

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Enterobacter cloacae) | BDBM50339868 (4-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339869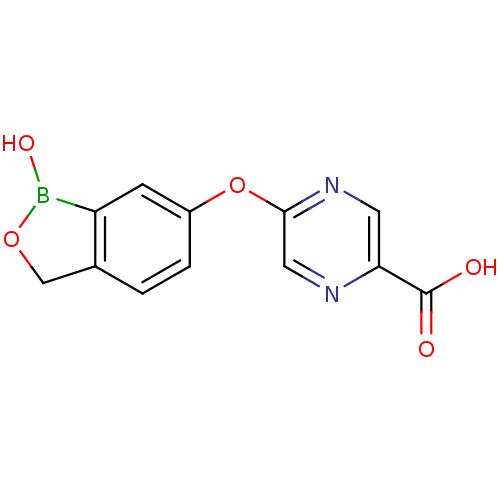 (5-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339870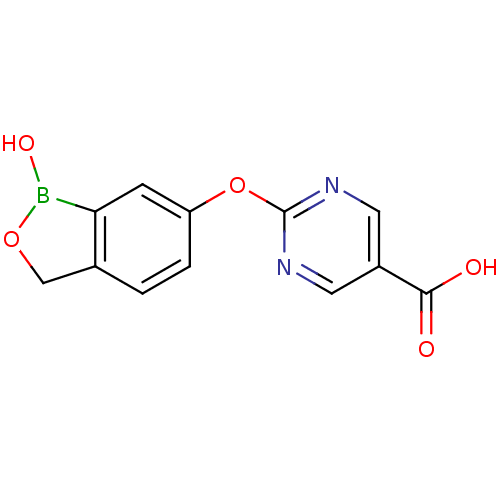 (2-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339846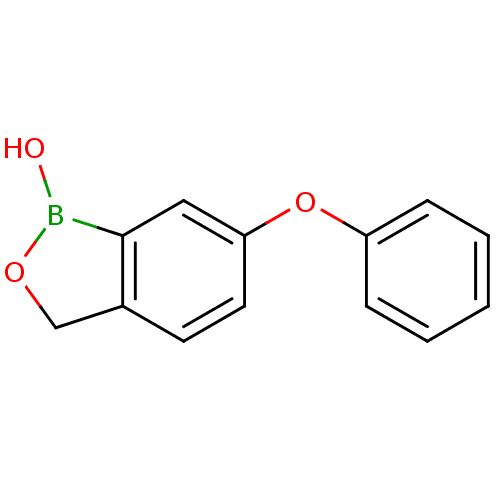 (6-phenoxybenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL17...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339856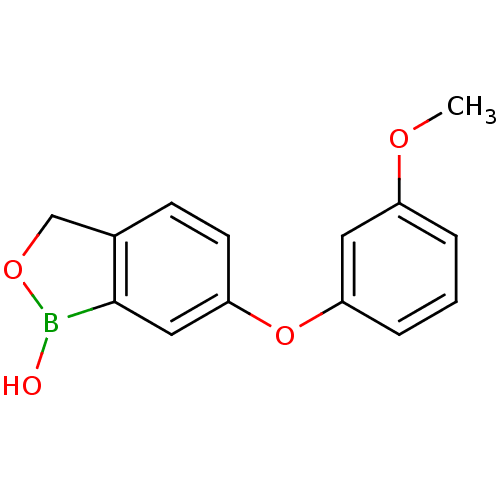 (6-(3-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339862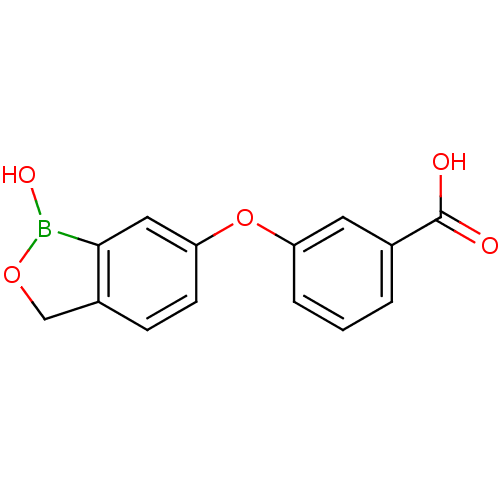 (3-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339860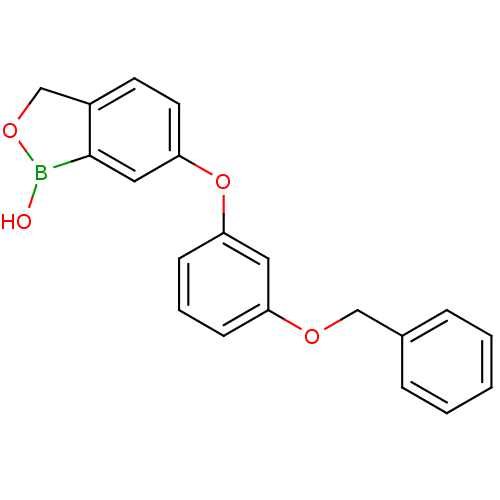 (6-(3-(benzyloxy)phenoxy)benzo[c][1,2]oxaborol-1(3H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339857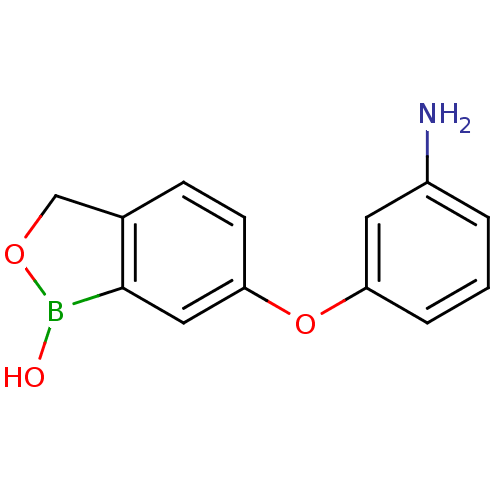 (6-(3-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339859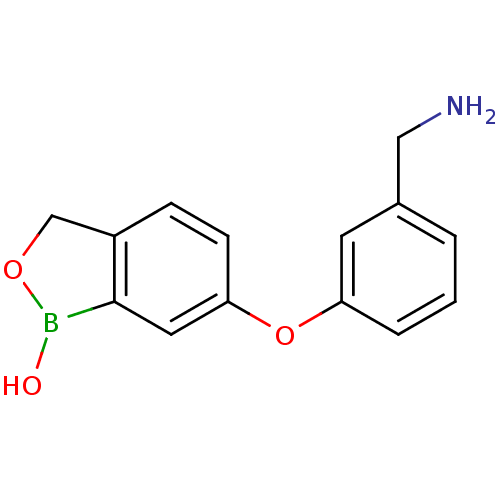 (6-(3-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339855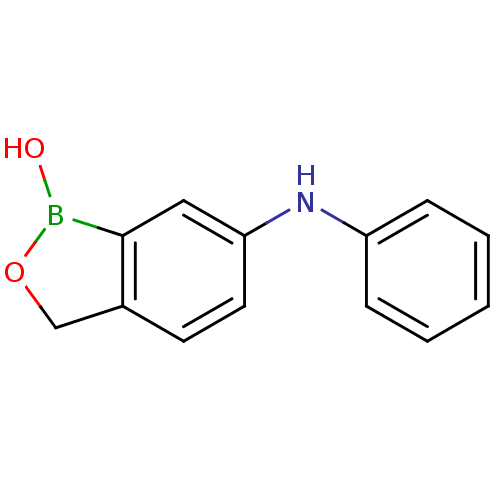 (6-(phenylamino)benzo[c][1,2]oxaborol-1(3H)-ol | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50280981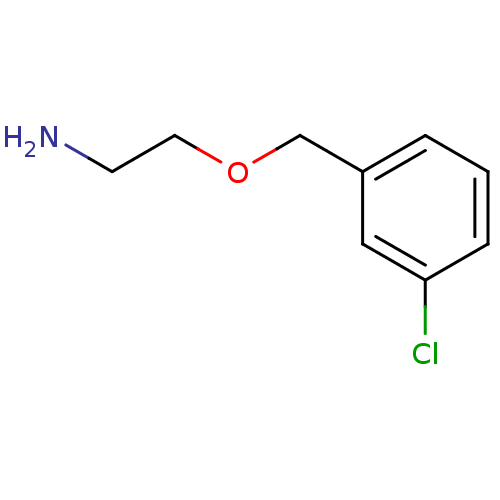 (2-(3-Chloro-benzyloxy)-ethylamine | CHEMBL65135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for enzyme affinity with purified bovine liver MAO B | Bioorg Med Chem Lett 3: 2077-2078 (1993) Article DOI: 10.1016/S0960-894X(01)81019-2 BindingDB Entry DOI: 10.7270/Q2PC329M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339867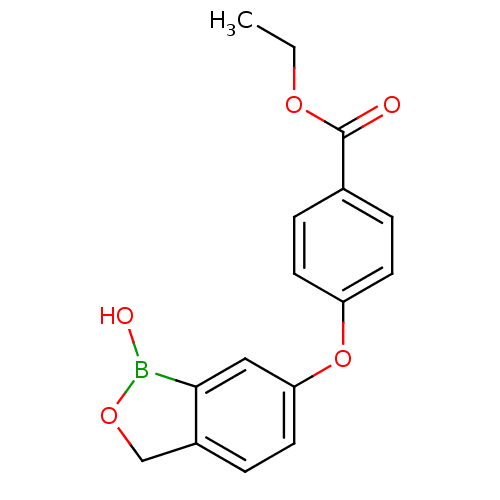 (CHEMBL1761273 | ethyl 