 Found 33910 hits with Last Name = 'wan' and Initial = 'h'
Found 33910 hits with Last Name = 'wan' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
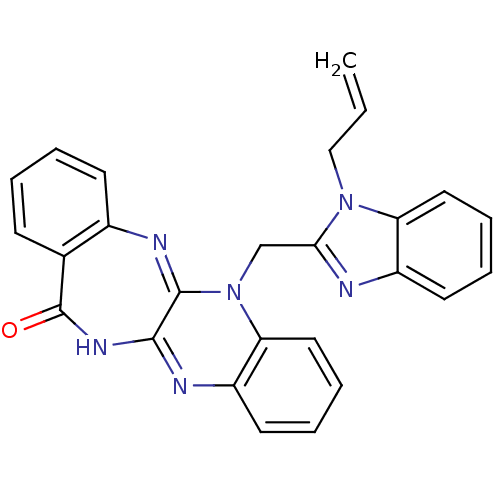
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
GTP-binding nuclear protein GSP1/CNR1
(Saccharomyces cerevisiae) | BDBM50176988
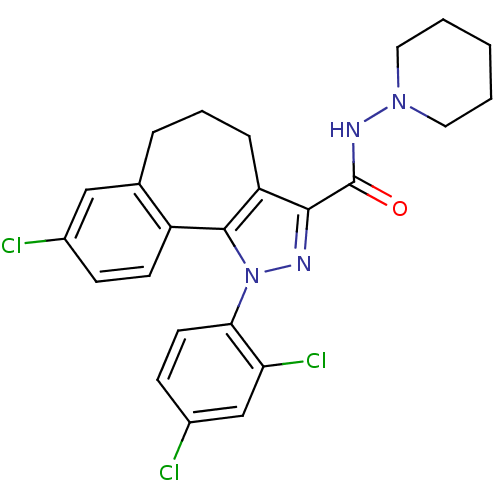
(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNS Biological Research
Curated by PDSP Ki Database
| |
J Med Chem 51: 2439-46 (2008)
Article DOI: 10.1021/jm701519h
BindingDB Entry DOI: 10.7270/Q22R3Q7S |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
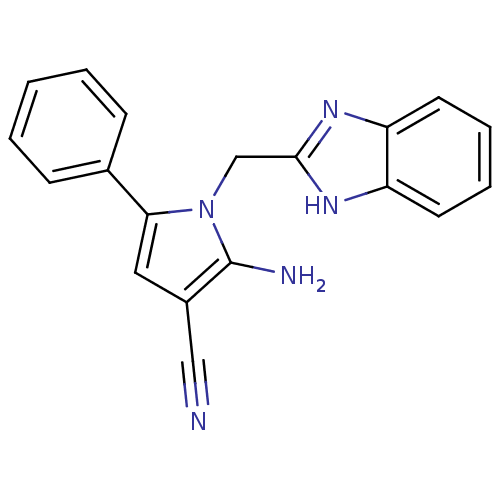
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
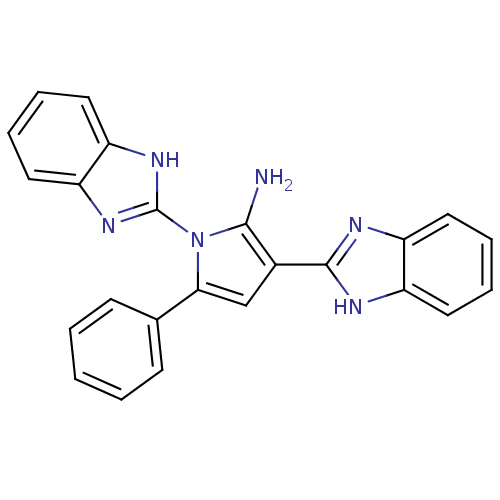
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
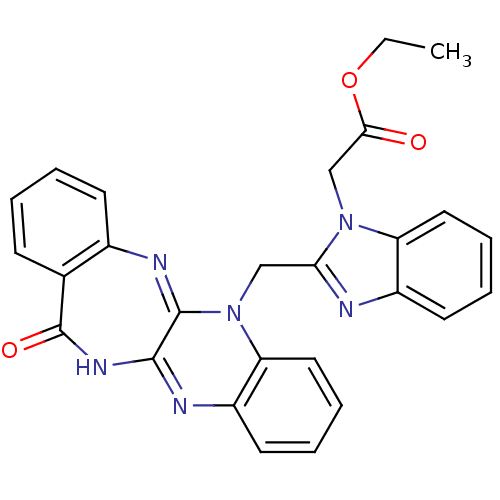
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
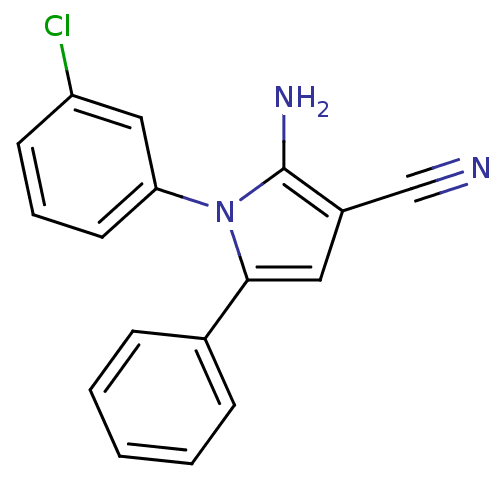
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
