

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176407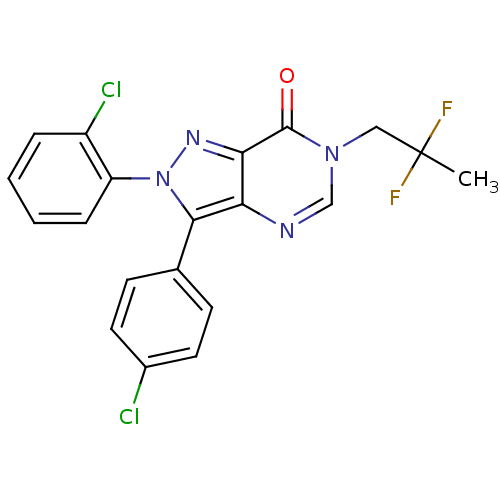 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176411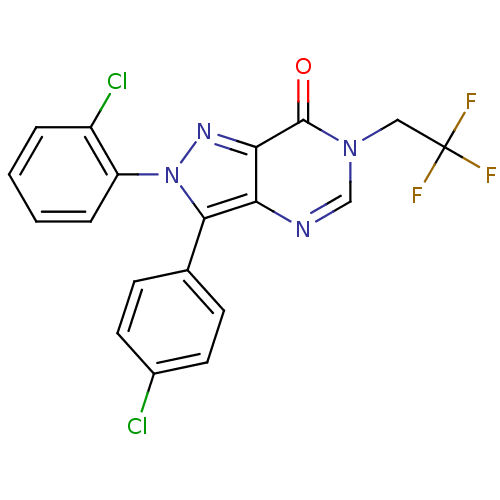 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176407 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176411 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120502 (2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120504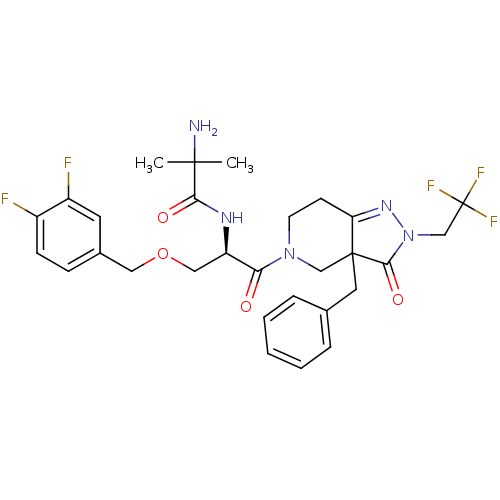 (2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176403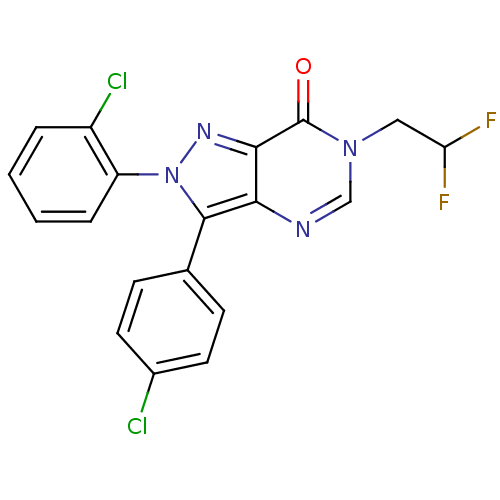 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061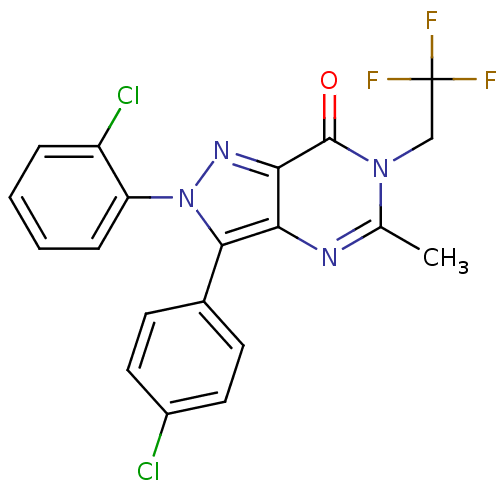 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176403 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176402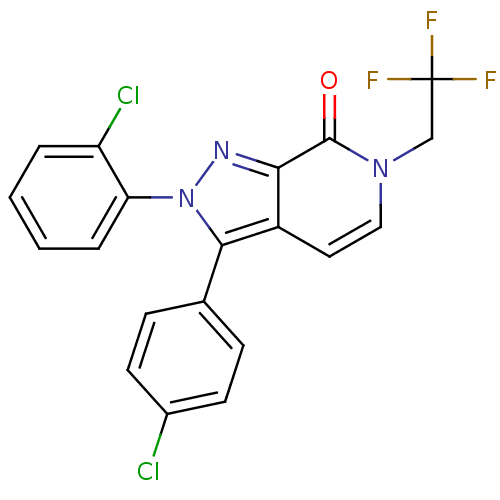 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176402 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342721 (CHEMBL1771259 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176418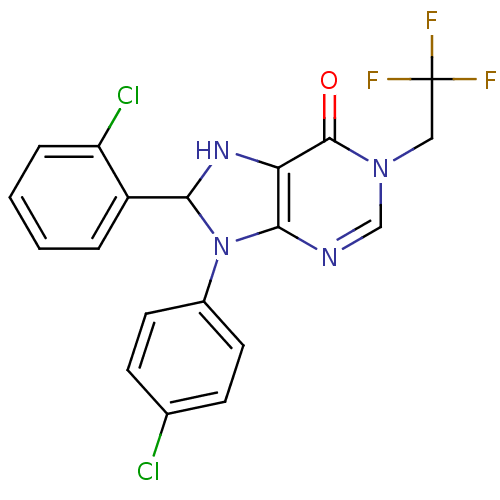 