

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50040929 (4,5-dianilinophthalimide | 5,6-bis(phenylamino)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description Competitive inhibition of ATP binding to EGF-R | J Med Chem 37: 1015-27 (1994) BindingDB Entry DOI: 10.7270/Q2M32TTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3014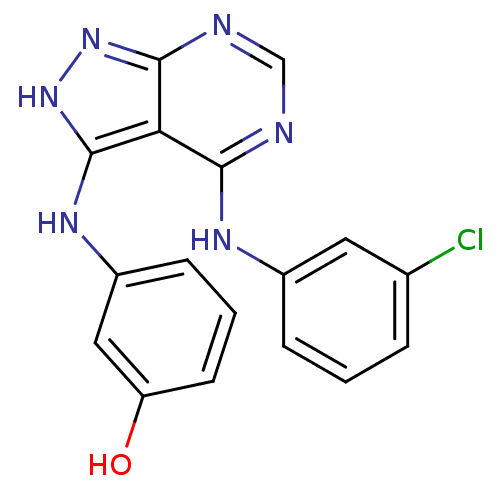 (3-((3-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3029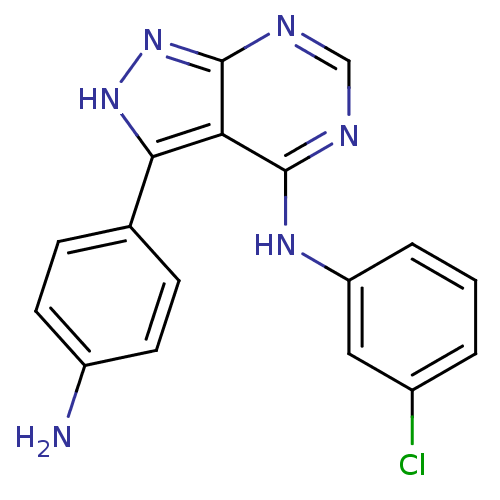 (3-(4-Aminophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549341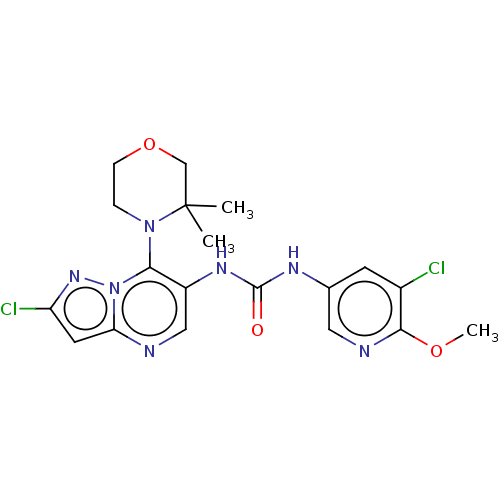 (CHEMBL4757361) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549337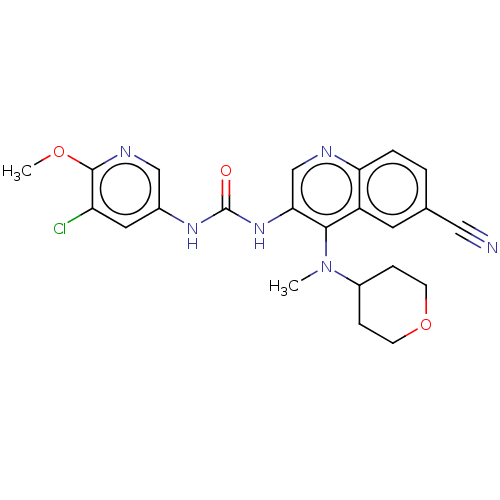 (CHEMBL4748505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3023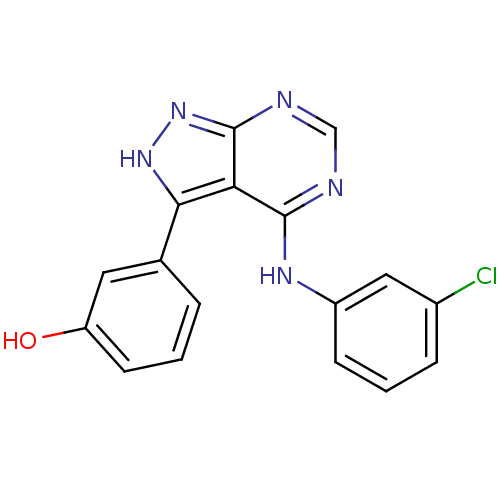 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against epidermal growth factor receptor | J Med Chem 39: 2285-92 (1996) Article DOI: 10.1021/jm960118j BindingDB Entry DOI: 10.7270/Q2J1028B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3016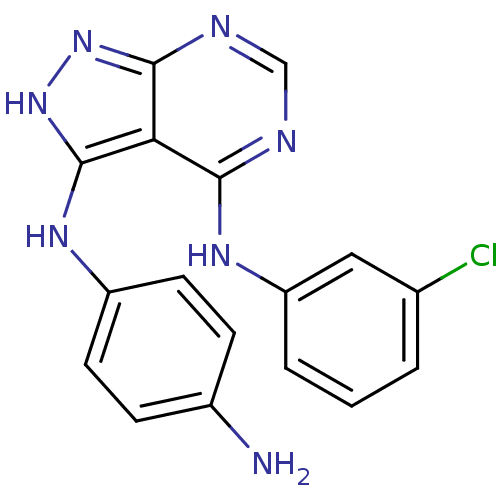 (3-((4-Aminophenyl)amino)-4-((3-chlorophenyl)amino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549339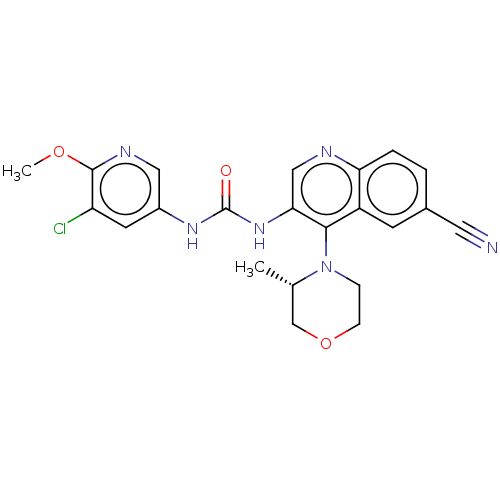 (CHEMBL4796575) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3027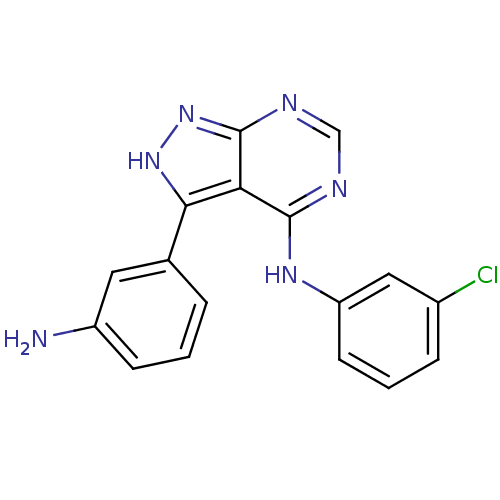 (3-(3-Aminophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3025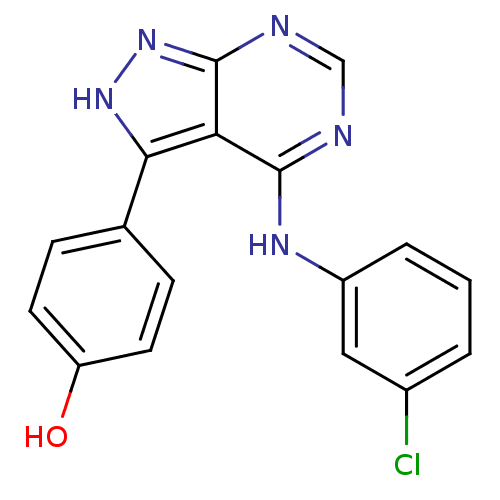 (3-(4-Hydroxyphenyl)-4-((3-chlorophenyl)amino)pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549338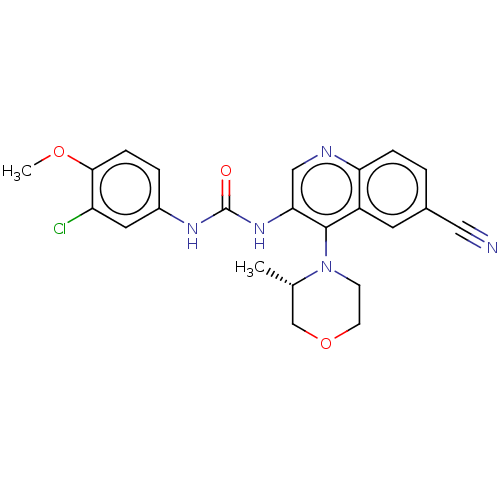 (CHEMBL4765158) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549342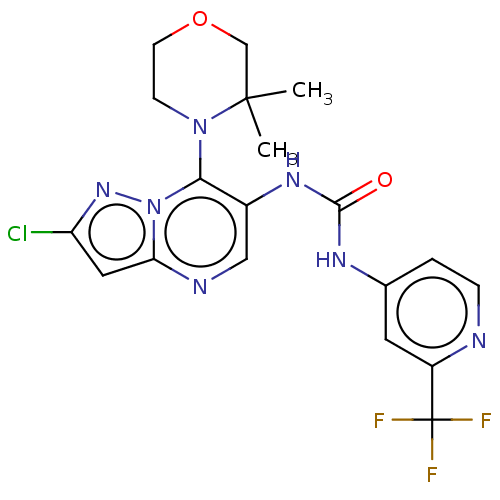 (CHEMBL4795909) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50051308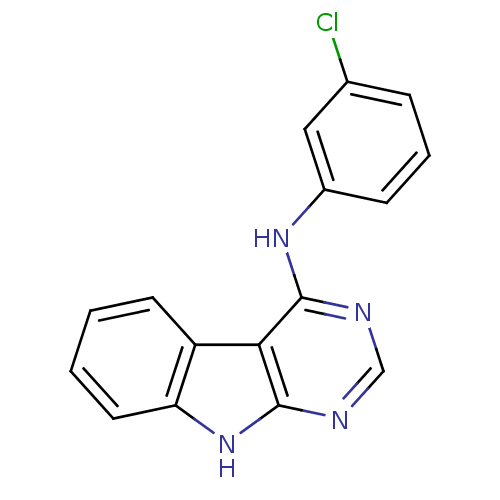 ((3-Chloro-phenyl)-(9H-pyrimido[4,5-b]indol-4-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against epidermal growth factor receptor | J Med Chem 39: 2285-92 (1996) Article DOI: 10.1021/jm960118j BindingDB Entry DOI: 10.7270/Q2J1028B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549343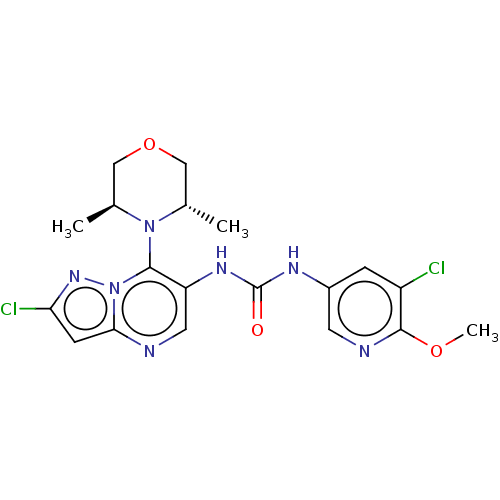 (CHEMBL4754690) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3018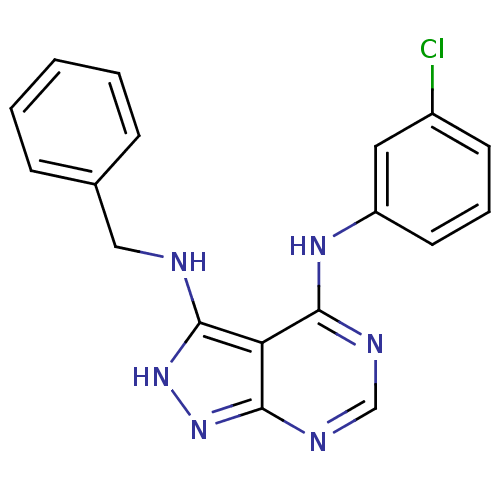 (3-(Benzylamino)-4-((3-chlorophenyl)amino)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3021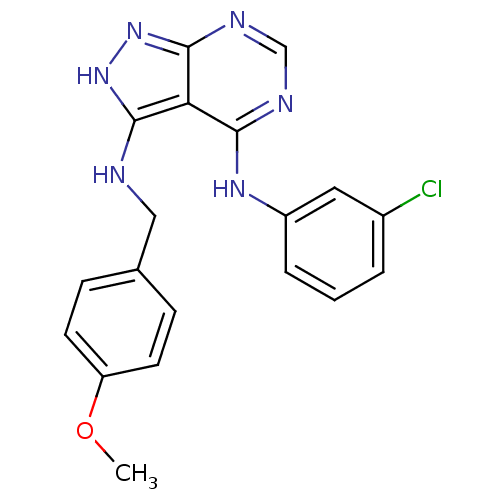 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3012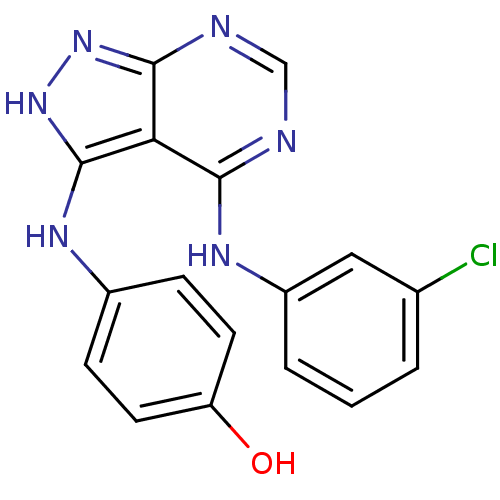 (3-((4-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549336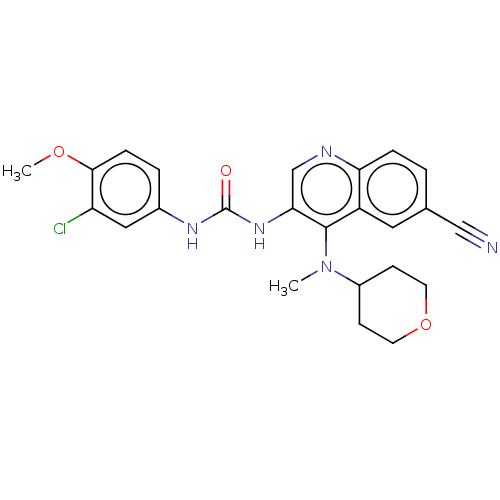 (CHEMBL4785146) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3013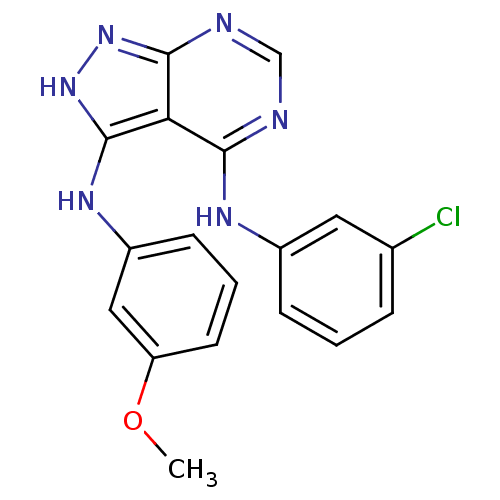 (3-((3-Methoxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4810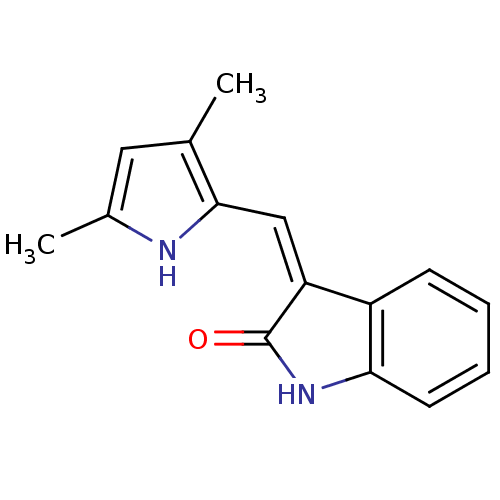 ((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3020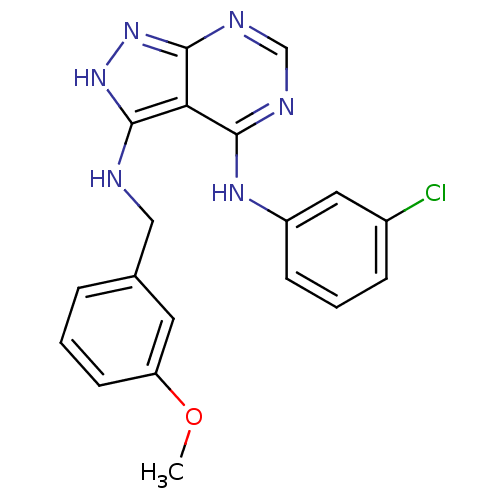 (3-((3-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549327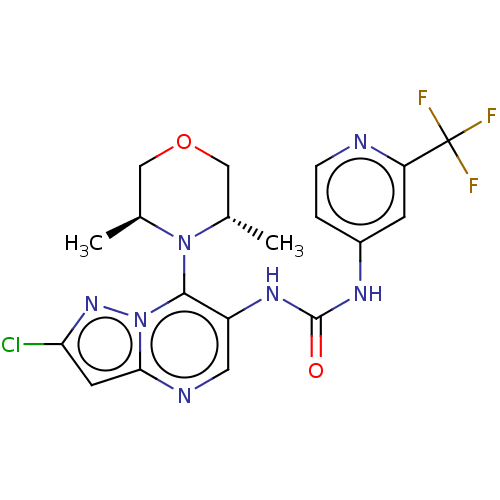 (CHEMBL4796225) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50287090 (4-Chloro-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the