 Found 8459 hits with Last Name = 'chi' and Initial = 'd'
Found 8459 hits with Last Name = 'chi' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50368723
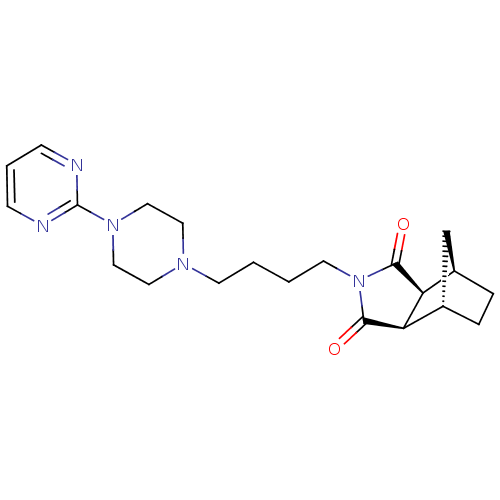
(Metanopirone | Sediel | TANDOSPIRONE HYDROCHLORIDE...)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1CCCCN1CCN(CC1)c1ncccn1 |r| Show InChI InChI=1S/C21H29N5O2/c27-19-17-15-4-5-16(14-15)18(17)20(28)26(19)9-2-1-8-24-10-12-25(13-11-24)21-22-6-3-7-23-21/h3,6-7,15-18H,1-2,4-5,8-14H2/t15-,16+,17+,18- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity by measuring displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus |
J Med Chem 36: 3526-32 (1994)
BindingDB Entry DOI: 10.7270/Q2D21Z74 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110577
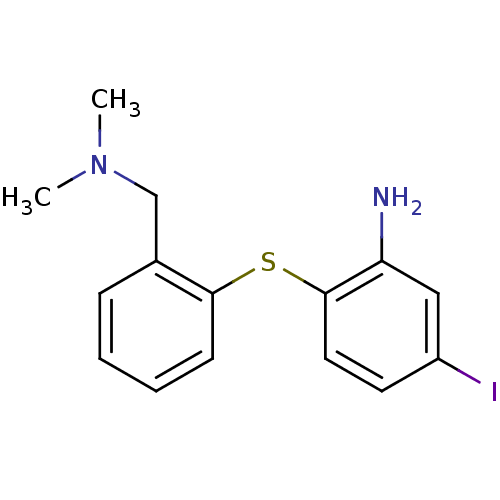
(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110578
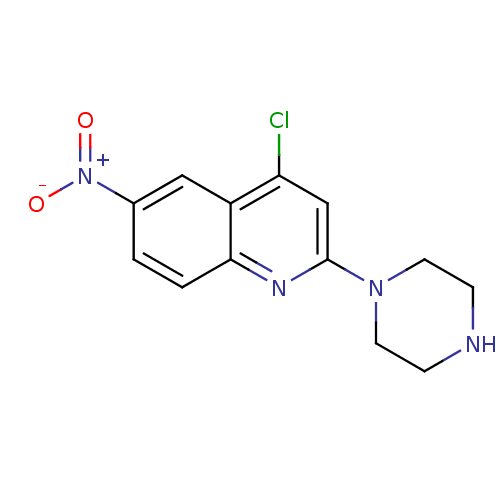
(4-Chloro-6-nitro-2-piperazin-1-yl-quinoline | CHEM...)Show InChI InChI=1S/C13H13ClN4O2/c14-11-8-13(17-5-3-15-4-6-17)16-12-2-1-9(18(19)20)7-10(11)12/h1-2,7-8,15H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176723
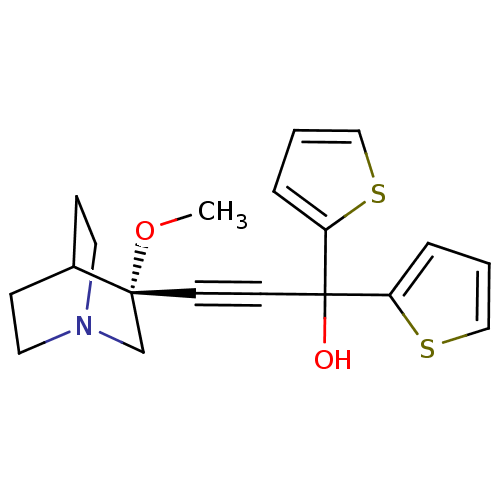
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176735
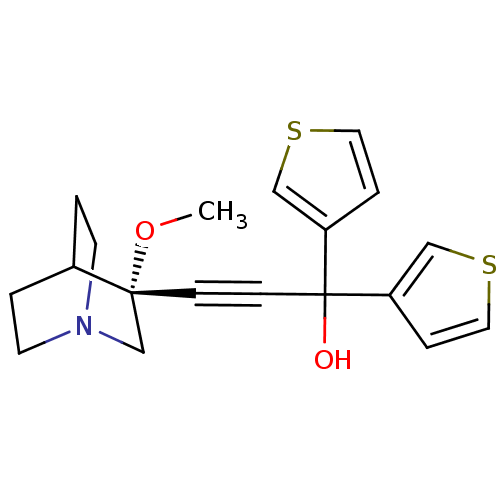
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165935
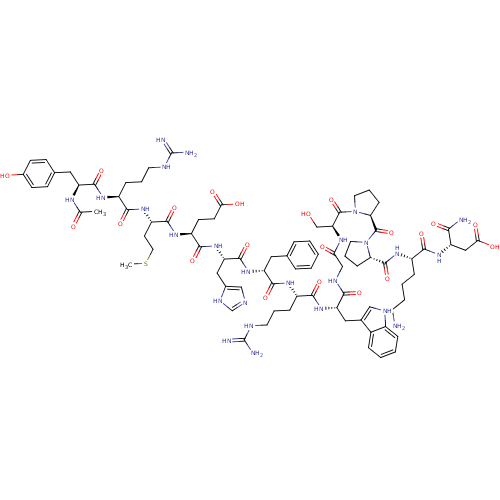
(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165931
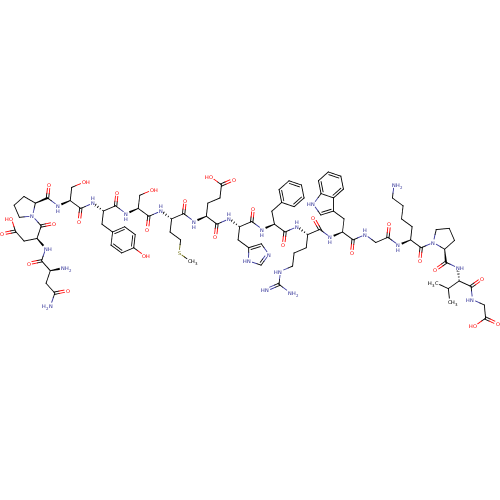
(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50131891
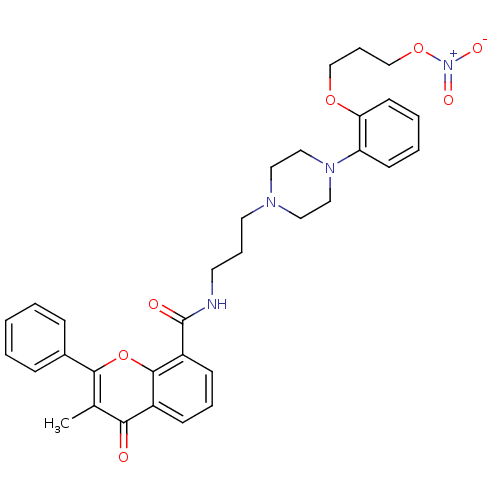
(3-Methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylic a...)Show SMILES Cc1c(oc2c(cccc2c1=O)C(=O)NCCCN1CCN(CC1)c1ccccc1OCCCO[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C33H36N4O7/c1-24-30(38)26-12-7-13-27(32(26)44-31(24)25-10-3-2-4-11-25)33(39)34-16-8-17-35-18-20-36(21-19-35)28-14-5-6-15-29(28)42-22-9-23-43-37(40)41/h2-7,10-15H,8-9,16-23H2,1H3,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-prazosin binding to cloned human Alpha-1A adrenergic receptor in CHO-cells (chinese hamster ovary cells) |
J Med Chem 46: 3762-5 (2003)
Article DOI: 10.1021/jm030825u
BindingDB Entry DOI: 10.7270/Q2BZ65FJ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50228471
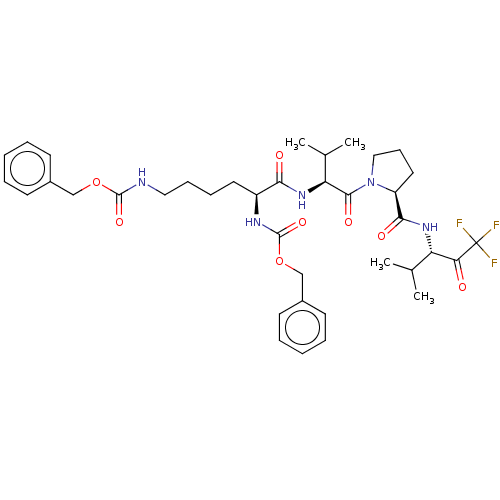
