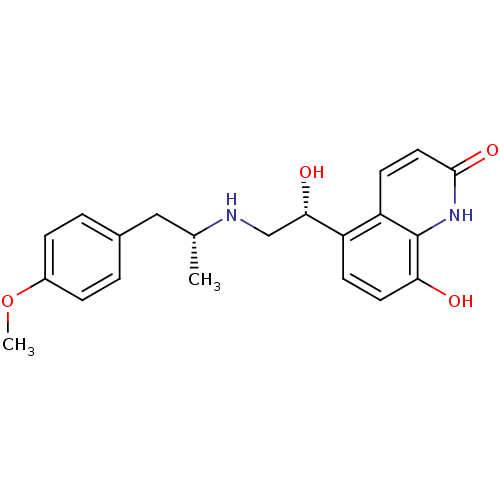 (CHEMBL1094785 | carmoterol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50151720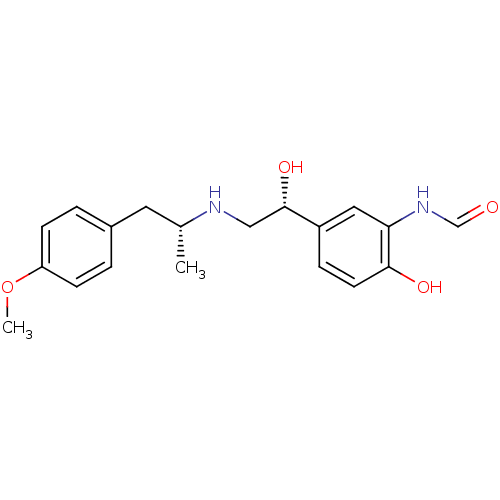 (ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159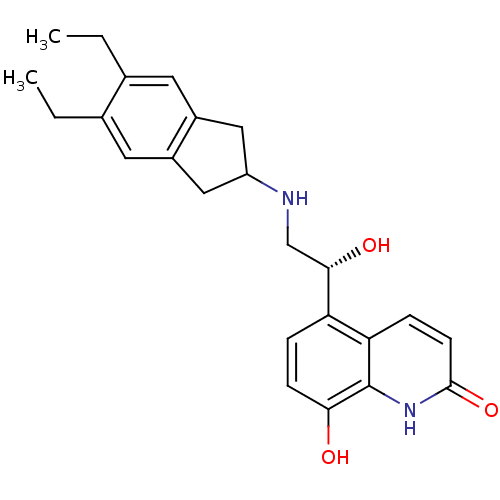 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318157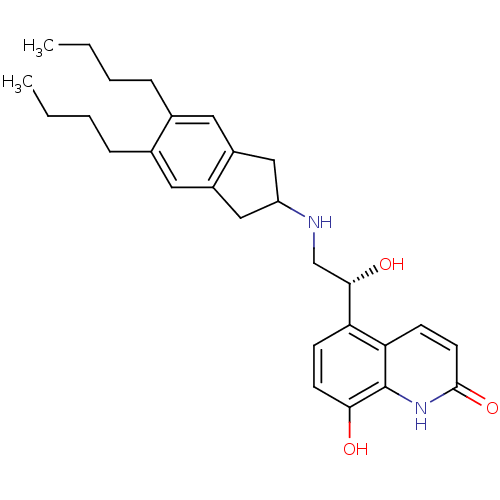 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-butylindan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318158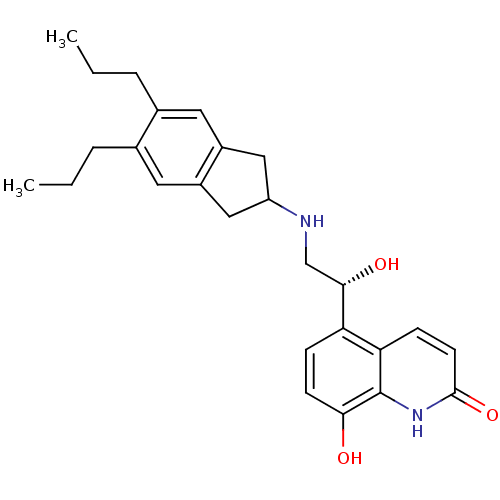 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-propylindan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318161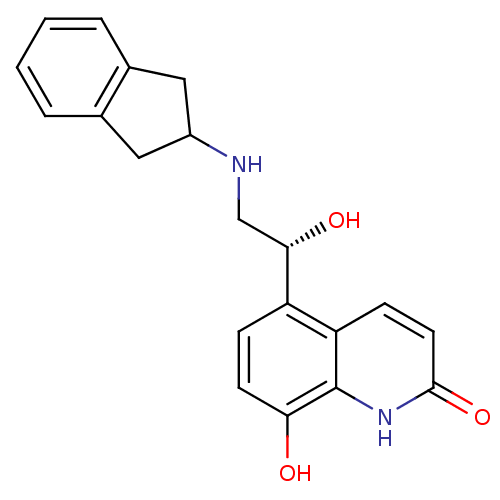 (8-Hydroxy-5-[(R)-1-hydroxy-2-(indan-2-ylamino)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318155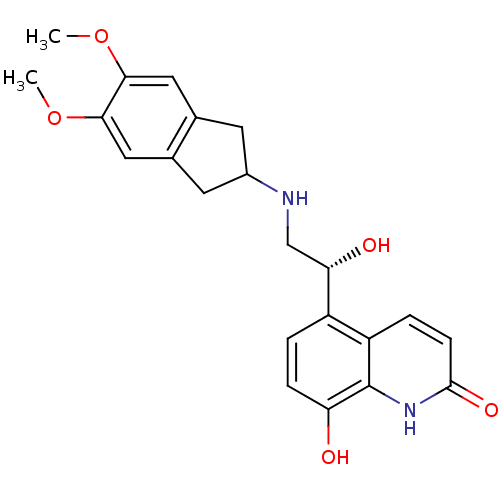 