platelet-derived growth factor receptor. | Bioorg Med Chem Lett 7: 187-192 (1997) Article DOI: 10.1016/S0960-894X(96)00601-4 BindingDB Entry DOI: 10.7270/Q23X86MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50051302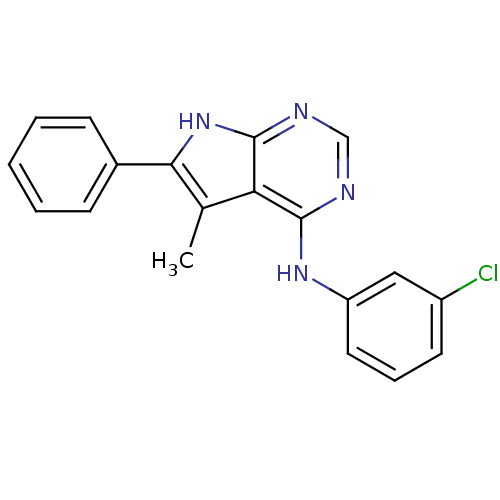 ((3-Chloro-phenyl)-(5-methyl-6-phenyl-7H-pyrrolo[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against epidermal growth factor receptor | J Med Chem 39: 2285-92 (1996) Article DOI: 10.1021/jm960118j BindingDB Entry DOI: 10.7270/Q2J1028B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50287090 (4-Chloro-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of tyrosine kinase(PDGF-R) | Bioorg Med Chem Lett 6: 1221-1226 (1996) Article DOI: 10.1016/0960-894X(96)00197-7 BindingDB Entry DOI: 10.7270/Q2348KBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50175491 (4-Methyl-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of tyrosine kinase(PDGF-R) | Bioorg Med Chem Lett 6: 1221-1226 (1996) Article DOI: 10.1016/0960-894X(96)00197-7 BindingDB Entry DOI: 