

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112574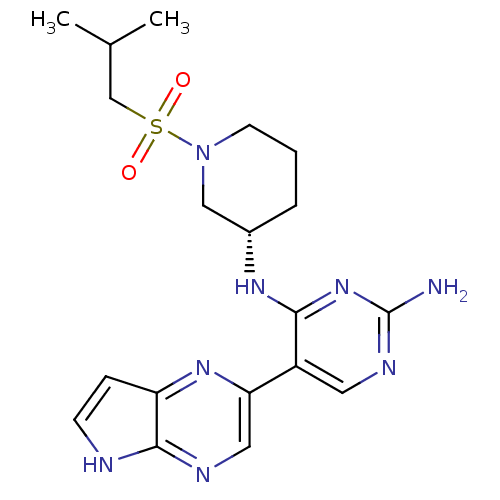 (US8618103, I-70) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000578 | n/a | 0.00115 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112546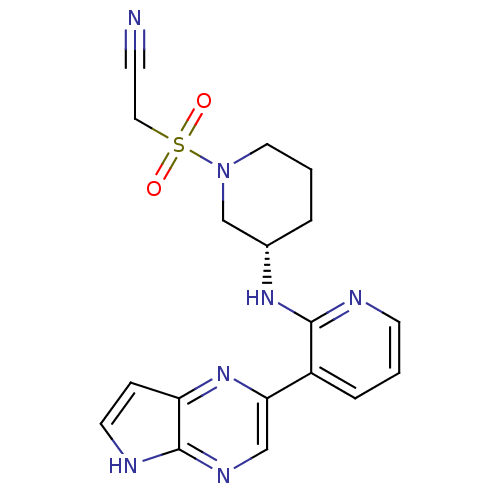 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000748 | n/a | 0.00149 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112576 (US8618103, I-72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000940 | n/a | 0.00188 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112573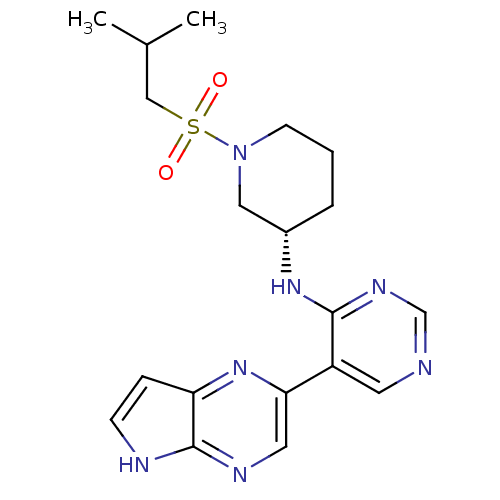 (US8618103, I-69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000980 | n/a | 0.00196 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106803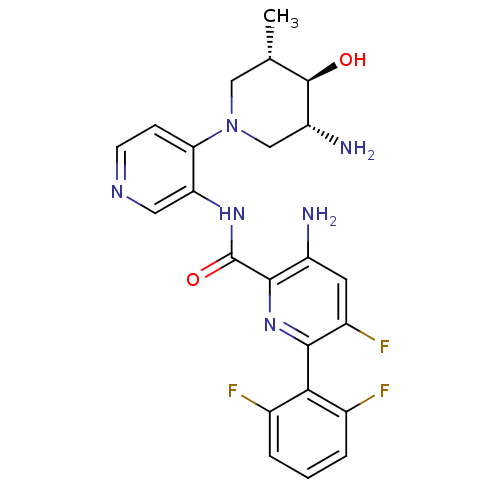 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234817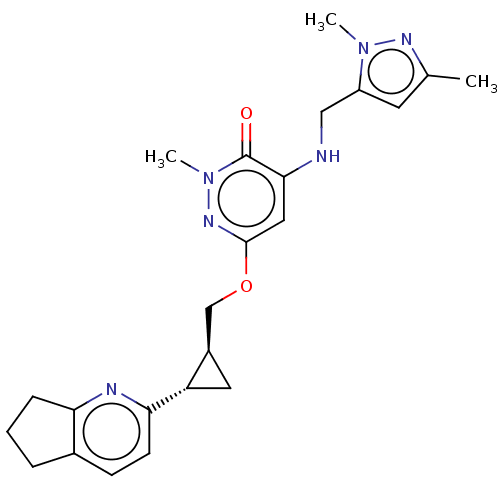 (US9353104, 121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234715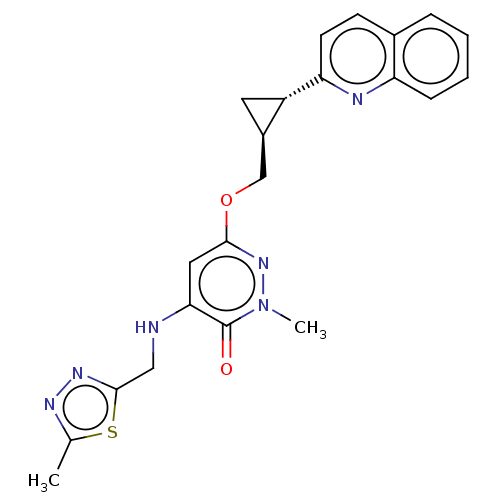 (US9353104, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112601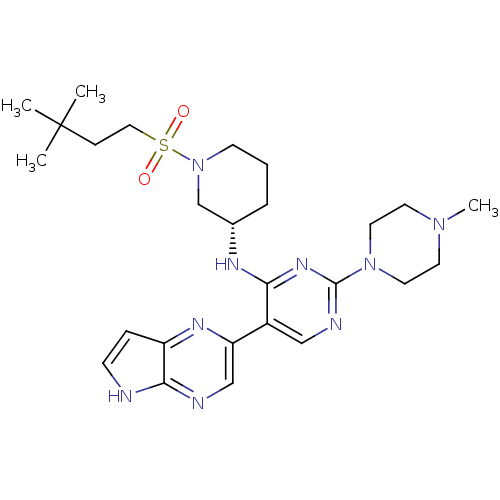 (US8618103, I-97) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00109 | n/a | 0.00218 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112536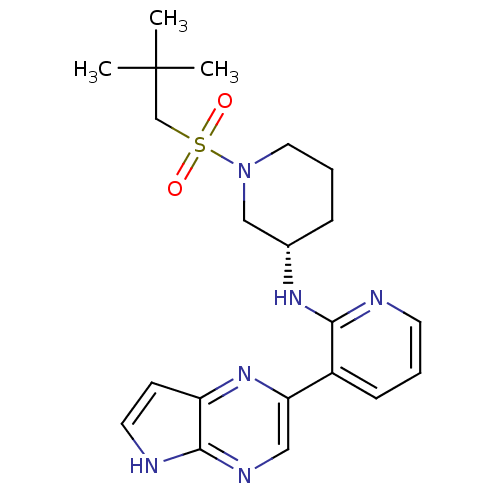 (US8618103, I-32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00120 | n/a | 0.00240 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112583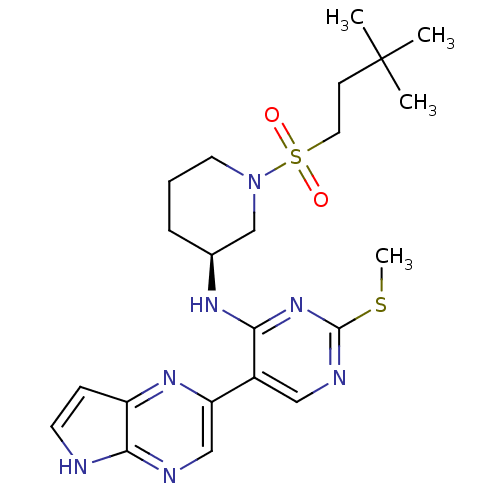 (US8618103, I-79) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00121 | n/a | 0.00242 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498523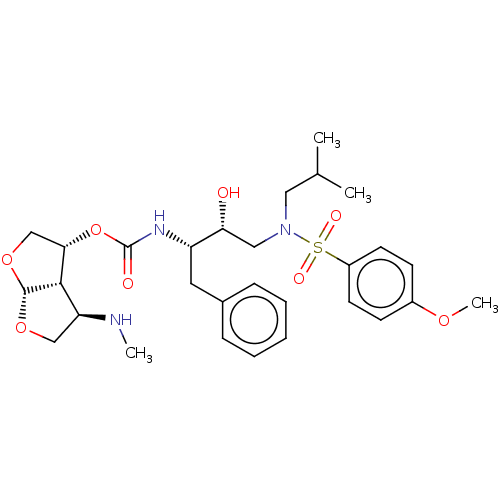 (CHEMBL3605643) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234762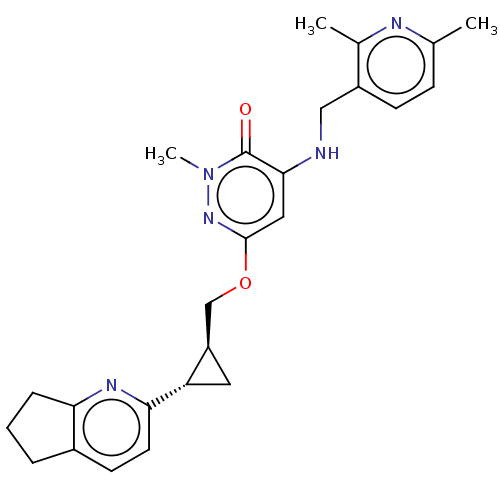 (US9353104, 56) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (RAT) | BDBM82253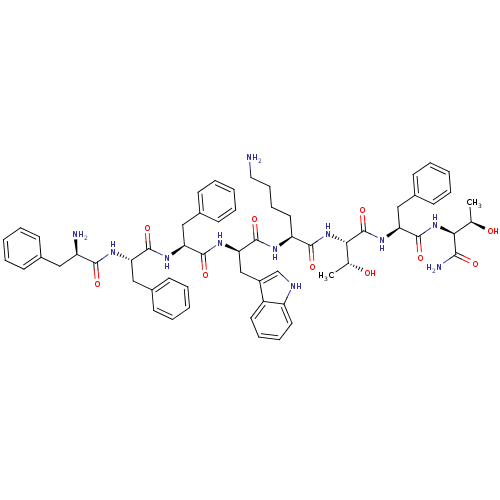 (BIM 23052 | CAS_133073-82-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by