(CHEMBL131548)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCNC(=O)OCc1ccccc1)NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C38H50F3N5O8/c1-24(2)30(32(47)38(39,40)41)44-34(49)29-19-13-21-46(29)35(50)31(25(3)4)45-33(48)28(43-37(52)54-23-27-16-9-6-10-17-27)18-11-12-20-42-36(51)53-22-26-14-7-5-8-15-26/h5-10,14-17,24-25,28-31H,11-13,18-23H2,1-4H3,(H,42,51)(H,43,52)(H,44,49)(H,45,48)/t28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against human leukocyte Elastase |
J Med Chem 33: 394-407 (1990)
BindingDB Entry DOI: 10.7270/Q26D5RZ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176732
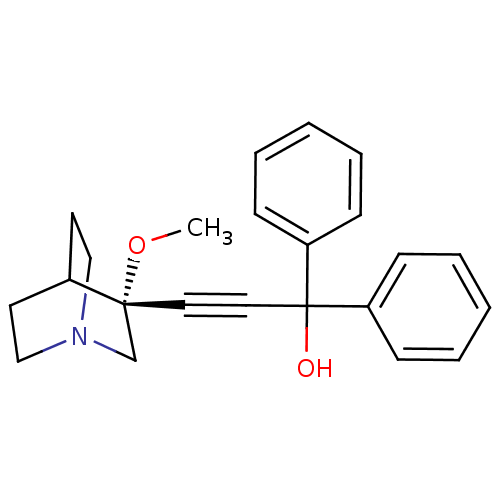
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-2.54,5.9,;-1.05,5.51,;-.64,4.02,;-.44,2.64,;1.09,3.3,;2.46,2.67,;2.18,4.06,;.83,4.66,;.9,6.3,;1.34,5.19,;-2.14,3.62,;-3.63,3.22,;-5.12,2.83,;-5.52,4.31,;-4.73,1.34,;-5.81,.25,;-5.42,-1.23,;-3.93,-1.63,;-2.84,-.54,;-3.24,.94,;-6.61,2.43,;-7.7,3.53,;-9.19,3.13,;-9.59,1.64,;-8.5,.54,;-7.01,.94,)| Show InChI InChI=1S/C23H25NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-11,19,25H,12-13,16-18H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165929
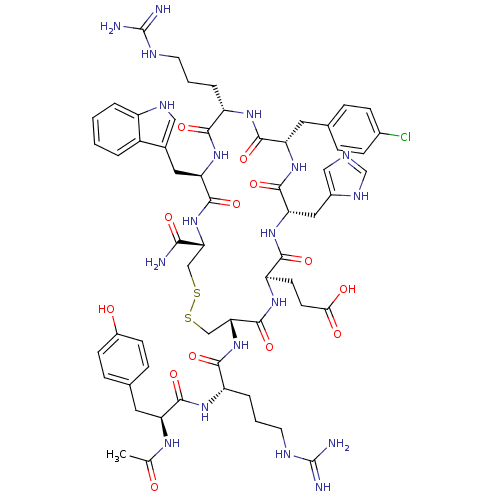
(Ac-YR[CEH(pCl-dF)RWC]-NH2 | CHEMBL415661)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H78ClN19O13S2/c1-31(81)72-43(22-33-12-16-37(82)17-13-33)54(89)73-41(9-5-21-69-60(65)66)52(87)80-48-29-95-94-28-47(50(62)85)79-56(91)45(24-34-26-70-39-7-3-2-6-38(34)39)77-51(86)40(8-4-20-68-59(63)64)74-55(90)44(23-32-10-14-35(61)15-11-32)76-57(92)46(25-36-27-67-30-71-36)78-53(88)42(75-58(48)93)18-19-49(83)84/h2-3,6-7,10-17,26-27,30,40-48,70,82H,4-5,8-9,18-25,28-29H2,1H3,(H2,62,85)(H,67,71)(H,72,81)(H,73,89)(H,74,90)(H,75,93)(H,76,92)(H,77,86)(H,78,88)(H,79,91)(H,80,87)(H,83,84)(H4,63,64,68)(H4,65,66,69)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50131889
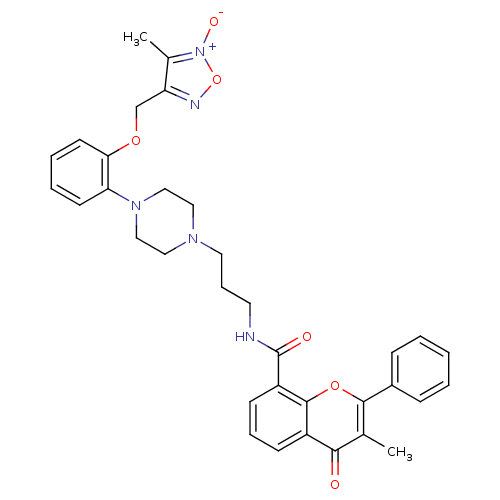
(3-Methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylic a...)Show SMILES Cc1c(COc2ccccc2N2CCN(CCCNC(=O)c3cccc4c3oc(-c3ccccc3)c(C)c4=O)CC2)no[n+]1[O-] Show InChI InChI=1S/C34H35N5O6/c1-23-31(40)26-12-8-13-27(33(26)44-32(23)25-10-4-3-5-11-25)34(41)35-16-9-17-37-18-20-38(21-19-37)29-14-6-7-15-30(29)43-22-28-24(2)39(42)45-36-28/h3-8,10-15H,9,16-22H2,1-2H3,(H,35,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-prazosin binding to cloned human Alpha-1A adrenergic receptor in CHO-cells (chinese hamster ovary cells) |
J Med Chem 46: 3762-5 (2003)
Article DOI: 10.1021/jm030825u
BindingDB Entry DOI: 10.7270/Q2BZ65FJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063266
(6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...)Show InChI InChI=1S/C13H14N4O2/c18-17(19)11-2-3-12-10(9-11)1-4-13(15-12)16-7-5-14-6-8-16/h1-4,9,14H,5-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063266