10.7270/Q2348KBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50088905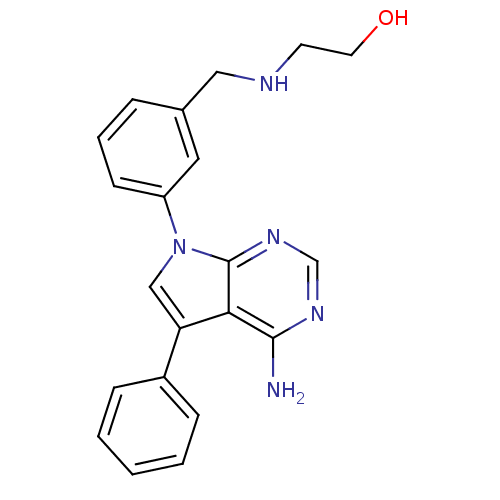 (2-(3-(4-amino-5-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Tested for inhibition of protein tyrosine kinase c-Src phosphorylation | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50175491 (4-Methyl-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the platelet-derived growth factor receptor. | Bioorg Med Chem Lett 7: 187-192 (1997) Article DOI: 10.1016/S0960-894X(96)00601-4 BindingDB Entry DOI: 10.7270/Q23X86MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549340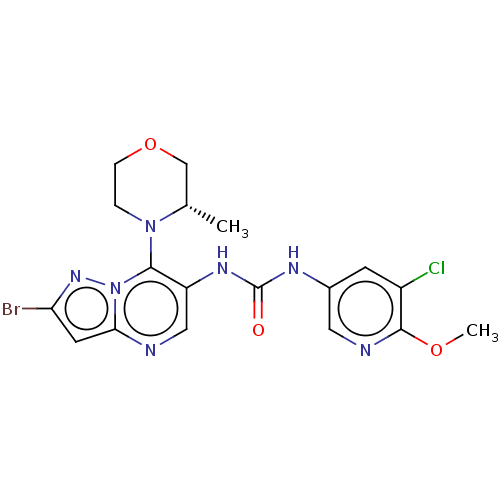 (CHEMBL4795854) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3022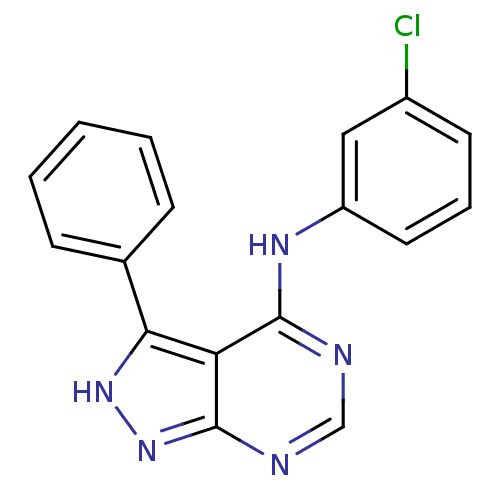 (4-(Phenylamino)pyrazolo[3,4-d]pyrimidine deriv. 23...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50088900 (5,7-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of PDGF-receptor kinase | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50088898 (2-(4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Tested for inhibition of protein