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-1-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176418 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-1-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176416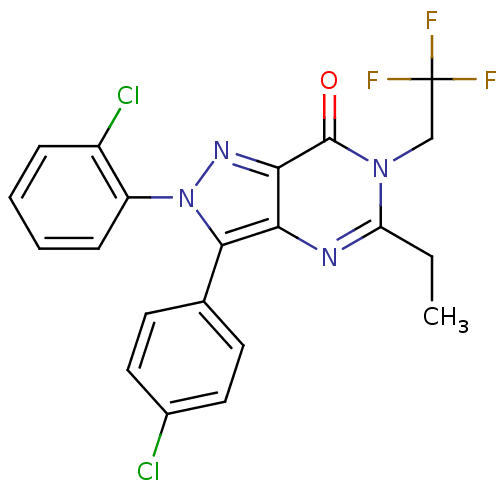 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-5-ethyl-6-(2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176415 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(3,3,3-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176413 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-propyl-2H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176413 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-propyl-2H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176410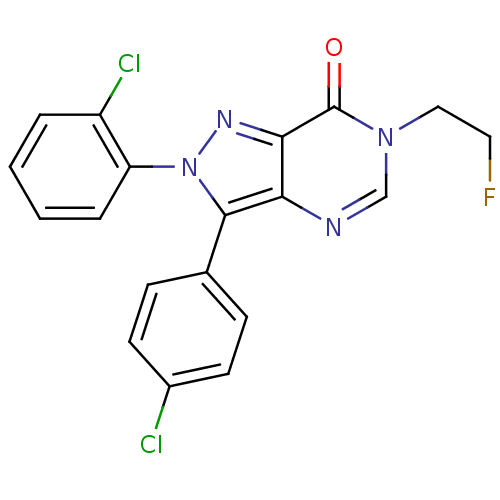 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2-fluoroe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176416 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-5-ethyl-6-(2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342728 (CHEMBL1771260 | N1-(3-(2,6-dichloro-4-ethoxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (RAT) | BDBM50342721 (CHEMBL1771259 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125]sauvagin from rat recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176415 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(3,3,3-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176409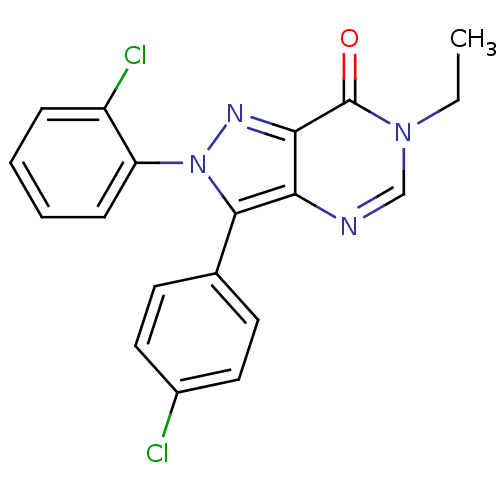 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-ethyl-2H-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176412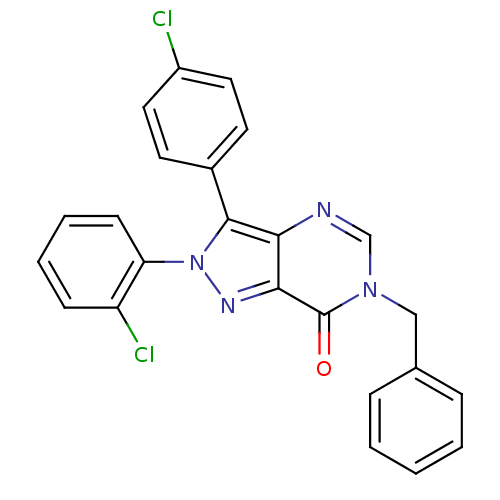 (6-benzyl-2-(2-chlorophenyl)-3-(4-chlorophenyl)-2H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176404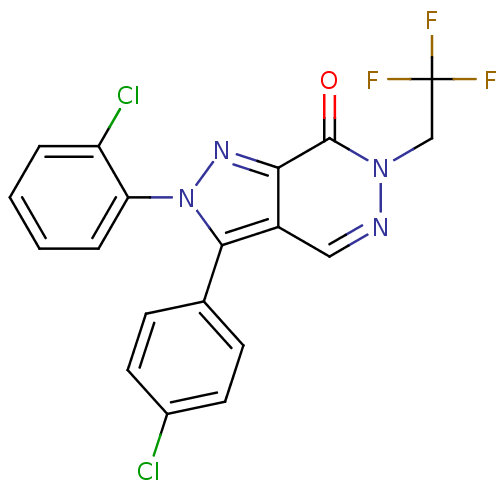 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176409 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-ethyl-2H-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342726 (CHEMBL1771262 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120505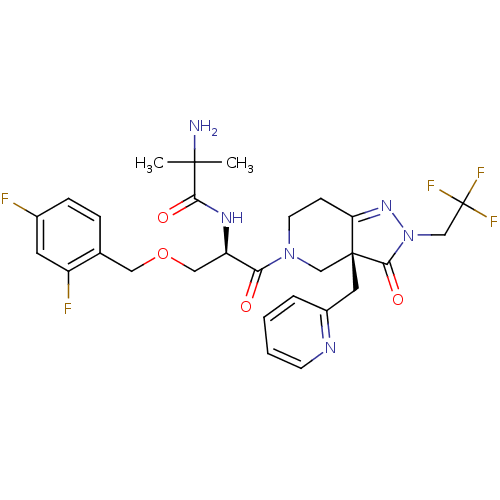 (2-Amino-N-{(R)-1-(2,4-difluoro-benzyloxymethyl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176410 