(6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...)Show InChI InChI=1S/C13H14N4O2/c18-17(19)11-2-3-12-10(9-11)1-4-13(15-12)16-7-5-14-6-8-16/h1-4,9,14H,5-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex |
Bioorg Med Chem Lett 10: 1559-62 (2000)
BindingDB Entry DOI: 10.7270/Q20P10J2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176723

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176718
(1-cyclohexyl-3-((R)-3-methoxyquinuclidin-3-yl)-1-p...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(C1CCCCC1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(11.35,5.74,;12.84,5.36,;13.25,3.86,;13.44,2.49,;14.97,3.14,;16.34,2.52,;16.06,3.9,;14.71,4.51,;14.78,6.14,;15.23,5.04,;11.75,3.47,;10.26,3.07,;8.77,2.68,;8.38,4.16,;9.16,1.19,;8.06,.11,;8.45,-1.37,;9.93,-1.78,;11.03,-.69,;10.64,.8,;7.28,2.28,;6.19,3.37,;4.71,2.98,;4.3,1.49,;5.39,.39,;6.88,.8,)| Show InChI InChI=1S/C23H31NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2,4-5,8-9,19,21,25H,3,6-7,10-13,16-18H2,1H3/t22-,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165932
(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063266

(6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...)Show InChI InChI=1S/C13H14N4O2/c18-17(19)11-2-3-12-10(9-11)1-4-13(15-12)16-7-5-14-6-8-16/h1-4,9,14H,5-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176708
((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#C[C@@](O)(c1ccccc1)c1cccnc1 |wU:12.14,2.1,wD:12.13,2.11,THB:1:2:5.6:9.8,(16.23,-16.03,;17.77,-16,;18.57,-17.32,;19.13,-18.59,;20.42,-17.54,;21.91,-17.77,;21.26,-16.51,;19.8,-16.3,;19.42,-14.71,;20.15,-15.65,;17.23,-18.11,;15.91,-18.89,;14.58,-19.68,;13.8,-18.36,;13.26,-20.47,;11.91,-19.72,;10.59,-20.5,;10.61,-22.05,;11.96,-22.8,;13.28,-22.01,;15.37,-21,;16.9,-20.98,;17.68,-22.31,;16.93,-23.64,;15.4,-23.66,;14.6,-22.35,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3/t21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176712
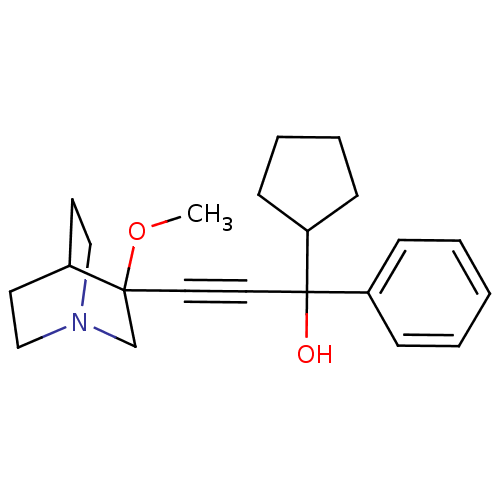
(1-cyclopentyl-3-(3-methoxyquinuclidin-3-yl)-1-phen...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(C1CCCC1)c1ccccc1 |THB:1:2:5.6:9.8,(26.6,5.4,;28.09,5.01,;28.5,3.51,;28.69,2.14,;30.23,2.79,;31.59,2.16,;31.32,3.55,;29.97,4.16,;30.04,5.8,;30.48,4.69,;27,3.12,;25.5,2.72,;24.01,2.33,;23.61,3.81,;24.41,.84,;23.43,-.35,;24.27,-1.64,;25.76,-1.25,;25.84,.29,;22.52,1.93,;21.42,3.02,;19.94,2.63,;19.53,1.13,;20.63,.04,;22.11,.44,)| Show InChI InChI=1S/C22H29NO2/c1-25-21(17-23-15-11-18(21)12-16-23)13-14-22(24,20-9-5-6-10-20)19-7-3-2-4-8-19/h2-4,7-8,18,20,24H,5-6,9-12,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM82423