tyrosine kinase c-Src phosphorylation | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50088909 ((4-(4-amino-5-phenyl-7H-pyrrolo[2,3-d]pyrimidin-7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Tested for inhibition of protein tyrosine kinase c-Src phosphorylation | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50051312 ((3-Bromo-phenyl)-(5,6-dimethyl-7H-pyrrolo[2,3-d]py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against epidermal growth factor receptor | J Med Chem 39: 2285-92 (1996) Article DOI: 10.1021/jm960118j BindingDB Entry DOI: 10.7270/Q2J1028B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3023 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3019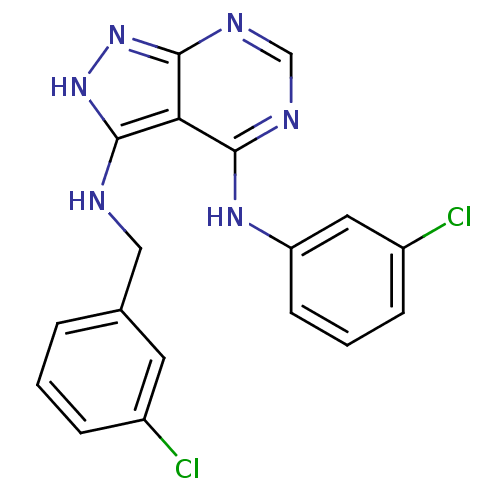 (3-((3-Chlorobenzyl)amino)-4-((3-chlorophenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549332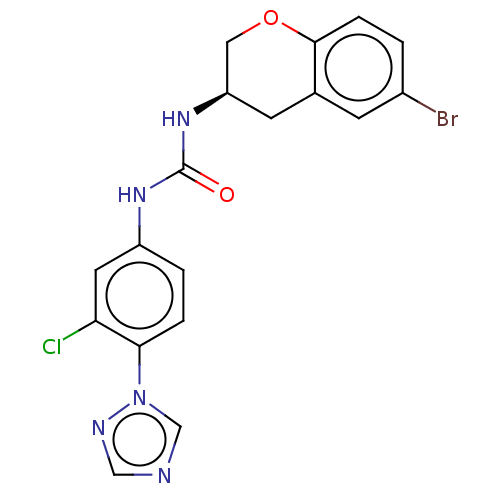 (CHEMBL4786633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50051317 ((3-Chloro-phenyl)-(5,6-dimethyl-7H-pyrrolo[2,3-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against epidermal growth