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethoxyindan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318160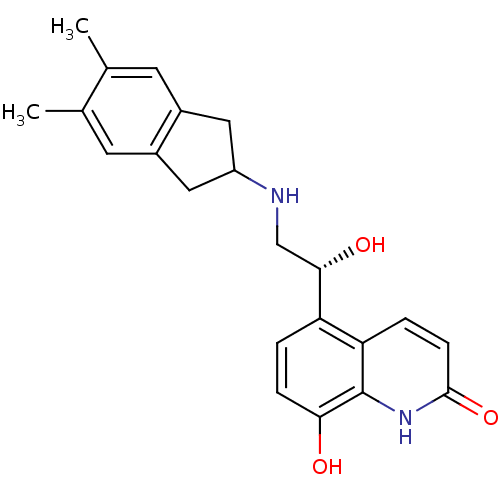 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethylindan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318154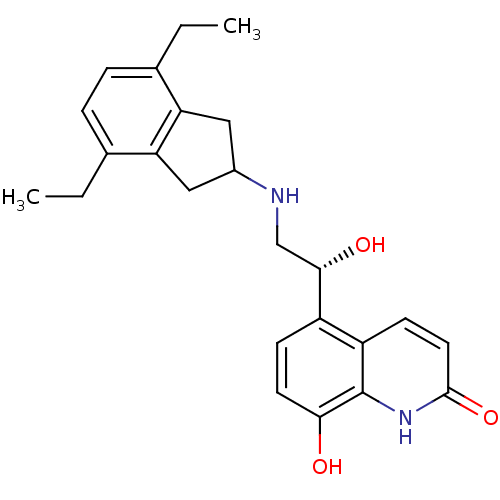 (8-Hydroxy-5-[(R)-1-hydroxy-2-(4,7-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25769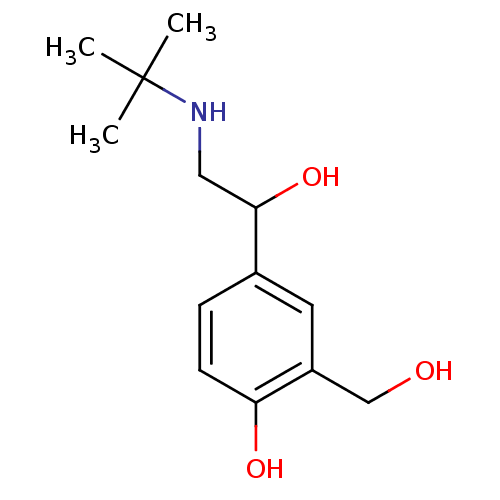 (4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50313618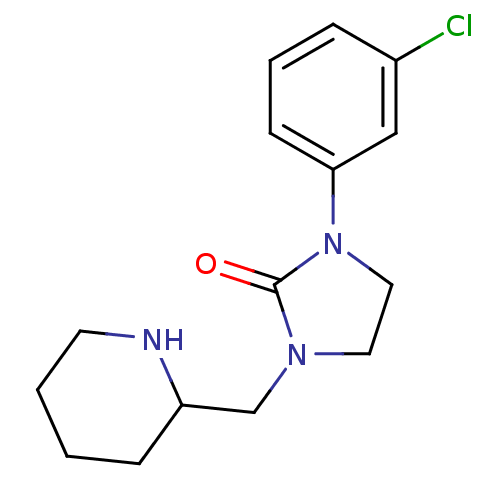 (1-(3-chlorophenyl)-3-(piperidin-2-ylmethyl)imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2013-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.090 BindingDB Entry DOI: 10.7270/Q27P8ZJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50207120 (3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of adenosine A1 receptor | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50313619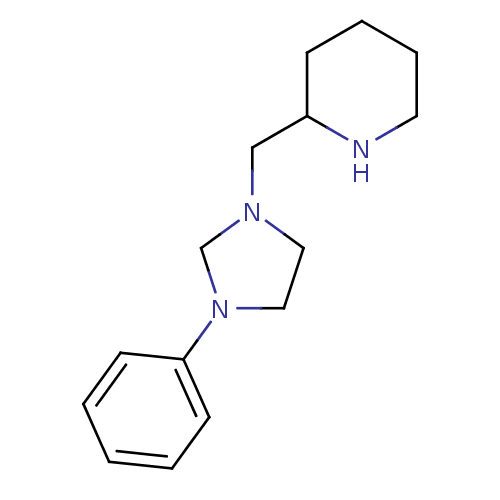 (2-((3-phenylimidazolidin-1-yl)methyl)piperidine | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity at histamine H1 receptor | Bioorg Med Chem Lett 20: 2013-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.090 BindingDB Entry DOI: 10.7270/Q27P8ZJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318153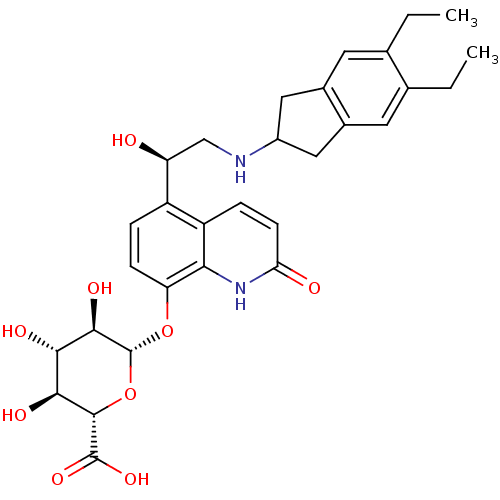 ((2S,3S,4S,5R,6S)-6-(5-((R)-2-(5,6-diethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318156 (CHEMBL1094785 | carmoterol) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50151720 (ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318161 (8-Hydroxy-5-[(R)-1-hydroxy-2-(indan-2-ylamino)-eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503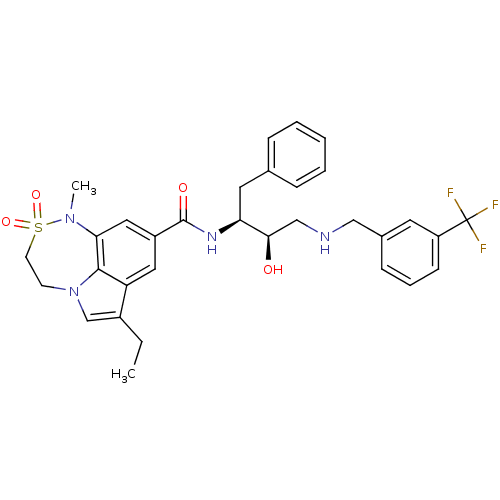 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207120 (3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26788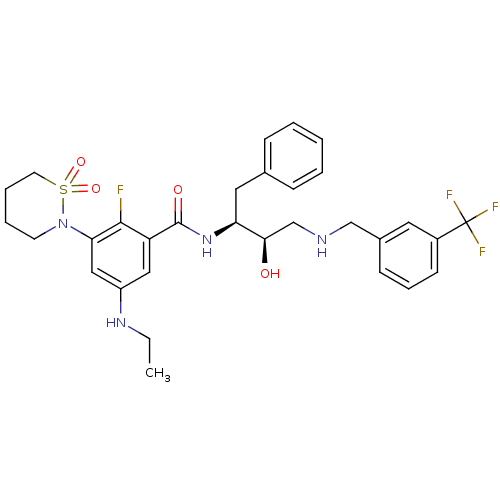 