4-(1-hydroxy-1,3-dihydrobenz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339863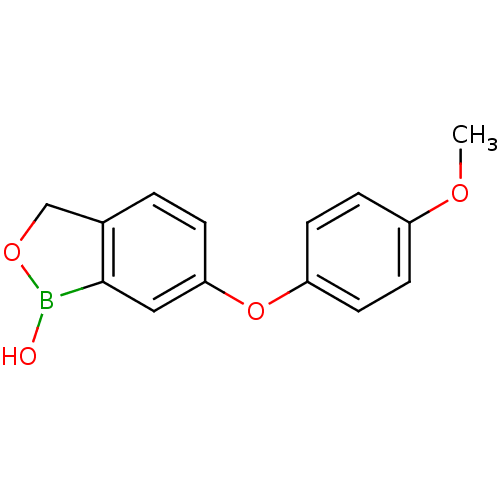 (6-(4-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339850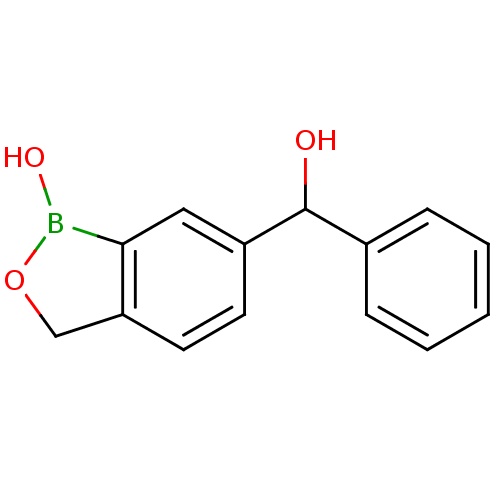 (6-(hydroxy(phenyl)methyl)benzo[c][1,2]oxaborol-1(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339861 (6-(3-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339858 (6-(3-(hydroxymethyl)phenoxy)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339864 (6-(4-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339847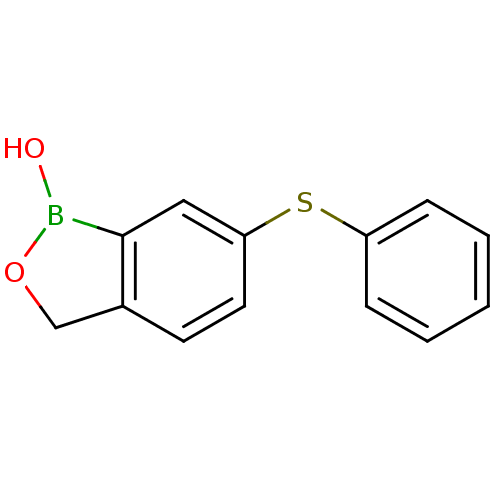 (6-(phenylthio)benzo[c][1,2]oxaborol-1(3H)-ol | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339865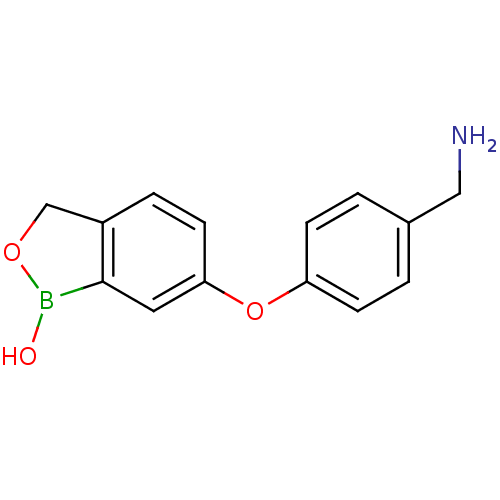 (6-(4-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339854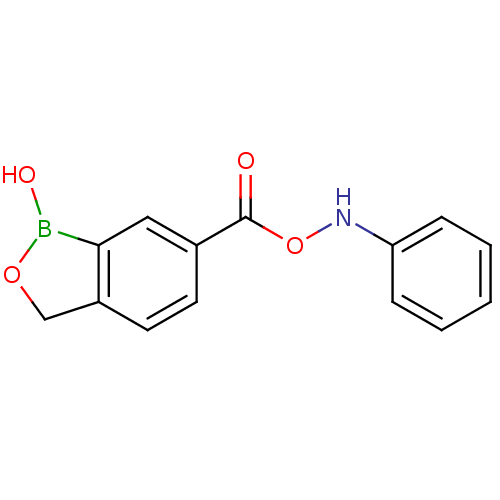 (6-((phenylaminooxy)carbonyl)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339848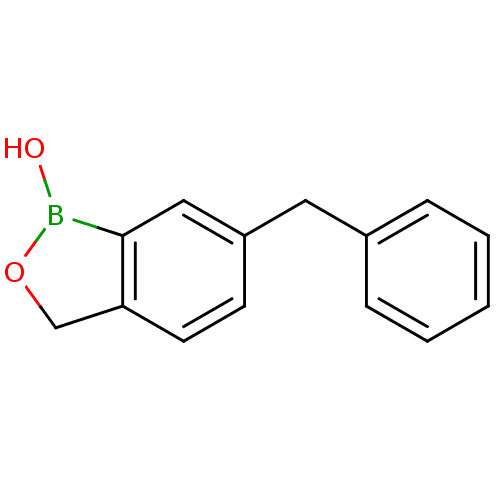 (6-benzylbenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL176...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339849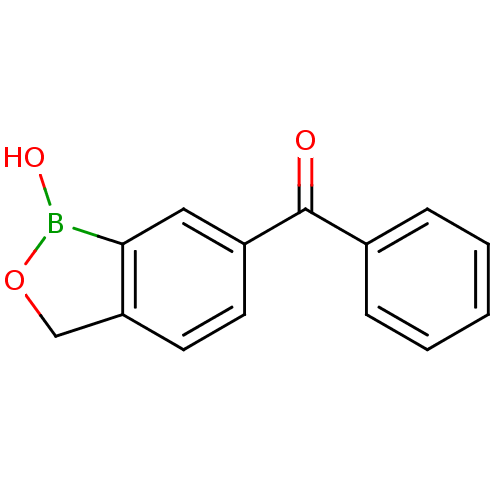 ((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339866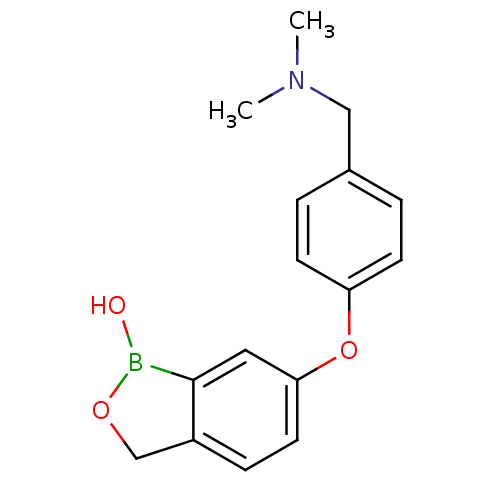 (6-(4-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339853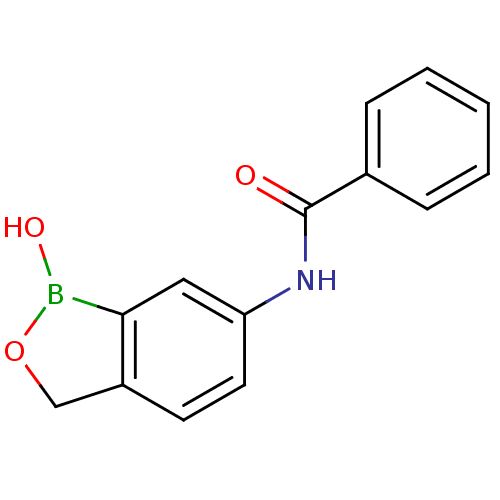 (CHEMBL1761258 | N-(1-hydroxy-1,3-dihydrobenzo[c][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339851 (6-(phenylsulfinyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339852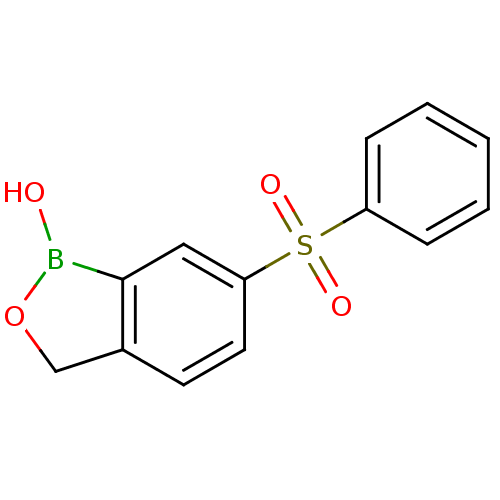 (6-(phenylsulfonyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50042588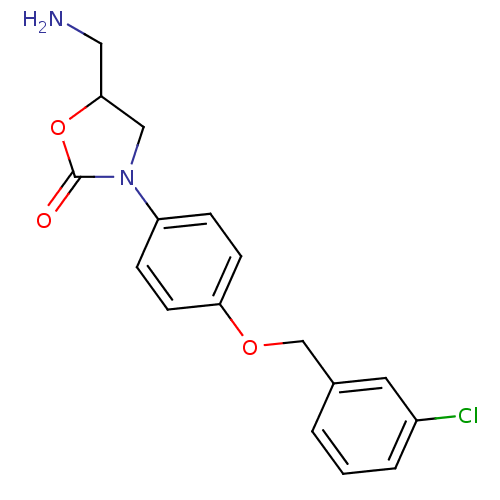 (5-Aminomethyl-3-[4-(3-chloro-benzyloxy)-phenyl]-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50042592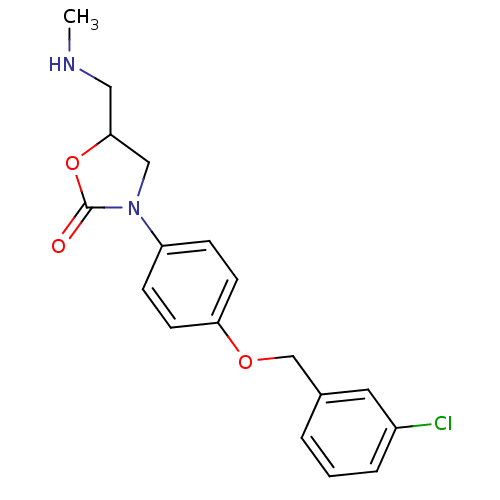 (3-[4-(3-Chloro-benzyloxy)-phenyl]-5-methylaminomet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50280981 (2-(3-Chloro-benzyloxy)-ethylamine | CHEMBL65135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for enzyme affinity with purified bovine liver MAO A | Bioorg Med Chem Lett 3: 2077-2078 (1993) Article DOI: 10.1016/S0960-894X(01)81019-2 BindingDB Entry DOI: 10.7270/Q2PC329M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50036731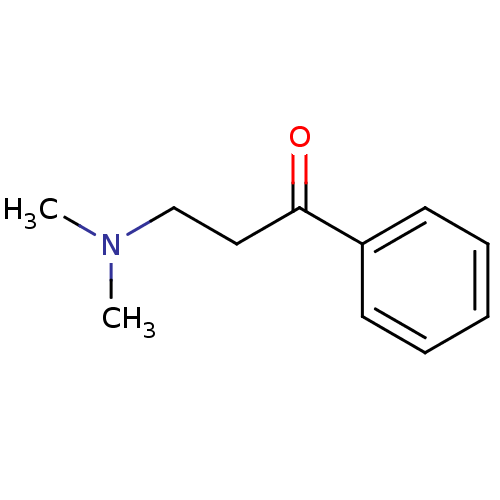 (3-Dimethylamino-1-phenyl-propan-1-one | CHEMBL5011...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50047897 (CHEMBL289199 | Dimethyl-(3-phenyl-propyl)-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50042589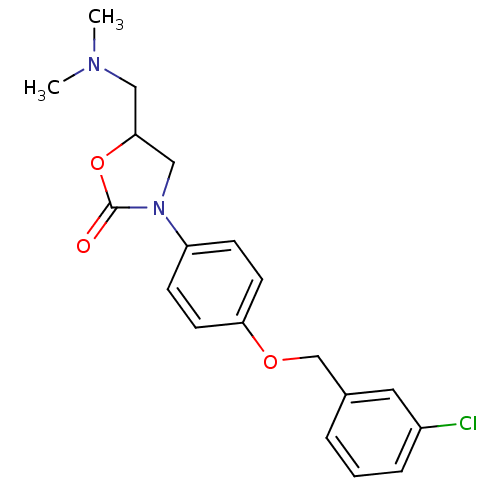 (3-[4-(3-Chloro-benzyloxy)-phenyl]-5-dimethylaminom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50047898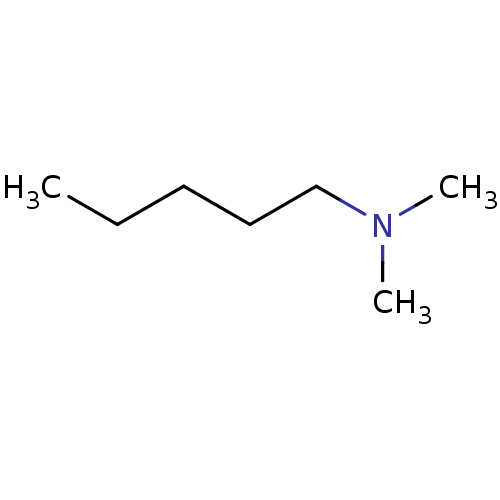 (CHEMBL47794 | Dimethyl-pentyl-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50047896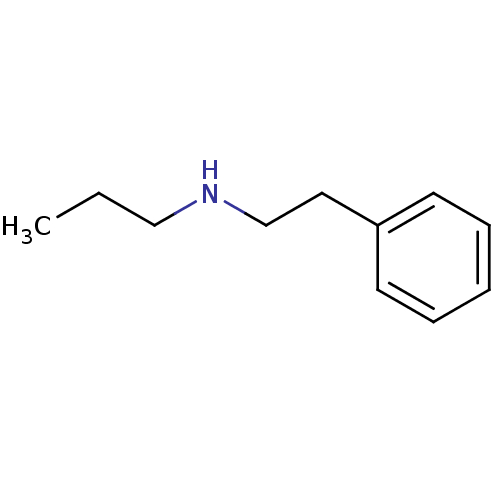 (CHEMBL46232 | Phenethyl-propyl-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50368739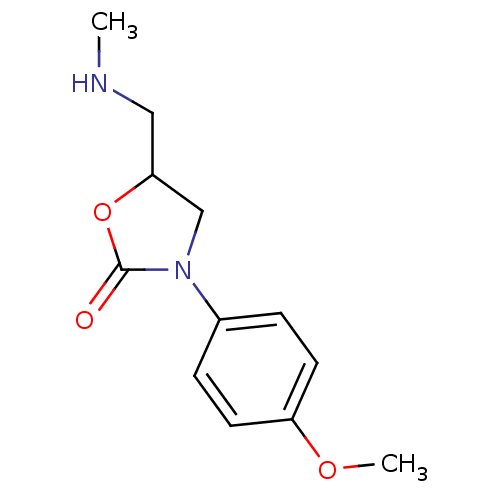 (CHEMBL1203212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50042587 (5-Aminomethyl-3-(4-methoxy-phenyl)-dihydro-furan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50042586 (4-Aminomethyl-1-(4-methoxy-phenyl)-pyrrolidin-2-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 6.