factor receptor | J Med Chem 39: 2285-92 (1996) Article DOI: 10.1021/jm960118j BindingDB Entry DOI: 10.7270/Q2J1028B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50051297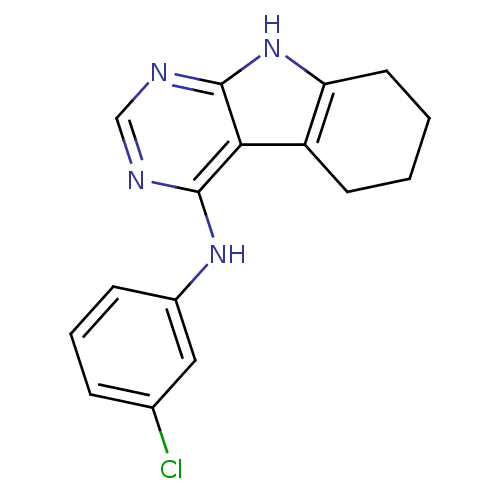 ((3-Chloro-phenyl)-(6,7,8,9-tetrahydro-5H-pyrimido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against epidermal growth factor receptor | J Med Chem 39: 2285-92 (1996) Article DOI: 10.1021/jm960118j BindingDB Entry DOI: 10.7270/Q2J1028B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549335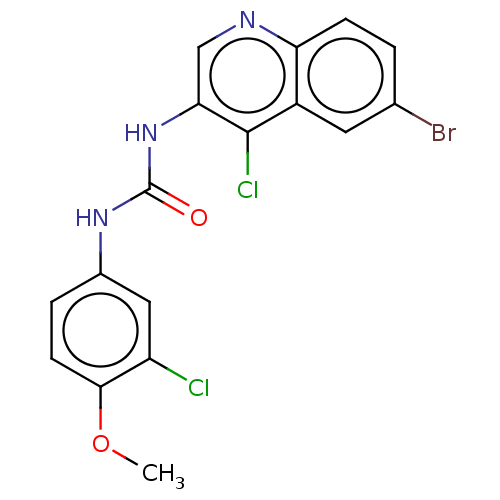 (CHEMBL4798110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3017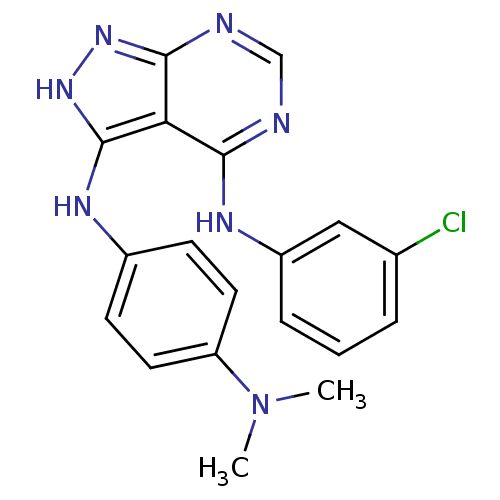 (3-((4-(Dimethylamino)phenyl)amino)-4-((3-chlorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50088899 (2-(4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Tested for inhibition of protein tyrosine kinase c-Src phosphorylation | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50088912 (2-(4-(4-amino-5-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Tested for inhibition of protein