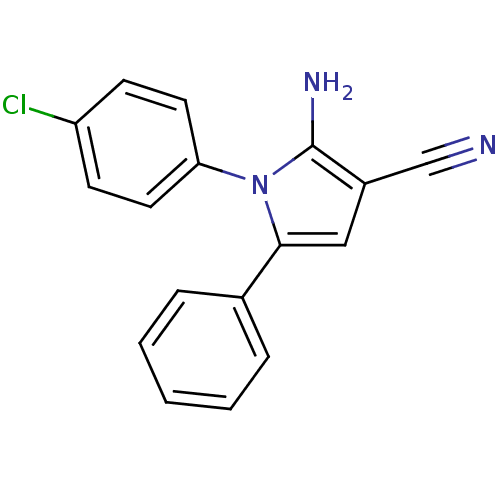
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
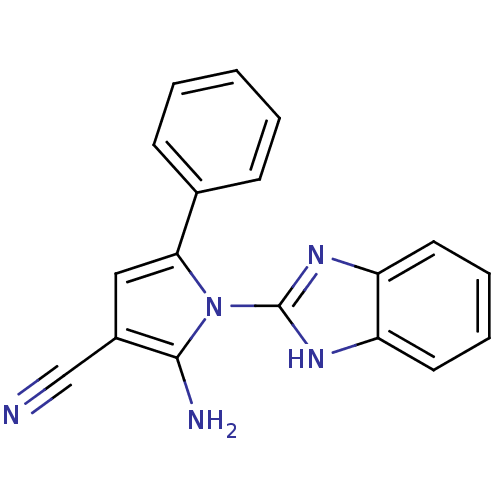
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
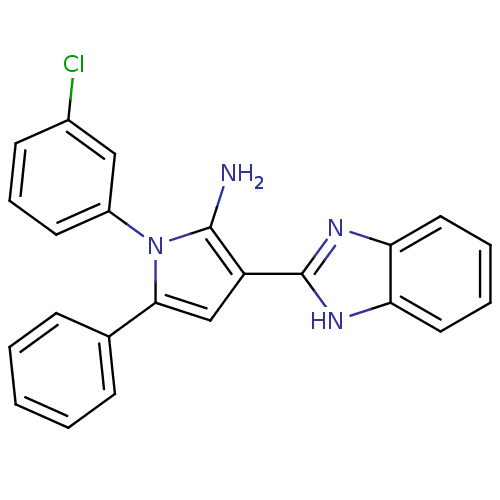
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
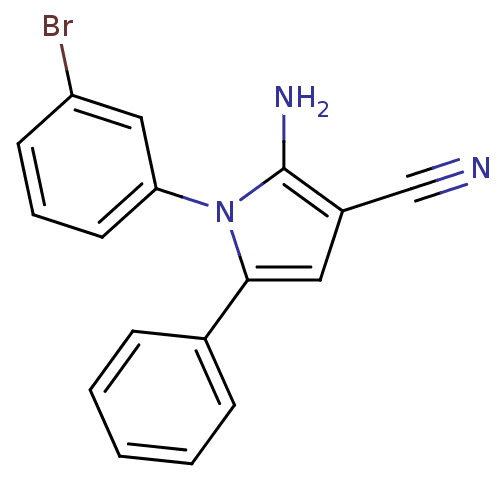
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
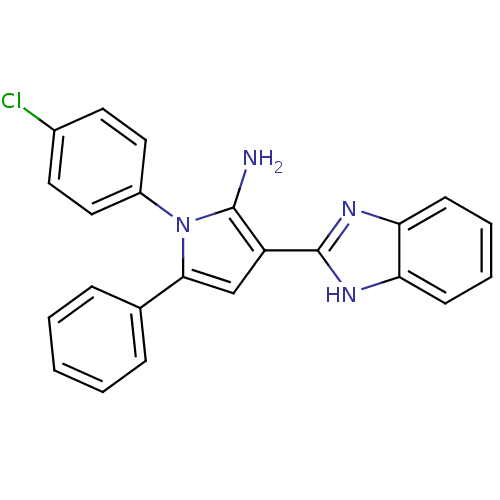
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50105033
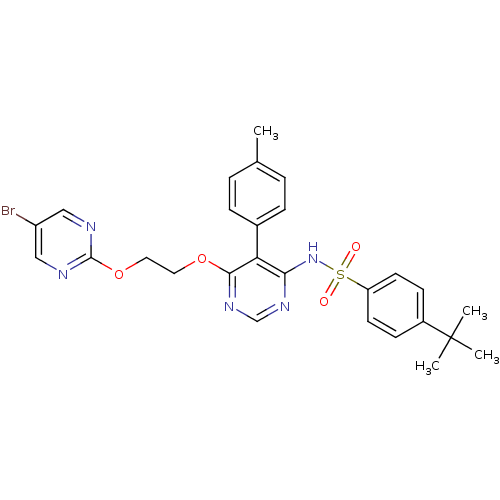
(CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...)Show SMILES Cc1ccc(cc1)-c1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)ncnc1OCCOc1ncc(Br)cn1 Show InChI InChI=1S/C27H28BrN5O4S/c1-18-5-7-19(8-6-18)23-24(33-38(34,35)22-11-9-20(10-12-22)27(2,3)4)31-17-32-25(23)36-13-14-37-26-29-15-21(28)16-30-26/h5-12,15-17H,13-14H2,1-4H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells |
J Med Chem 44: 3369-77 (2001)
BindingDB Entry DOI: 10.7270/Q27M08P8 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