PDSP Ki Database | Mol Pharmacol 44: 385-92 (1993) BindingDB Entry DOI: 10.7270/Q2X065KZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (RAT) | BDBM50097783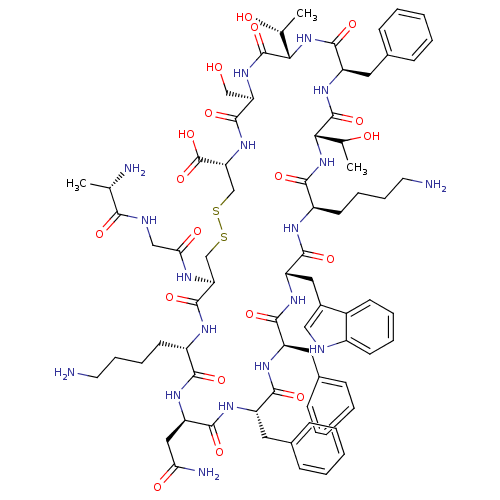 (CHEMBL438401 | D-Trp8 SST-14 | SOMATOSTATIN | SRIF...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by PDSP Ki Database | Mol Pharmacol 44: 385-92 (1993) BindingDB Entry DOI: 10.7270/Q2X065KZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234827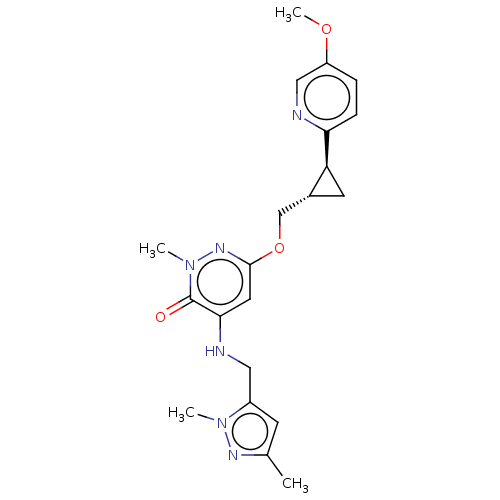 (US9353104, 132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234819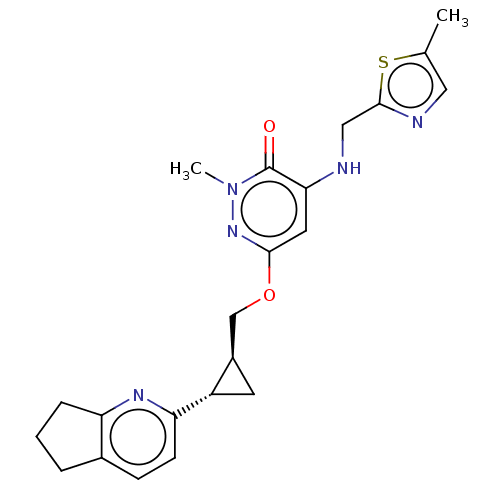 (US9353104, 124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234818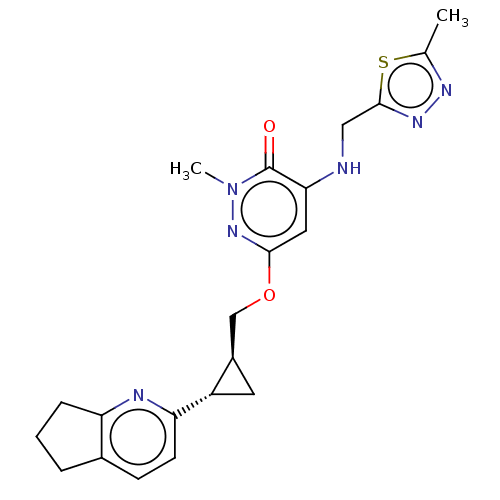 (US9353104, 123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445124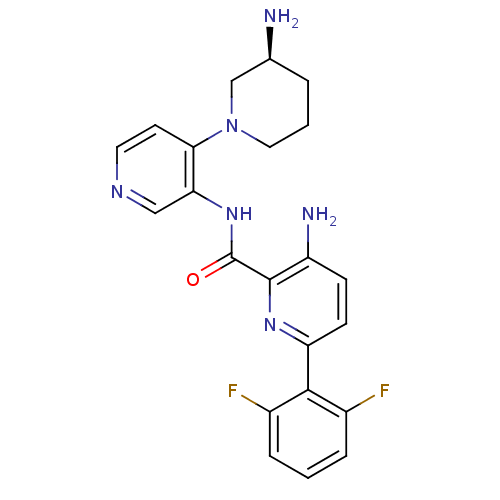 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445133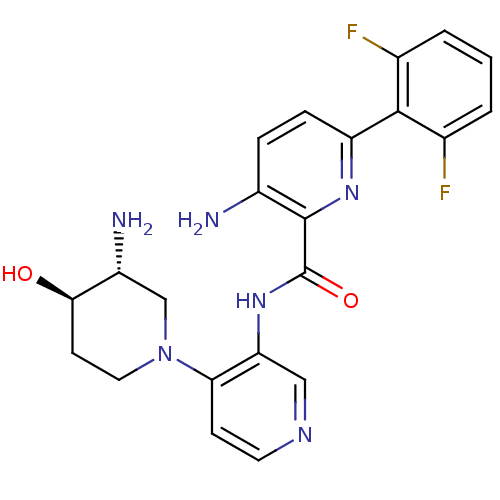 