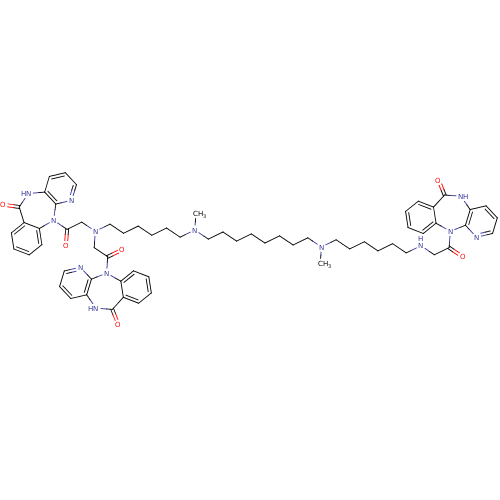
(CAS_132947 | NSC_132947 | TRIPITRAMINE)Show SMILES CN(CCCCCCCCN(C)CCCCCCN(CC(=O)N1c2ccccc2C(=O)Nc2cccnc12)CC(=O)N1c2ccccc2C(=O)Nc2cccnc12)CCCCCCNCC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C64H77N13O6/c1-72(41-20-8-5-17-35-65-44-56(78)75-53-32-14-11-26-47(53)62(81)69-50-29-23-36-66-59(50)75)39-18-6-3-4-7-19-40-73(2)42-21-9-10-22-43-74(45-57(79)76-54-33-15-12-27-48(54)63(82)70-51-30-24-37-67-60(51)76)46-58(80)77-55-34-16-13-28-49(55)64(83)71-52-31-25-38-68-61(52)77/h11-16,23-34,36-38,65H,3-10,17-22,35,39-46H2,1-2H3,(H,69,81)(H,70,82)(H,71,83) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by PDSP Ki Database
| |
Eur J Pharmacol 268: 459-62 (1994)
Article DOI: 10.1016/0922-4106(94)90075-2
BindingDB Entry DOI: 10.7270/Q2348HWT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165927
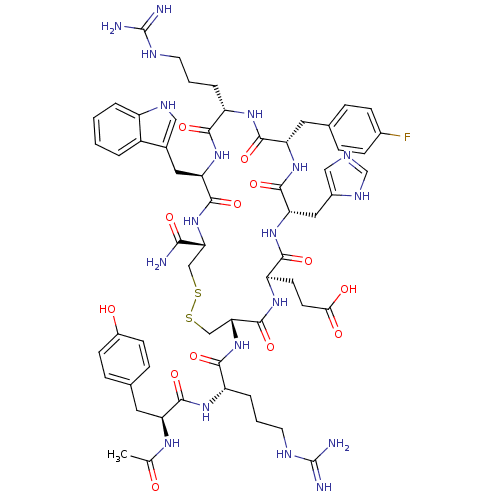
(Ac-YR[CEH(pF-dF)RWC]-NH2 | CHEMBL407809)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H78FN19O13S2/c1-31(81)72-43(22-33-12-16-37(82)17-13-33)54(89)73-41(9-5-21-69-60(65)66)52(87)80-48-29-95-94-28-47(50(62)85)79-56(91)45(24-34-26-70-39-7-3-2-6-38(34)39)77-51(86)40(8-4-20-68-59(63)64)74-55(90)44(23-32-10-14-35(61)15-11-32)76-57(92)46(25-36-27-67-30-71-36)78-53(88)42(75-58(48)93)18-19-49(83)84/h2-3,6-7,10-17,26-27,30,40-48,70,82H,4-5,8-9,18-25,28-29H2,1H3,(H2,62,85)(H,67,71)(H,72,81)(H,73,89)(H,74,90)(H,75,93)(H,76,92)(H,77,86)(H,78,88)(H,79,91)(H,80,87)(H,83,84)(H4,63,64,68)(H4,65,66,69)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281469

(CHEMBL313955 | [3-Benzylcarbamoyl-3,3-difluoro-1-(...)Show SMILES CC(C)(C)OC(=O)NC(Cc1ccc(O)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C23H26F2N2O5/c1-22(2,3)32-21(31)27-18(13-15-9-11-17(28)12-10-15)19(29)23(24,25)20(30)26-14-16-7-5-4-6-8-16/h4-12,18,28H,13-14H2,1-3H3,(H,26,30)(H,27,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against the recombinant HIV-1 protease |
Bioorg Med Chem Lett 3: 253-258 (1993)
Article DOI: 10.1016/S0960-894X(01)80887-8
BindingDB Entry DOI: 10.7270/Q2RX9C0Q |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165924