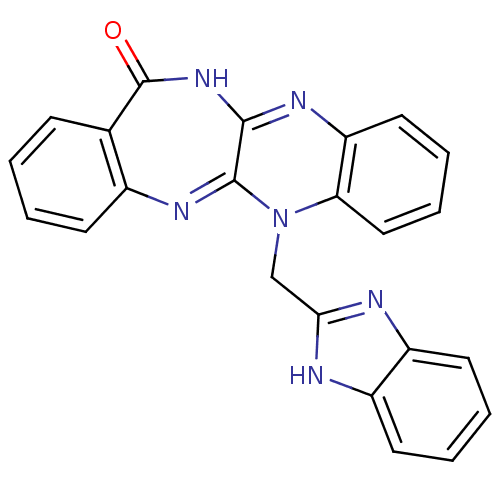
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
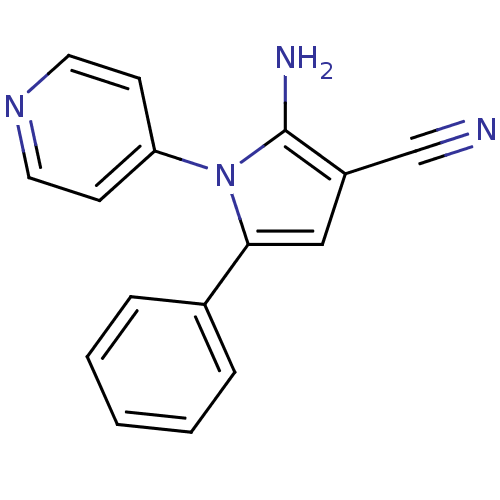
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251]
(Staphylococcus aureus) | BDBM97445
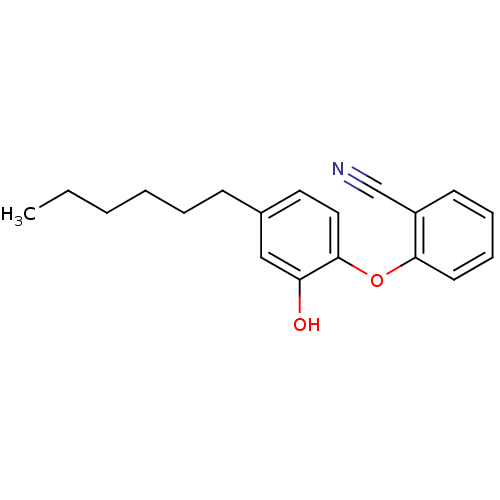
(PT119)Show InChI InChI=1S/C19H21NO2/c1-2-3-4-5-8-15-11-12-19(17(21)13-15)22-18-10-7-6-9-16(18)14-20/h6-7,9-13,21H,2-5,8H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus enoyl ACP reductase |
Eur J Med Chem 88: 66-73 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.008
BindingDB Entry DOI: 10.7270/Q25T3N3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385
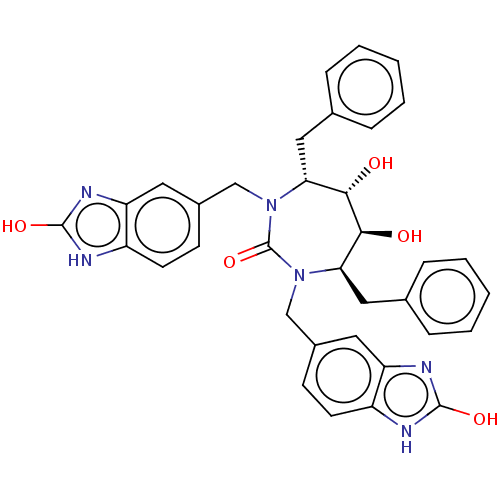
(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385

(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36648
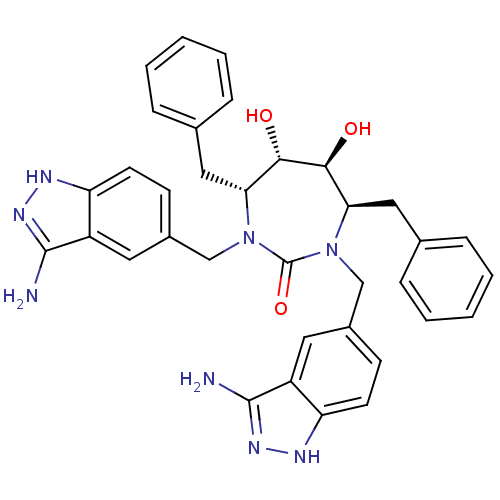
(3-alkylaminoindazole cyclic