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50018453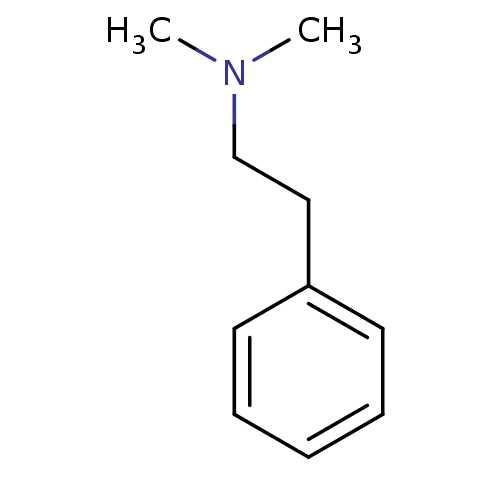 (CHEMBL46278 | Dimethyl-phenethyl-amine | N,N-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50016907 (CHEMBL46841 | Dimethyl-(4-phenyl-butyl)-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50368566 (CHEMBL1203213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.25E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50368737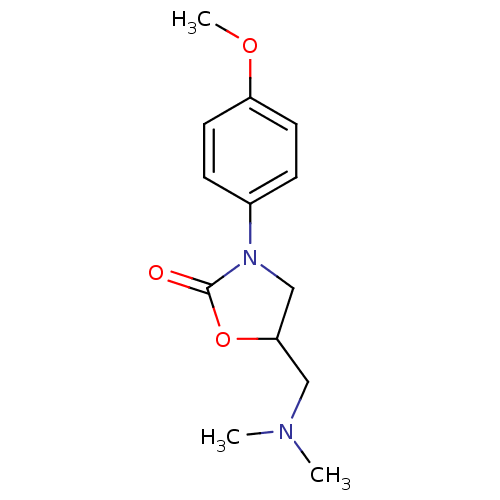 (CHEMBL1203215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.33E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50368741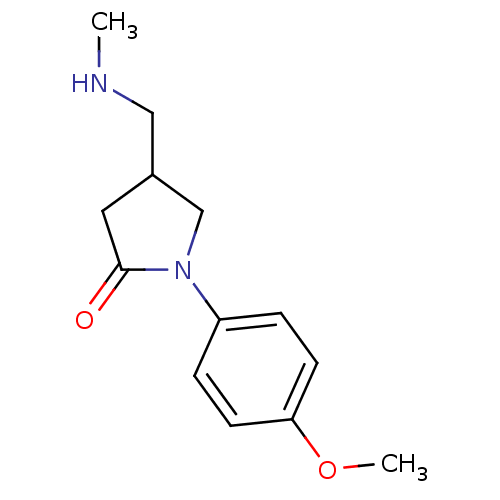 (CHEMBL1203214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50368738 (CHEMBL1203211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50368740 (CHEMBL1203210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant for inactivation of MAO B from replot of half-lives of inactivation | J Med Chem 36: 3606-10 (1994) BindingDB