(Ac-YR[CEH(d-2alpha-Nal)RWC]-NH2 | CHEMBL412523)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C64H81N19O13S2/c1-34(84)75-47(25-35-15-18-41(85)19-16-35)58(92)76-45(13-7-23-72-64(68)69)56(90)83-52-32-98-97-31-51(54(65)88)82-60(94)49(27-39-29-73-43-11-5-4-10-42(39)43)80-55(89)44(12-6-22-71-63(66)67)77-59(93)48(26-36-14-17-37-8-2-3-9-38(37)24-36)79-61(95)50(28-40-30-70-33-74-40)81-57(91)46(78-62(52)96)20-21-53(86)87/h2-5,8-11,14-19,24,29-30,33,44-52,73,85H,6-7,12-13,20-23,25-28,31-32H2,1H3,(H2,65,88)(H,70,74)(H,75,84)(H,76,92)(H,77,93)(H,78,96)(H,79,95)(H,80,89)(H,81,91)(H,82,94)(H,83,90)(H,86,87)(H4,66,67,71)(H4,68,69,72)/t44-,45-,46+,47-,48-,49+,50-,51+,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176706
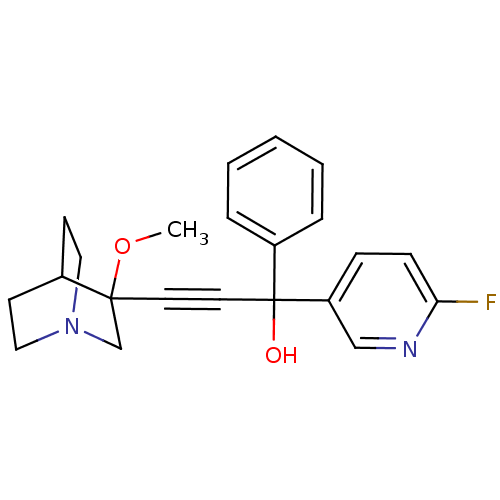
(1-(6-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccc(F)nc1 |THB:1:2:5.6:9.8,(7.82,-23.84,;9.35,-23.8,;10.15,-25.13,;10.72,-26.4,;12.01,-25.35,;13.49,-25.58,;12.85,-24.32,;11.39,-24.11,;11.01,-22.52,;11.74,-23.46,;8.82,-25.91,;7.5,-26.7,;6.17,-27.49,;5.39,-26.17,;4.85,-28.28,;3.5,-27.53,;2.18,-28.31,;2.2,-29.85,;3.55,-30.61,;4.87,-29.82,;6.96,-28.81,;8.48,-28.79,;9.27,-30.12,;8.52,-31.45,;9.31,-32.77,;6.98,-31.47,;6.19,-30.16,)| Show InChI InChI=1S/C22H23FN2O2/c1-27-21(16-25-13-9-17(21)10-14-25)11-12-22(26,18-5-3-2-4-6-18)19-7-8-20(23)24-15-19/h2-8,15,17,26H,9-10,13-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110584

(3-(3-Fluoro-propyl)-6-nitro-2-piperazin-1-yl-quino...)Show InChI InChI=1S/C16H19FN4O2/c17-5-1-2-12-10-13-11-14(21(22)23)3-4-15(13)19-16(12)20-8-6-18-7-9-20/h3-4,10-11,18H,1-2,5-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50057465
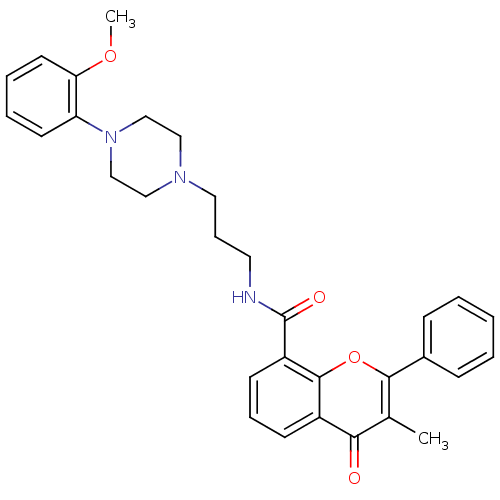
(3-Methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylic a...)Show SMILES COc1ccccc1N1CCN(CCCNC(=O)c2cccc3c2oc(c(C)c3=O)-c2ccccc2)CC1 Show InChI InChI=1S/C31H33N3O4/c1-22-28(35)24-12-8-13-25(30(24)38-29(22)23-10-4-3-5-11-23)31(36)32-16-9-17-33-18-20-34(21-19-33)26-14-6-7-15-27(26)37-2/h3-8,10-15H,9,16-21H2,1-2H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Torino
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-prazosin binding to cloned human Alpha-1A adrenergic receptor in CHO-cells (chinese hamster ovary cells) |
J Med Chem 46: 3762-5 (2003)
Article DOI: 10.1021/jm030825u
BindingDB Entry DOI: 10.7270/Q2BZ65FJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110574
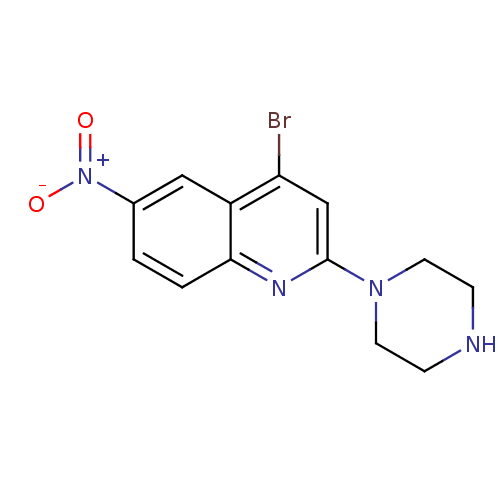
(4-Bromo-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...)Show InChI InChI=1S/C13H13BrN4O2/c14-11-8-13(17-5-3-15-4-6-17)16-12-2-1-9(18(19)20)7-10(11)12/h1-2,7-8,15H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165936
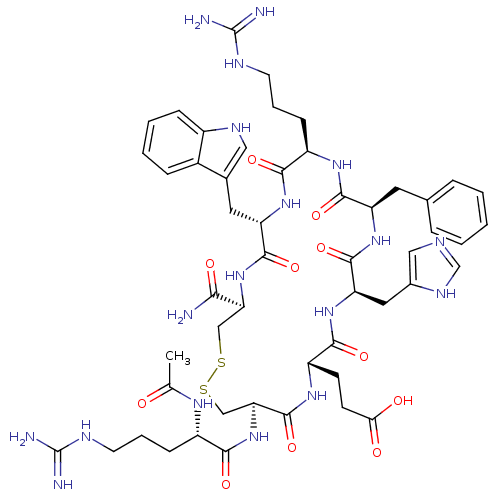
(Ac-dR[CEHdFRWC]-NH2 | CHEMBL267900)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34+,35-,36+,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176735