urea, (H))Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(N)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C35H36N8O3/c36-33-25-15-23(11-13-27(25)38-40-33)19-42-29(17-21-7-3-1-4-8-21)31(44)32(45)30(18-22-9-5-2-6-10-22)43(35(42)46)20-24-12-14-28-26(16-24)34(37)41-39-28/h1-16,29-32,44-45H,17-20H2,(H3,36,38,40)(H3,37,39,41)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50073223
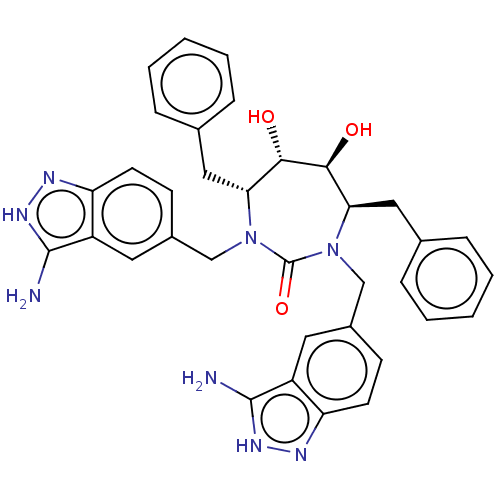
(CHEMBL73240)Show SMILES Nc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(N)c5c4)C3=O)cc12 Show InChI InChI=1S/C41H60N6O6S/c1-26(2)36(44-40(51)53-23-31-25-54-39(42-31)27(3)4)38(50)43-33(18-28-12-9-8-10-13-28)35(48)21-47-17-16-46(20-34(47)37(49)45-41(5,6)7)19-29-14-11-15-30-22-52-24-32(29)30/h8-13,15,25-27,29,33-36,48H,14,16-24H2,1-7H3,(H,43,50)(H,44,51)(H,45,49)/t29?,33-,34-,35+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36647
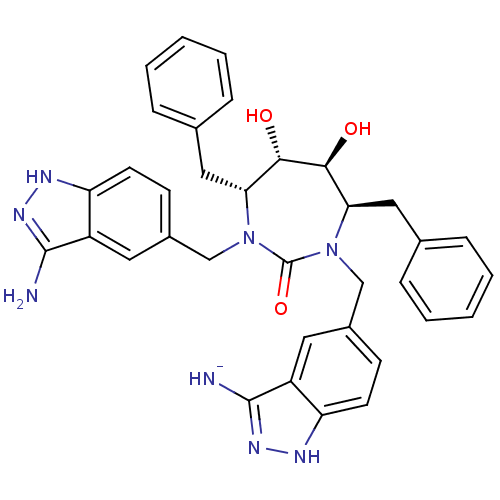
(3-Aminoindazole, 2)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc([NH-])c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C35H35N8O3/c36-33-25-15-23(11-13-27(25)38-40-33)19-42-29(17-21-7-3-1-4-8-21)31(44)32(45)30(18-22-9-5-2-6-10-22)43(35(42)46)20-24-12-14-28-26(16-24)34(37)41-39-28/h1-16,29-32,44-45H,17-20H2,(H5-,36,37,38,39,40,41)/q-1/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