tyrosine kinase c-Src phosphorylation | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3011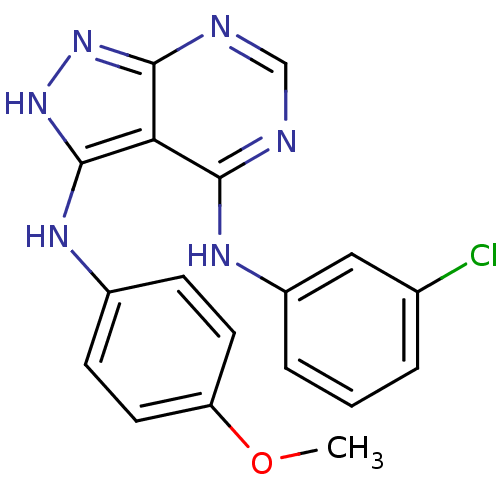 (3-((4-Methoxyphenyl)-amino)-4-((3-chlorophenyl)ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4876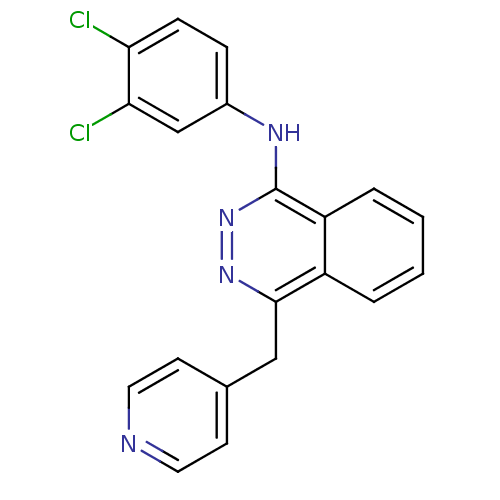 (1-(3,4-Dichloroanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50088903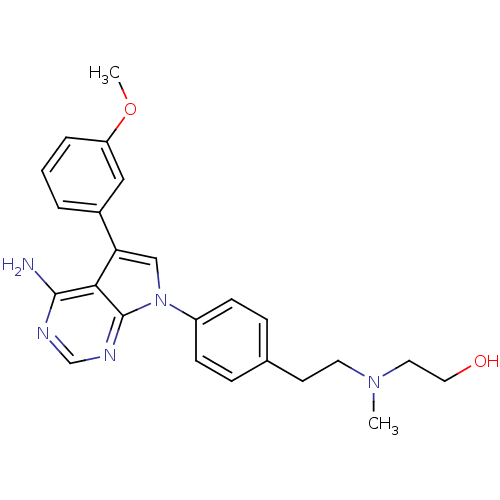 (2-((4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Tested for inhibition of protein tyrosine kinase c-Src phosphorylation | Bioorg Med Chem Lett 10: 945-9 (2000) BindingDB Entry DOI: 10.7270/Q2R78DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50549333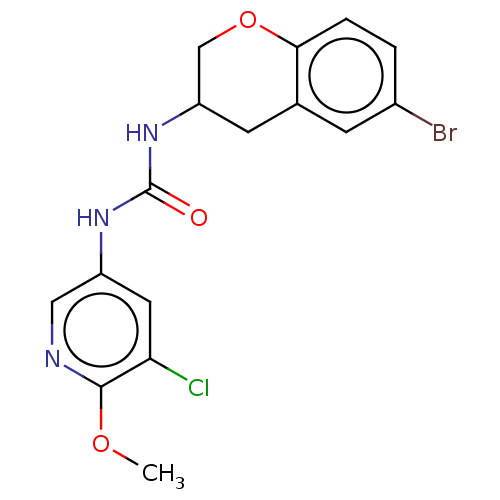 (CHEMBL4747104) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human wild-type full-length MALT1 (329 to 824 residues) protease activity using Ac-Leu-Arg-Ser-Arg Rh110-dPro as substrate p... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01245 BindingDB Entry DOI: 10.7270/Q2S1864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 944 total ) | Next | Last >> |