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207130 (8-((6,7-dimethoxy-1-methylisoquinolin-4-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318155 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethoxyindan-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322883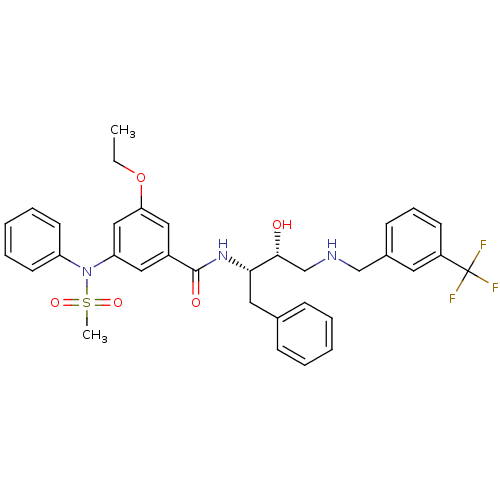 (3-ethoxy-N-((2S,3R)-3-hydroxy-1-phenyl-4-(3-(trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322882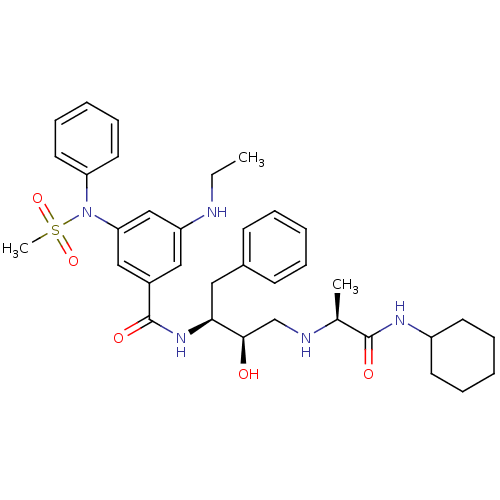 (CHEMBL1210359 | N-((1S,2R)-3-(((1S)-2-(CYCLOHEXYLA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM25771 (1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322916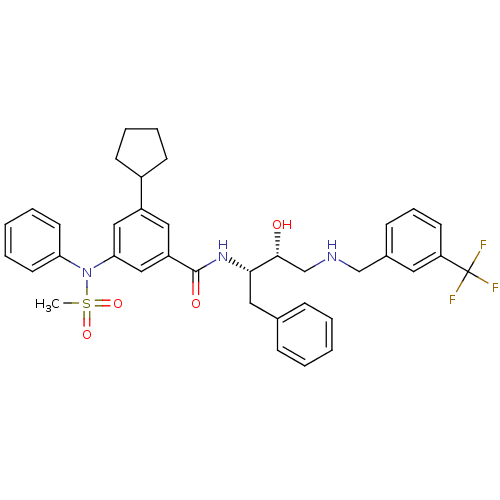 (3-cyclopentyl-N-((2S,3R)-3-hydroxy-1-phenyl-4-(3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29815 (sulfonamide tricyclic analogue, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322884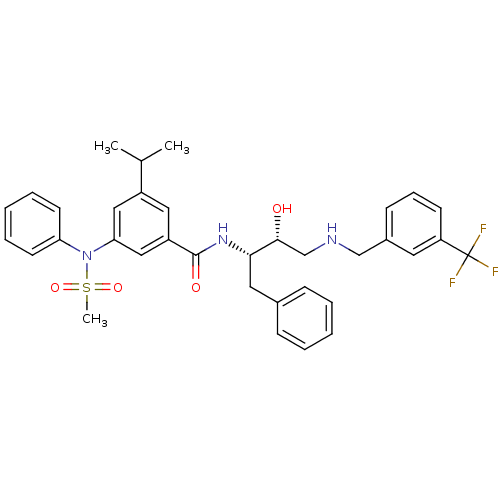 (CHEMBL1210361 | N-((2S,3R)-3-hydroxy-1-phenyl-4-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26506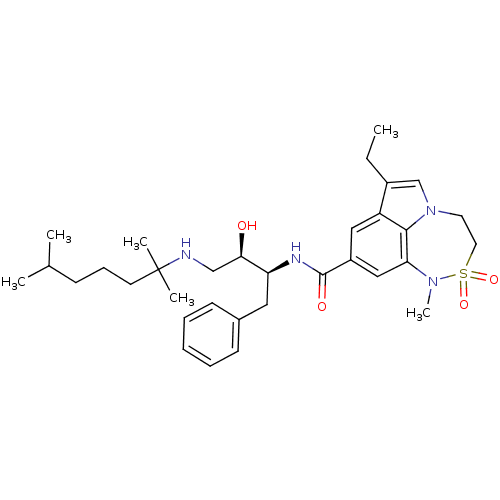 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207121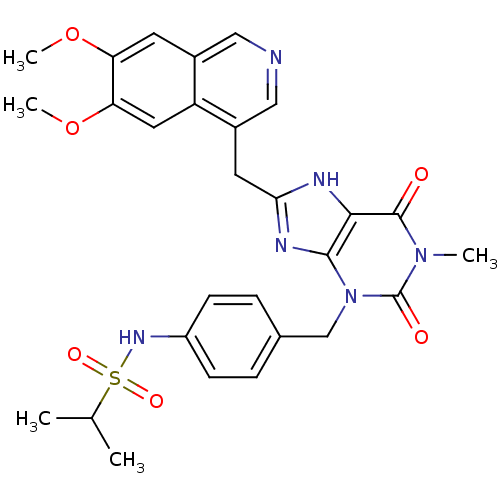 (CHEMBL245648 | N-(4-((8-((6,7-dimethoxyisoquinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29770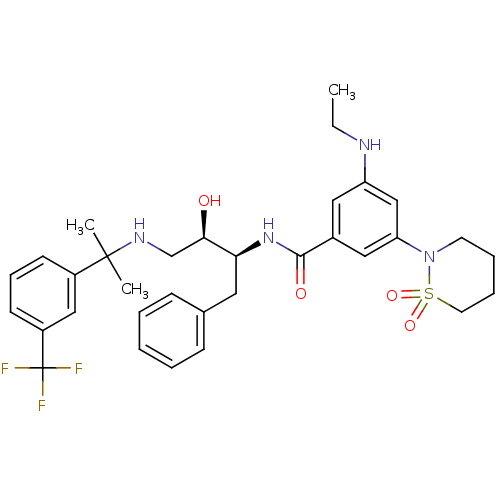 (hydroxyethylamine derivative, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322886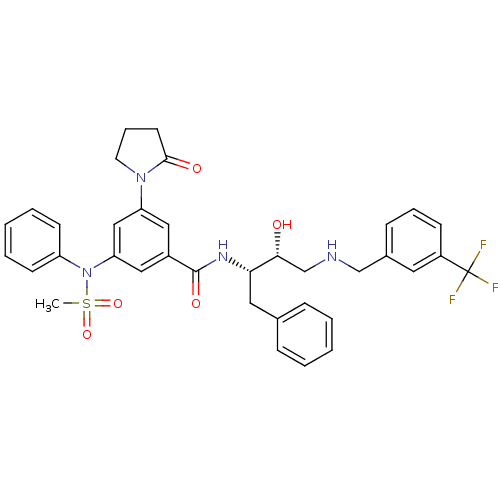 (CHEMBL1210363 | N-((2S,3R)-3-hydroxy-1-phenyl-4-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207123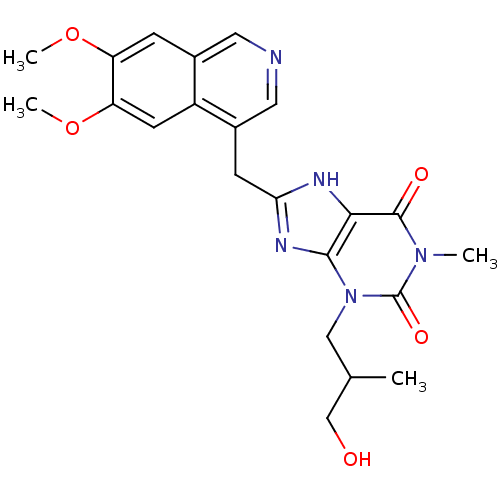 (8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-3-(3-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29805 (sulfone tricyclic analogue, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322891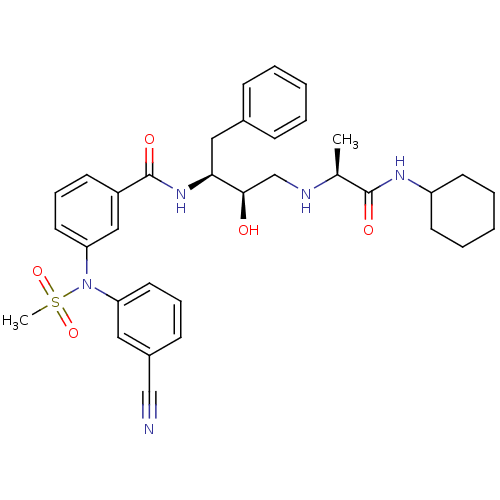 (3-(N-(3-cyanophenyl)methylsulfonamido)-N-((2S,3R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207127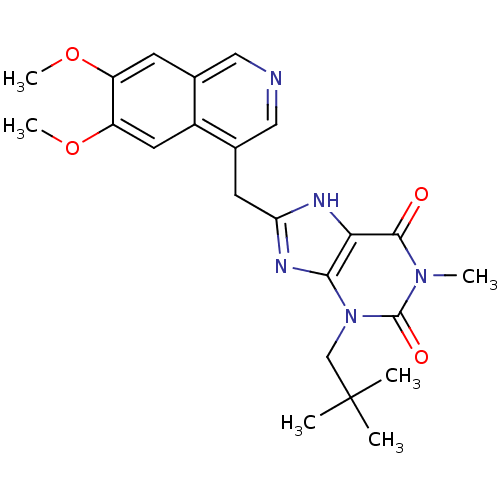 (8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29809 (sulfonamide tricyclic analogue, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29804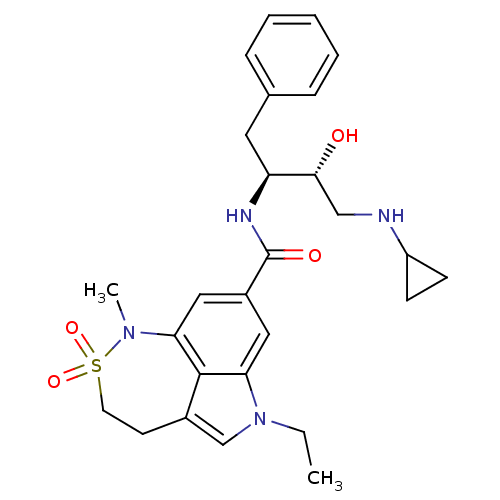 (sulfonamide tricyclic analogue, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29755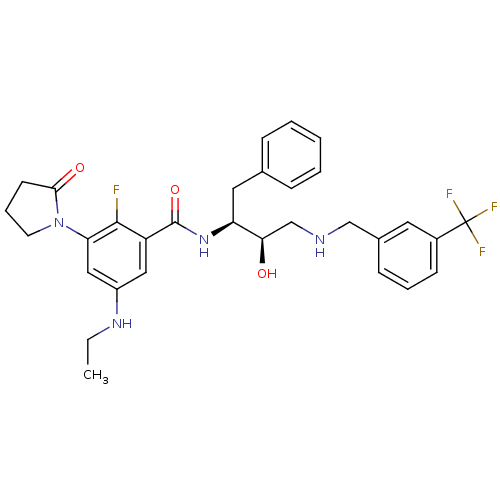 (hydroxyethylamine derivative, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26508 (BMCL193669 Compound 26 | N-[(2S,3R)-4-(cyclohexyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207132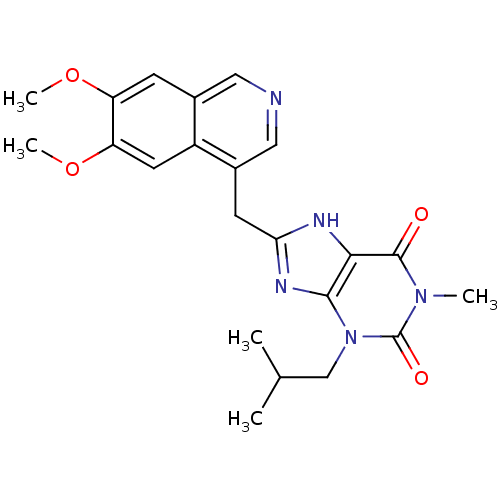 (8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-3-isobut...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 372 total ) | Next | Last >> |