Entry DOI: 10.7270/Q20R9Q09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM504412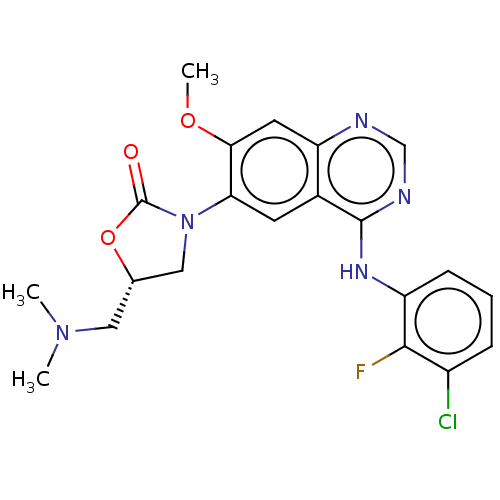 (US11040984, Compound 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM504408 (US11040984, Compound 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM504411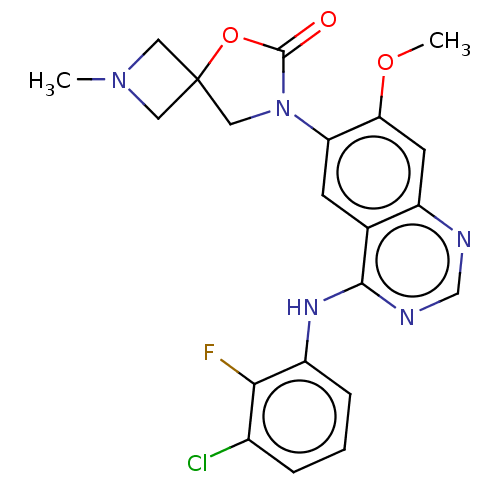 (US11040984, Compound 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM504410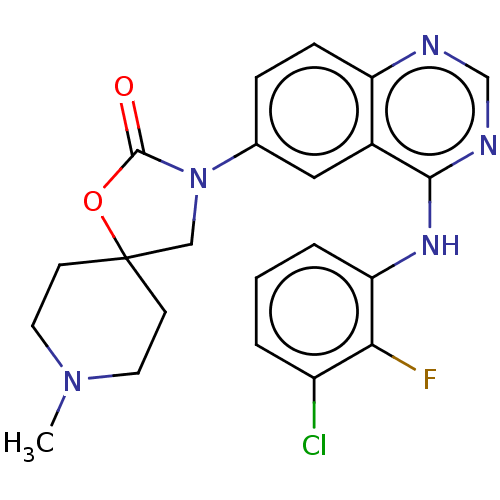 (US11040984, Compound 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-745,751-1210] (Homo sapiens (Human)) | BDBM504403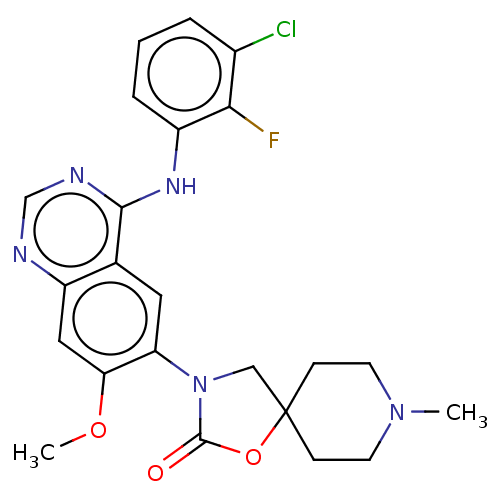 (US11040984, Compound 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM504415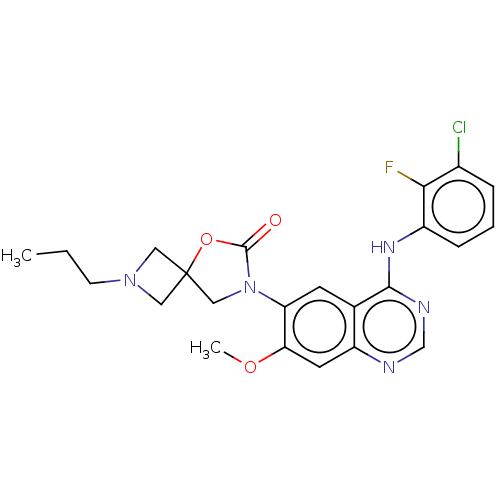 (US11040984, Compound 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1449 total ) | Next | Last >> |