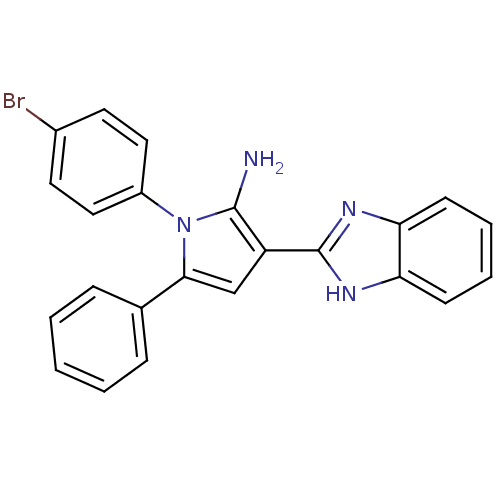
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
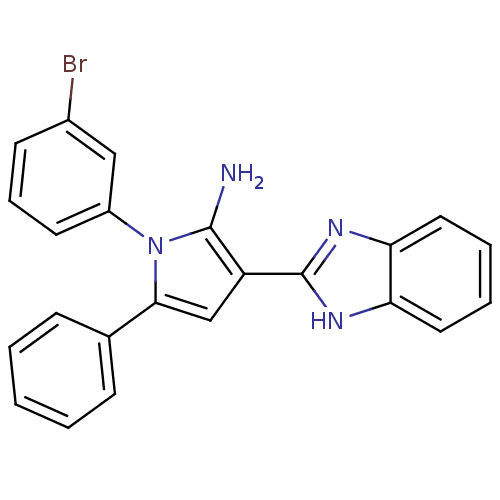
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369953
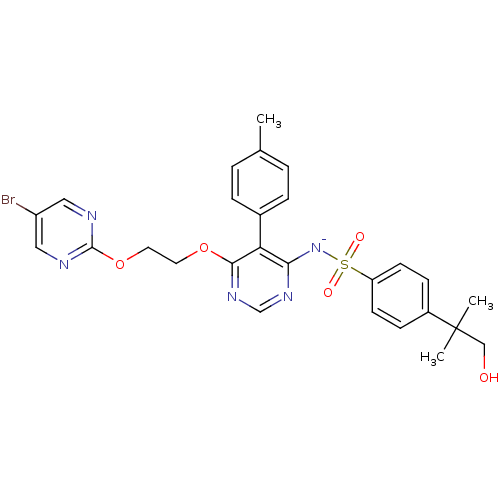
(CHEMBL1627022)Show SMILES Cc1ccc(cc1)-c1c([N-]S(=O)(=O)c2ccc(cc2)C(C)(C)CO)ncnc1OCCOc1ncc(Br)cn1 Show InChI InChI=1S/C27H27BrN5O5S/c1-18-4-6-19(7-5-18)23-24(33-39(35,36)22-10-8-20(9-11-22)27(2,3)16-34)31-17-32-25(23)37-12-13-38-26-29-14-21(28)15-30-26/h4-11,14-15,17,34H,12-13,16H2,1-3H3/q-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells |
J Med Chem 44: 3369-77 (2001)
BindingDB Entry DOI: 10.7270/Q27M08P8 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36656

(Cyclobutylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(CC4CCC4)C3=O)cc12 |r| Show InChI