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2-fluoroe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176412 (6-benzyl-2-(2-chlorophenyl)-3-(4-chlorophenyl)-2H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342724 (CHEMBL1771264 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176414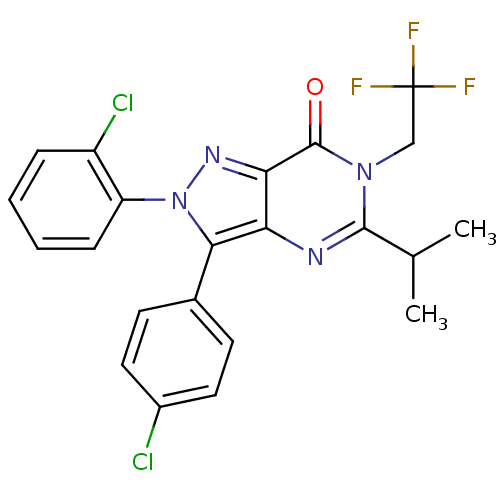 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-5-isopropyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342729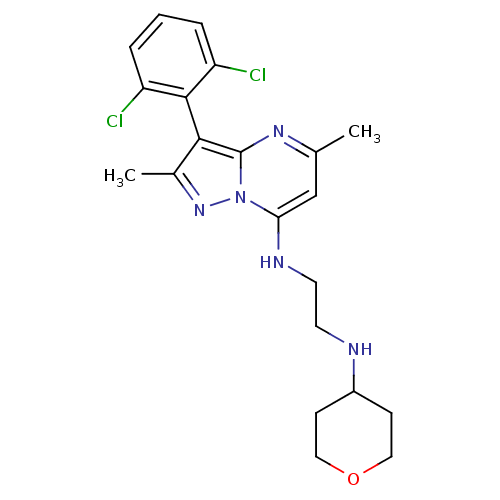 (CHEMBL1771258 | N1-(3-(2,6-dichlorophenyl)-2,5-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50083974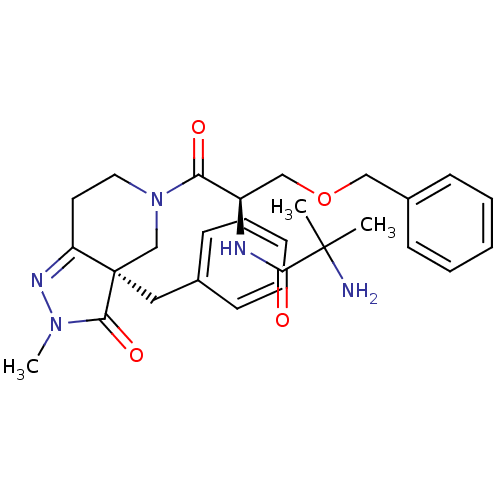 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386955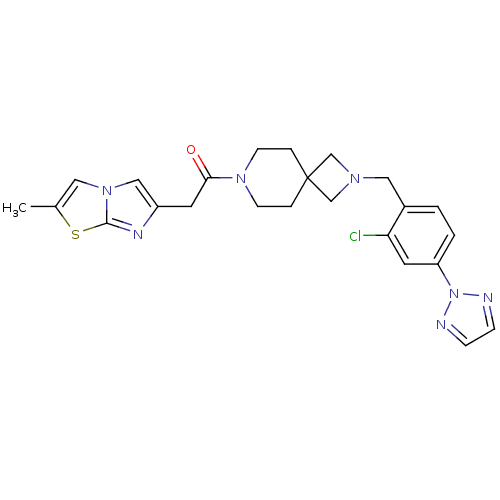 (CHEMBL2048820) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176404 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342727 (CHEMBL1771261 | N1-(3-(2,6-dichloro-4-propoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386949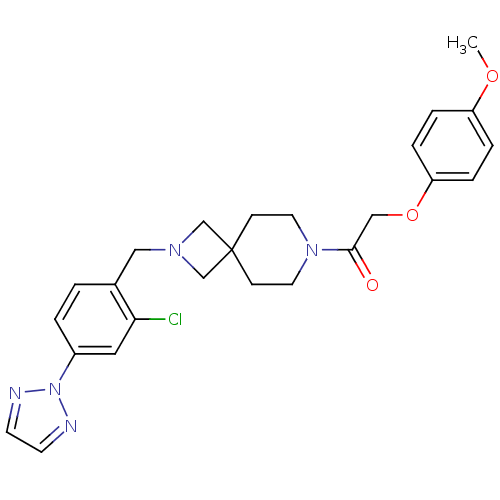 (CHEMBL2048814) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176406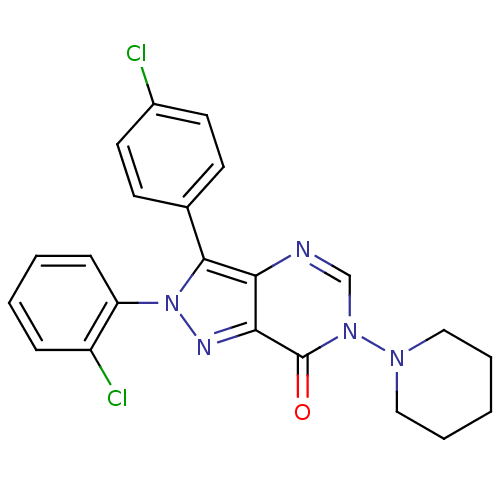 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(piperidin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176414 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-5-isopropyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386952 (CHEMBL2048817) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386957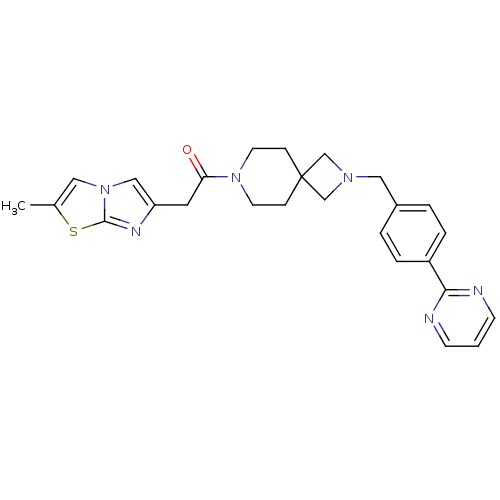 (CHEMBL2048822) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386948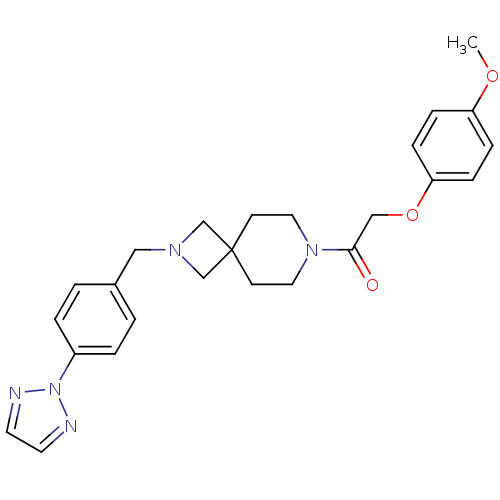 (CHEMBL2048813) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386953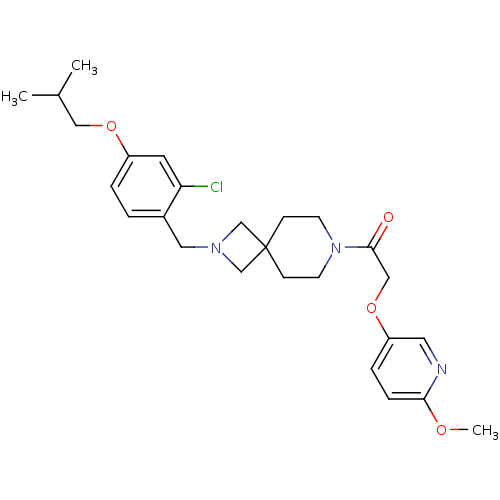 (CHEMBL2048818) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120503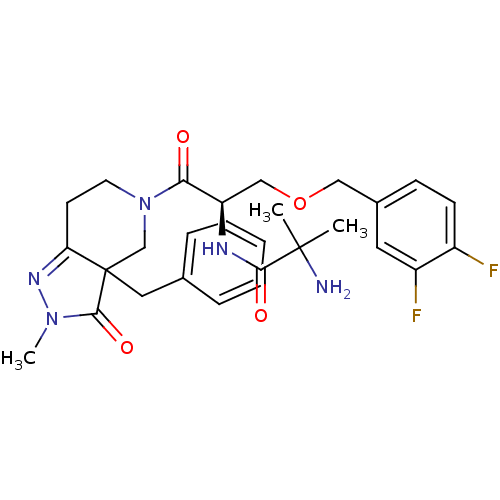 (2-Amino-N-[(R)-2-(3a-benzyl-2-methyl-3-oxo-2,3,3a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176417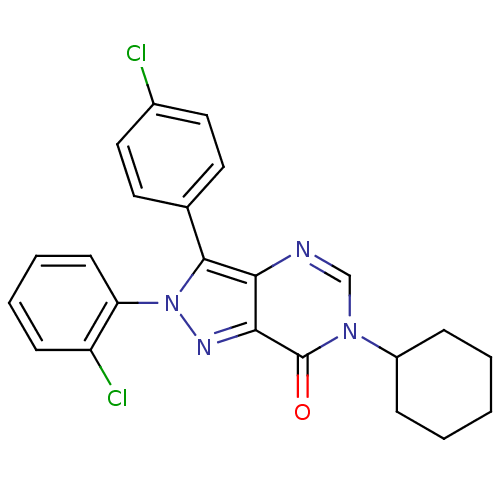 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-cyclohexyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 369 total ) | Next | Last >> |