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176729
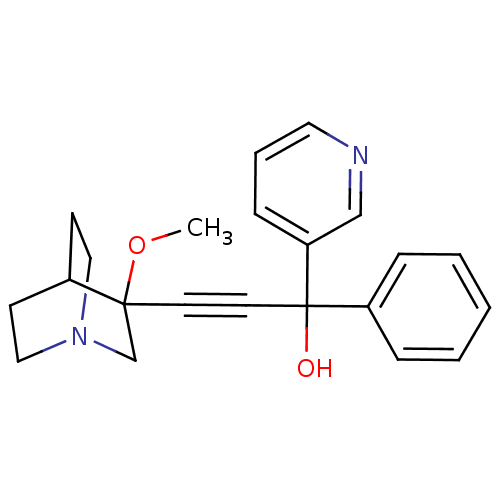
(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyridin-...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1 |THB:1:2:5.6:9.8,(-5.03,-13.2,;-3.49,-13.16,;-2.69,-14.49,;-2.13,-15.76,;-.83,-14.71,;.65,-14.94,;0,-13.68,;-1.46,-13.46,;-1.84,-11.87,;-1.11,-12.82,;-4.02,-15.27,;-5.35,-16.06,;-6.67,-16.85,;-7.46,-15.53,;-8,-17.64,;-9.35,-16.88,;-10.67,-17.67,;-10.65,-19.21,;-9.3,-19.97,;-7.98,-19.18,;-5.89,-18.17,;-4.36,-18.15,;-3.58,-19.47,;-4.32,-20.81,;-5.86,-20.83,;-6.65,-19.52,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283355
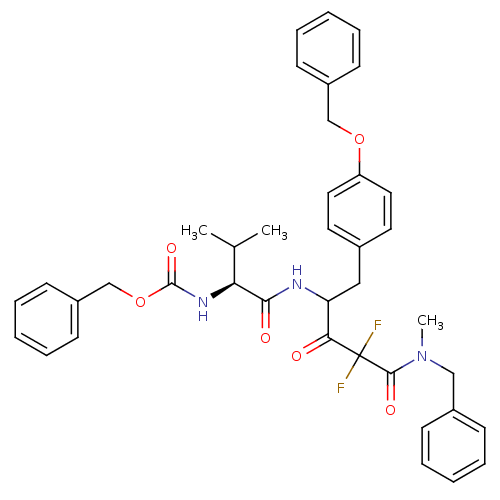
(CHEMBL311418 | {(S)-1-[3-(Benzyl-methyl-carbamoyl)...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C39H41F2N3O6/c1-27(2)34(43-38(48)50-26-31-17-11-6-12-18-31)36(46)42-33(23-28-19-21-32(22-20-28)49-25-30-15-9-5-10-16-30)35(45)39(40,41)37(47)44(3)24-29-13-7-4-8-14-29/h4-22,27,33-34H,23-26H2,1-3H3,(H,42,46)(H,43,48)/t33?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50208770

(2-[2-(ethoxymethyl)piperazin-1-yl]-6-nitroquinolin...)Show SMILES CCOCC1CNCCN1c1ccc2cc(ccc2n1)[N+]([O-])=O |w:4.3| Show InChI InChI=1S/C16H20N4O3/c1-2-23-11-14-10-17-7-8-19(14)16-6-3-12-9-13(20(21)22)4-5-15(12)18-16/h3-6,9,14,17H,2,7-8,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50208769
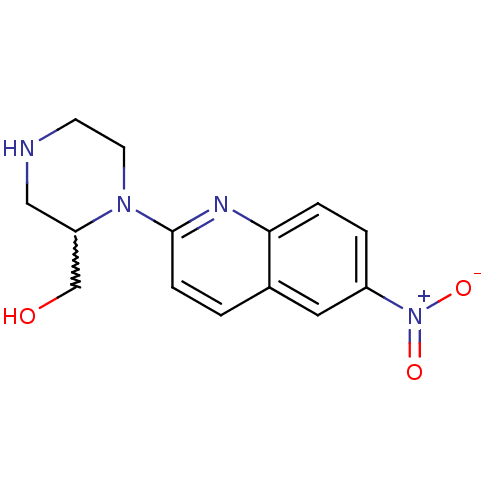
(2-[2-(hydroxymethyl)piperazin-1-yl]-6-nitroquinoli...)Show SMILES OCC1CNCCN1c1ccc2cc(ccc2n1)[N+]([O-])=O |w:2.1| Show InChI InChI=1S/C14H16N4O3/c19-9-12-8-15-5-6-17(12)14-4-1-10-7-11(18(20)21)2-3-13(10)16-14/h1-4,7,12,15,19H,5-6,8-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from Sprague-Dawley rat SERT |
Bioorg Med Chem 15: 3499-504 (2007)
Article DOI: 10.1016/j.bmc.2007.03.001
BindingDB Entry DOI: 10.7270/Q2SN08N4 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165941

(Ac-R[CEHdFRWC]-NH2 | CHEMBL408257)Show SMILES CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34-,35+,36-,37+,38-,39+,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244370
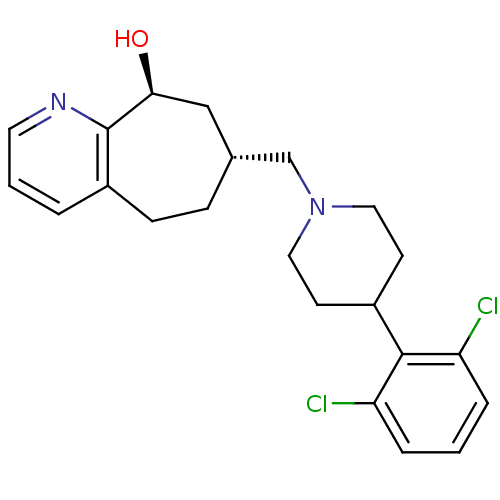
((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2c(Cl)cccc2Cl)CCc2cccnc12 |r| Show InChI InChI=1S/C22H26Cl2N2O/c23-18-4-1-5-19(24)21(18)16-8-11-26(12-9-16)14-15-6-7-17-3-2-10-25-22(17)20(27)13-15/h1-5,10,15-16,20,27H,6-9,11-14H2/t15-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165933
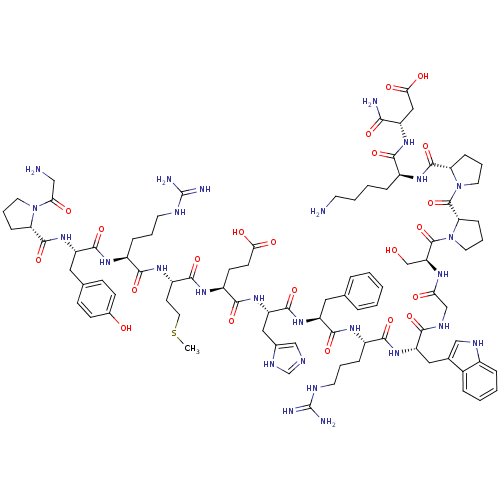