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112616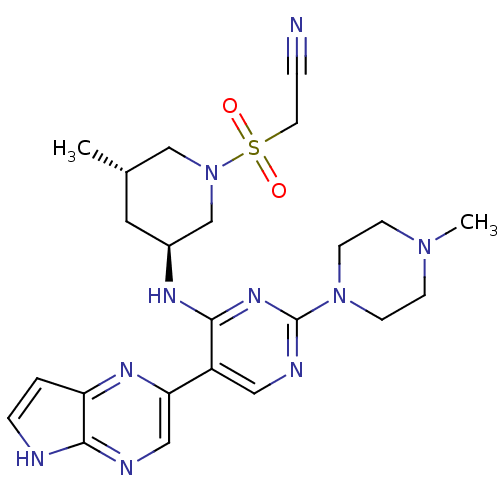 (US8618103, I-112) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00207 | n/a | 0.00415 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112568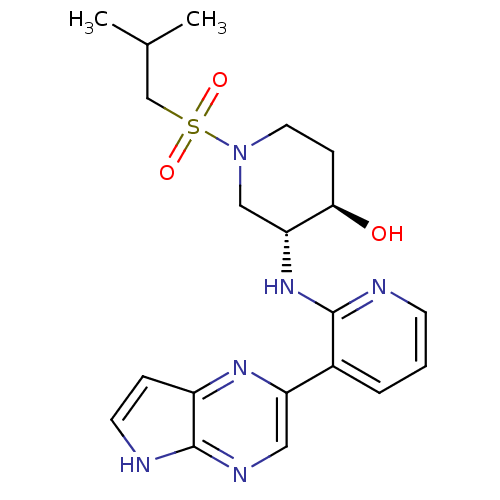 (US8618103, I-64) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00210 | n/a | 0.00421 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112575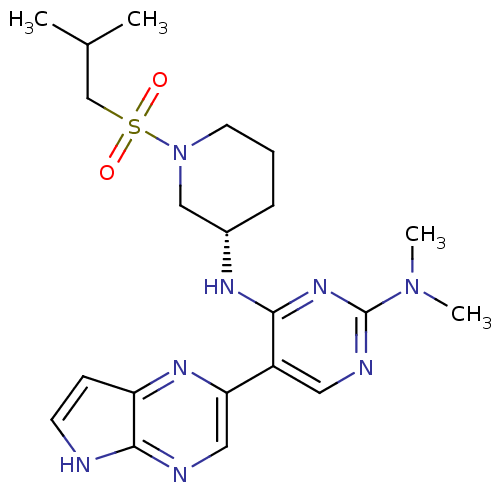 (US8618103, I-71) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00248 | n/a | 0.00496 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50056769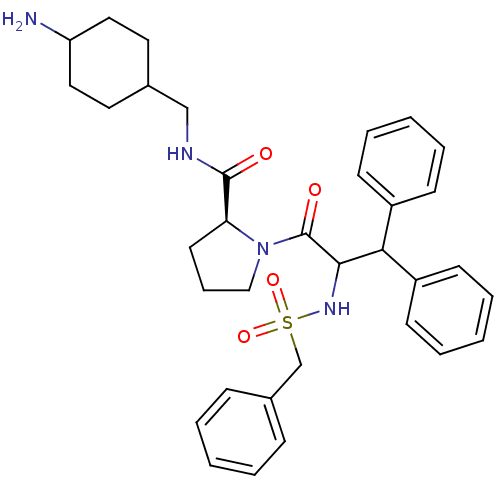 ((S)-1-(3,3-Diphenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Binding affinity (Ki) at calf intestinal adenosine deaminase. | J Med Chem 35: 4180-4 (1992) Checked by Author BindingDB Entry DOI: 10.7270/Q2SQ9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112622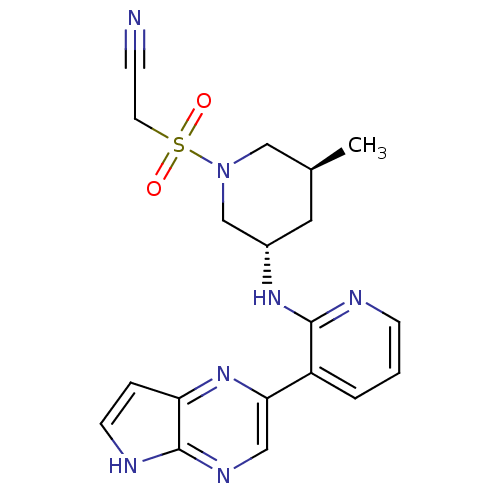 (US8618103, I-118) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00260 | n/a | 0.00521 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112597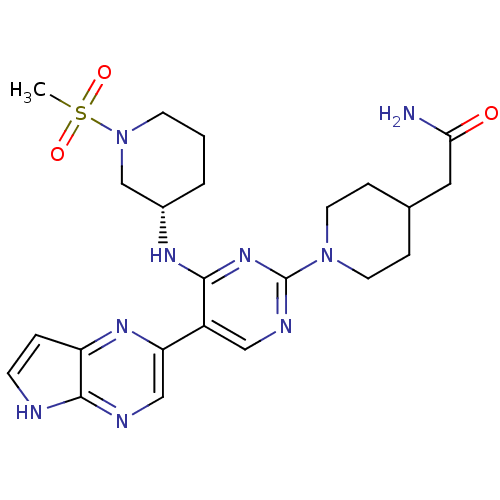 (US8618103, I-93) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00265 | n/a | 0.00530 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM128418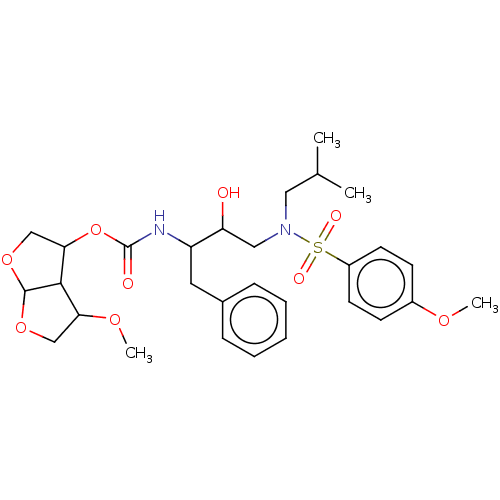 (US8802724, 23a | US8802724, 23c) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 0.00290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Research Foundation US Patent | Assay Description The enzyme inhibitory activity (Ki) was determined according to an assay protocol reported by Toth and Marshall (Toth, M. V.; Marshall, G. R. Int. J.... | US Patent US8802724 (2014) BindingDB Entry DOI: 10.7270/Q2445K5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50553771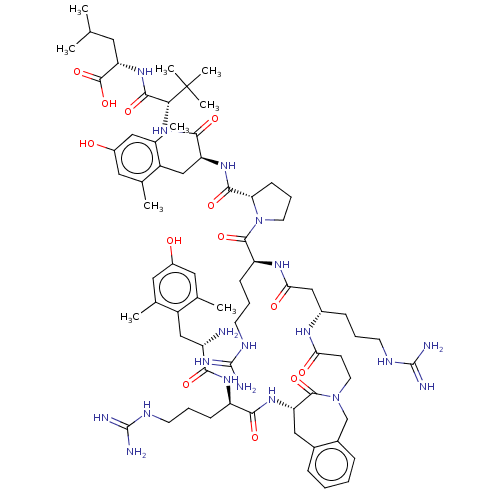 (CHEMBL4776986) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-neurotensin from human recombinant NTS2 stably expressed in human 1321N1 cell