(CHEMBL428326 | GPYRMEHFRWGSPPKD-NH2)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C89H127N27O22S/c1-139-37-30-60(107-76(127)57(19-9-32-98-88(93)94)104-82(133)63(39-50-24-26-53(118)27-25-50)113-84(135)67-21-11-34-114(67)71(120)43-91)80(131)106-59(28-29-72(121)122)79(130)112-65(41-52-45-97-48-102-52)83(134)110-62(38-49-14-3-2-4-15-49)81(132)105-58(20-10-33-99-89(95)96)78(129)111-64(40-51-44-100-55-17-6-5-16-54(51)55)75(126)101-46-70(119)103-66(47-117)86(137)116-36-13-23-69(116)87(138)115-35-12-22-68(115)85(136)108-56(18-7-8-31-90)77(128)109-61(74(92)125)42-73(123)124/h2-6,14-17,24-27,44-45,48,56-69,100,117-118H,7-13,18-23,28-43,46-47,90-91H2,1H3,(H2,92,125)(H,97,102)(H,101,126)(H,103,119)(H,104,133)(H,105,132)(H,106,131)(H,107,127)(H,108,136)(H,109,128)(H,110,134)(H,111,129)(H,112,130)(H,113,135)(H,121,122)(H,123,124)(H4,93,94,98)(H4,95,96,99)/t56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165926

(Ac-YR[CEHdFRWC]SPPKD-NH2 | CHEMBL2373515)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O |wU:103.110,32.96,27.27,4.3,72.76,114.121,61.64,50.53,wD:110.118,94.99,16.16,123.130,36.37,82.87,(-1.2,-14.32,;-.65,-12.88,;.87,-12.64,;-1.63,-11.7,;-1.07,-10.25,;-2.62,-10.24,;-3.96,-9.47,;-5.2,-10.39,;-6.61,-9.78,;-6.79,-8.24,;-8.2,-7.63,;-5.55,-7.32,;-4.14,-7.93,;.45,-10.01,;1.41,-11.19,;.99,-8.56,;.02,-7.37,;1.51,-6.97,;3.03,-6.87,;3.9,-8.14,;5.44,-8.04,;6.29,-9.31,;7.83,-9.22,;5.61,-10.7,;-1.49,-7.62,;-2.04,-9.06,;-2.48,-6.43,;-1.91,-4.99,;-.42,-5.33,;1.11,-5.27,;2.58,-4.78,;3.85,-3.92,;4.85,-2.75,;5.49,-1.36,;5.74,.16,;7.28,.13,;5.56,1.69,;7.06,2.08,;8.14,3.18,;9.06,4.42,;10.51,3.93,;10.5,2.38,;11.63,1.35,;11.28,-.15,;9.82,-.62,;8.7,.44,;9.03,1.94,;4.98,3.11,;4.04,4.33,;5.12,5.43,;2.81,5.24,;3.54,6.6,;5.09,6.63,;5.82,7.98,;7.36,8,;8.1,9.36,;9.64,9.4,;7.3,10.67,;1.37,5.79,;-.16,5.95,;-.23,7.47,;-1.68,5.67,;-2.16,7.12,;-1.14,8.26,;.38,7.93,;1.39,9.08,;.91,10.56,;-.6,10.86,;-1.62,9.71,;-3.06,4.97,;-4.21,3.95,;-5.38,4.96,;-5.04,2.66,;-6.44,3.31,;-6.58,4.84,;-5.42,5.85,;-6.02,7.26,;-7.56,7.12,;-7.89,5.62,;-5.48,1.19,;-5.52,-.35,;-7.05,-.52,;-5.13,-1.84,;-6.56,-2.42,;-7.8,-1.48,;-9.21,-2.06,;-10.42,-1.11,;-9.42,-3.59,;-4.38,-3.17,;-3.27,-4.25,;-4.19,-5.49,;6.15,-3.59,;6.08,-5.12,;7.51,-2.88,;8.81,-3.7,;8.73,-5.24,;10.04,-6.07,;10.17,-3,;10.25,-1.46,;11.47,-3.71,;10.78,-2.02,;11.57,-.8,;12.9,-1.58,;12.83,-3.12,;14.14,-3.95,;14.07,-5.49,;15.34,-3.36,;14.94,-4.86,;15.22,-6.01,;16.72,-5.61,;16.79,-4.06,;18.17,-3.36,;18.22,-1.83,;19.46,-4.2,;20.82,-3.48,;20.9,-1.95,;22.25,-1.24,;22.33,.3,;23.7,1,;23.77,2.55,;22.11,-4.31,;22.04,-5.85,;23.49,-3.61,;24.79,-4.43,;24.71,-5.98,;26.01,-6.8,;27.51,-7.2,;25.94,-8.33,;26.15,-3.73,;27.45,-4.56,;26.22,-2.19,)| Show