membranes incubated for 60 mins by gamma counter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01376 BindingDB Entry DOI: 10.7270/Q2H41W2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234726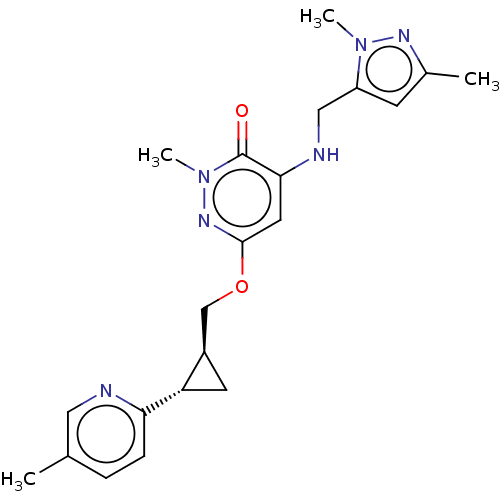 (US9353104, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234756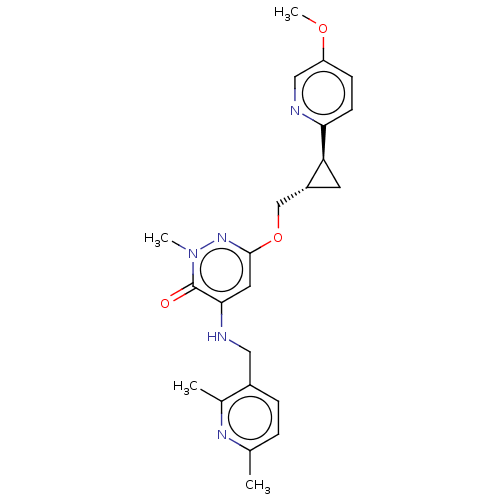 (US9353104, 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112614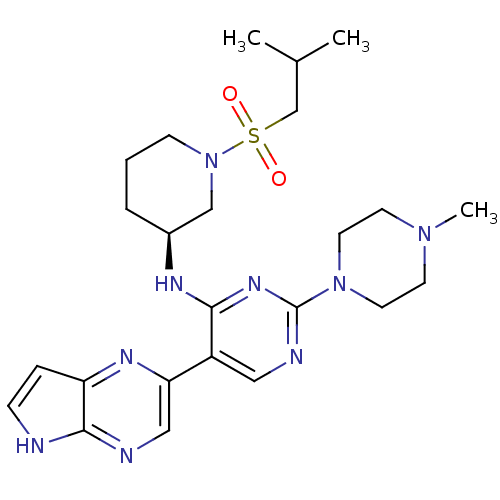 (US8618103, I-110) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00337 | n/a | 0.00675 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112530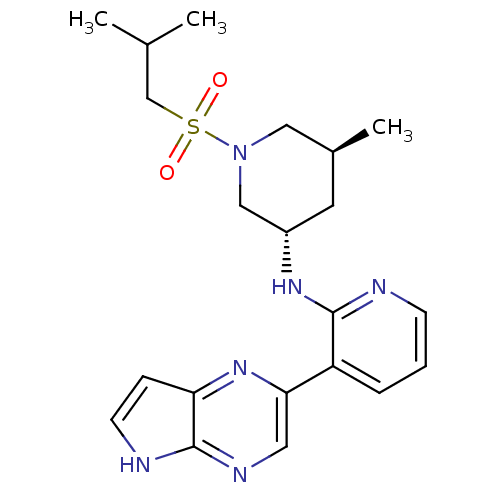 (US8618103, I-26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00344 | n/a | 0.00670 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112542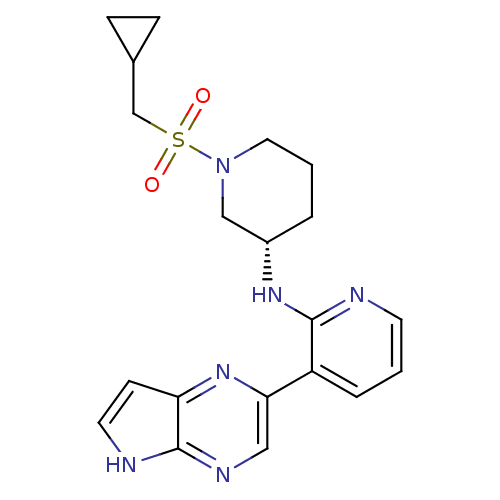 (US8618103, I-38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00373 | n/a | 0.00747 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112569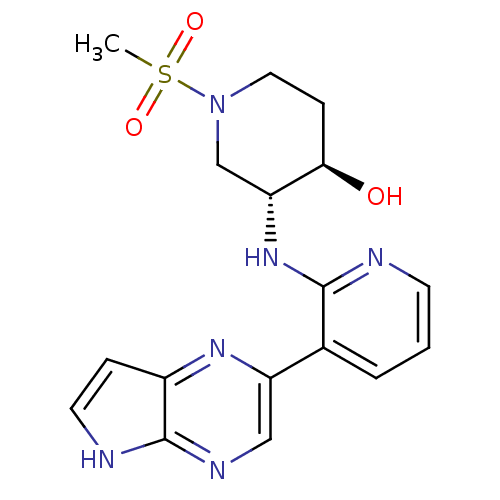 (US8618103, I-65) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00380 | n/a | 0.00762 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112567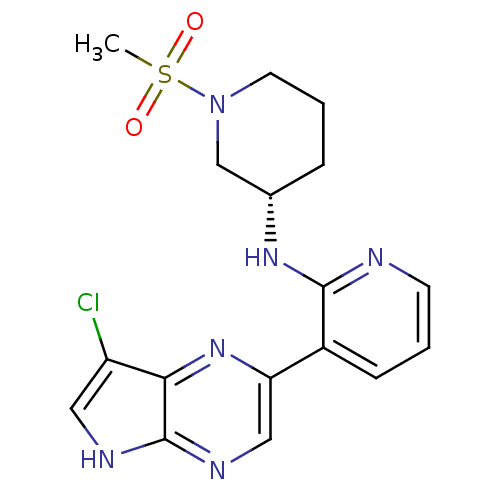 (US8618103, I-63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00386 | n/a | 0.00773 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498524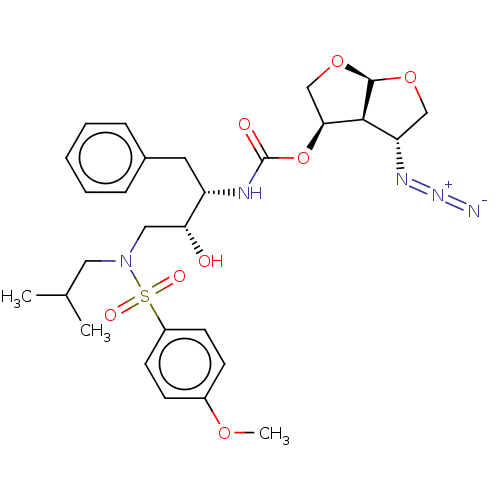 (CHEMBL3605638) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112529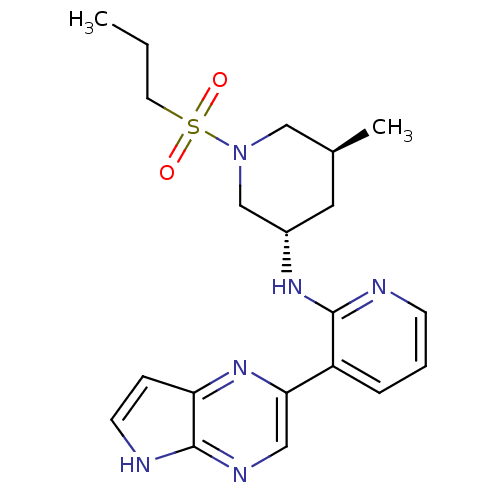 (US8618103, I-25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00398 | n/a | 0.00776 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM209756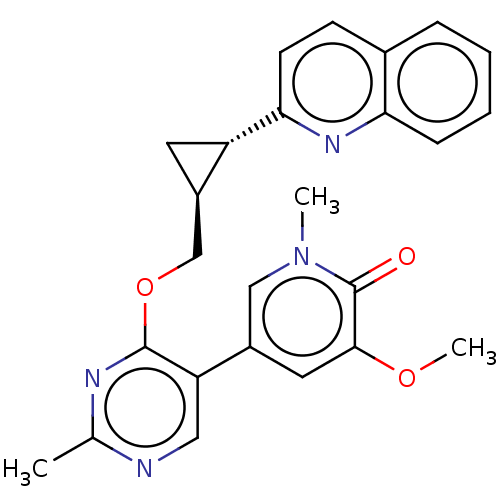 (US9273033, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | -65.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP FP kit supplied by Molecular Devices, Sunnyv... | US Patent US9273033 (2016) BindingDB Entry DOI: 10.7270/Q26Q1W2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234836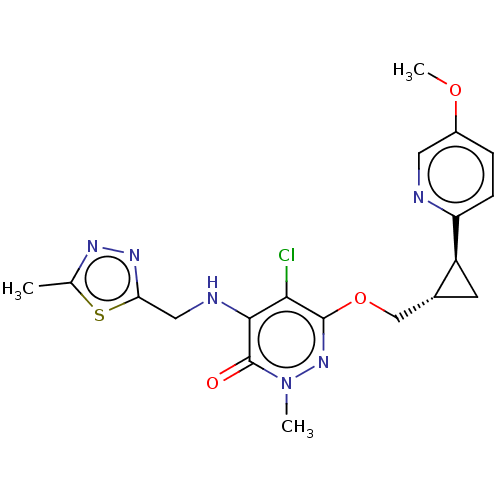 (US9353104, 141) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234763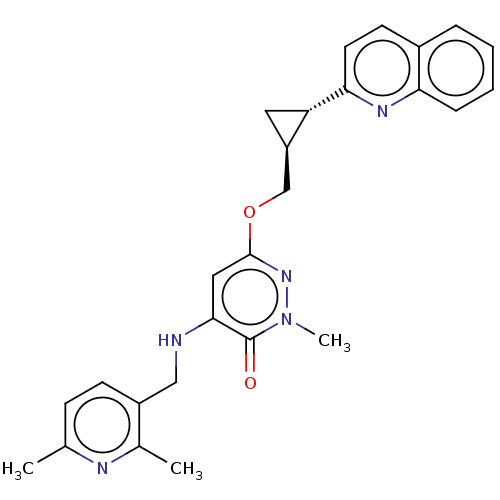 (US9353104, 57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234825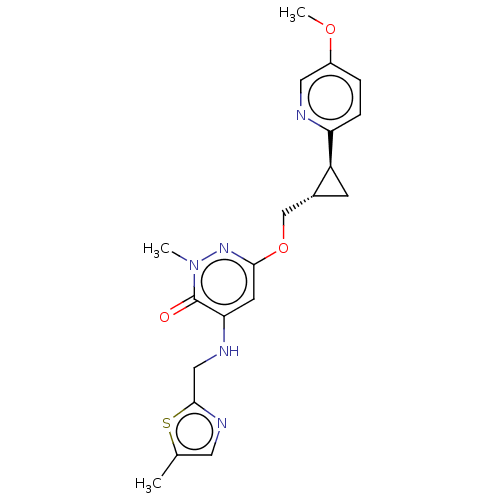 (US9353104, 130) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM234835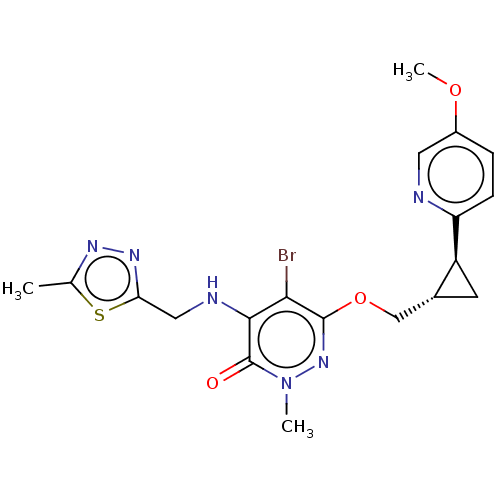 (US9353104, 140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny... | US Patent US9353104 (2016) BindingDB Entry DOI: 10.7270/Q2ZP4517 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112589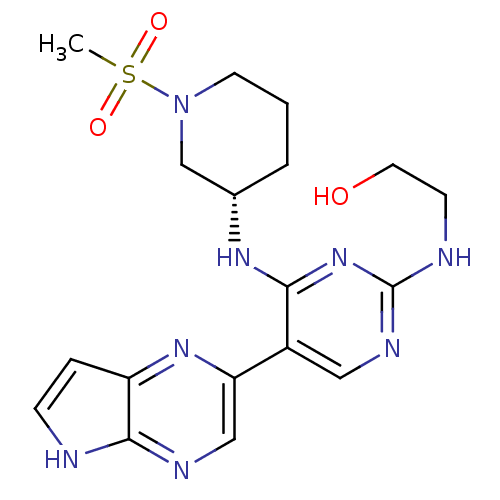 (US8618103, I-85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00402 | n/a | 0.00805 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112599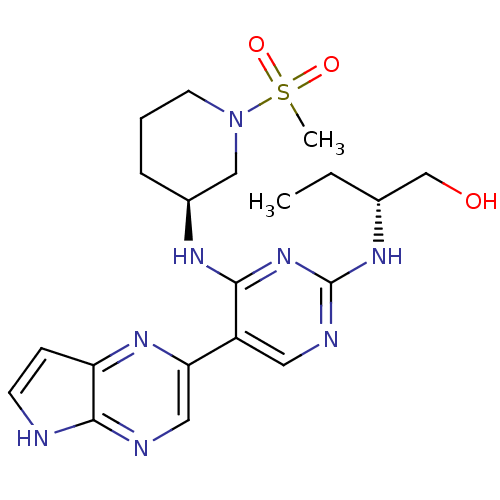 (US8618103, I-95) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00429 | n/a | 0.00858 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143305 total ) | Next | Last >> |