InChI InChI=1S/C83H115N25O21S2/c1-44(110)95-57(34-46-22-24-49(111)25-23-46)73(121)96-54(19-10-30-92-83(88)89)71(119)105-62-41-130-131-42-63(78(126)104-61(40-109)80(128)108-32-12-21-65(108)81(129)107-31-11-20-64(107)79(127)99-52(17-7-8-28-84)69(117)100-56(68(85)116)37-67(114)115)106-75(123)59(35-47-38-93-51-16-6-5-15-50(47)51)102-70(118)53(18-9-29-91-82(86)87)97-74(122)58(33-45-13-3-2-4-14-45)101-76(124)60(36-48-39-90-43-94-48)103-72(120)55(98-77(62)125)26-27-66(112)113/h2-6,13-16,22-25,38-39,43,52-65,93,109,111H,7-12,17-21,26-37,40-42,84H2,1H3,(H2,85,116)(H,90,94)(H,95,110)(H,96,121)(H,97,122)(H,98,125)(H,99,127)(H,100,117)(H,101,124)(H,102,118)(H,103,120)(H,104,126)(H,105,119)(H,106,123)(H,112,113)(H,114,115)(H4,86,87,91)(H4,88,89,92)/t52-,53-,54?,55+,56-,57-,58-,59+,60-,61-,62+,63+,64-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165930
(Ac-YR[CEHdFRWC]-NH2 | CHEMBL264352)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C60H79N19O13S2/c1-32(80)71-43(24-34-15-17-37(81)18-16-34)54(88)72-41(14-8-22-68-60(64)65)52(86)79-48-30-94-93-29-47(50(61)84)78-56(90)45(25-35-27-69-39-12-6-5-11-38(35)39)76-51(85)40(13-7-21-67-59(62)63)73-55(89)44(23-33-9-3-2-4-10-33)75-57(91)46(26-36-28-66-31-70-36)77-53(87)42(74-58(48)92)19-20-49(82)83/h2-6,9-12,15-18,27-28,31,40-48,69,81H,7-8,13-14,19-26,29-30H2,1H3,(H2,61,84)(H,66,70)(H,71,80)(H,72,88)(H,73,89)(H,74,92)(H,75,91)(H,76,85)(H,77,87)(H,78,90)(H,79,86)(H,82,83)(H4,62,63,67)(H4,64,65,68)/t40-,41-,42+,43-,44-,45+,46-,47+,48+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex |
Bioorg Med Chem Lett 10: 1559-62 (2000)
BindingDB Entry DOI: 10.7270/Q20P10J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50165932

(Ac-YRMEHdFRWG-NH2 | CHEMBL266879)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(N)=O Show InChI InChI=1S/C61H83N19O13S/c1-34(81)73-46(27-36-16-18-39(82)19-17-36)57(91)74-42(14-8-23-68-60(63)64)53(87)77-45(22-25-94-2)56(90)76-44(20-21-51(84)85)55(89)80-49(29-38-31-67-33-72-38)59(93)78-47(26-35-10-4-3-5-11-35)58(92)75-43(15-9-24-69-61(65)66)54(88)79-48(52(86)71-32-50(62)83)28-37-30-70-41-13-7-6-12-40(37)41/h3-7,10-13,16-19,30-31,33,42-49,70,82H,8-9,14-15,20-29,32H2,1-2H3,(H2,62,83)(H,67,72)(H,71,86)(H,73,81)(H,74,91)(H,75,92)(H,76,90)(H,77,87)(H,78,93)(H,79,88)(H,80,89)(H,84,85)(H4,63,64,68)(H4,65,66,69)/t42-,43-,44-,45-,46-,47+,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data