InChI=1S/C32H37N5O3/c33-31-25-16-24(14-15-26(25)34-35-31)20-37-28(18-22-10-5-2-6-11-22)30(39)29(38)27(17-21-8-3-1-4-9-21)36(32(37)40)19-23-12-7-13-23/h1-6,8-11,14-16,23,27-30,38-39H,7,12-13,17-20H2,(H3,33,34,35)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288430

((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3c[nH]nc3c2)C(=O)N(Cc2ccc3c[nH]nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-27-19-36-38-29(27)15-25)35(44)41(22-26-12-14-28-20-37-39-30(28)16-26)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161
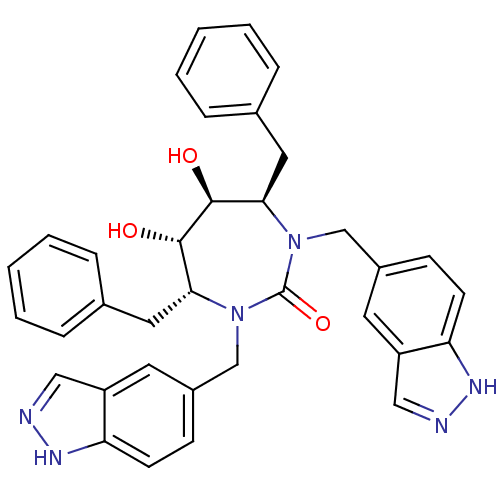
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM161

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50069033
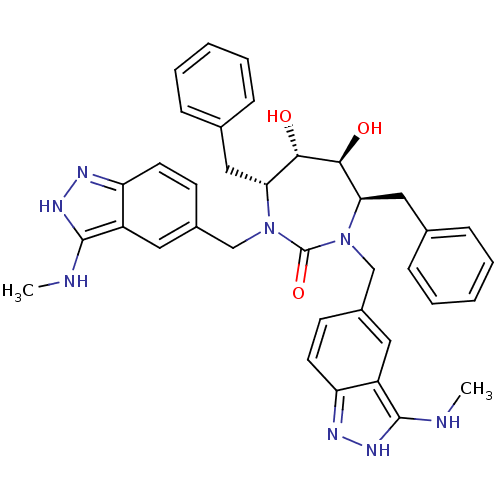
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES CNc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(NC)c5c4)C3=O)cc12 Show InChI InChI=1S/C37H40N8O3/c1-38-35-27-17-25(13-15-29(27)40-42-35)21-44-31(19-23-9-5-3-6-10-23)33(46)34(47)32(20-24-11-7-4-8-12-24)45(37(44)48)22-26-14-16-30-28(18-26)36(39-2)43-41-30/h3-18,31-34,46-47H,19-22H2,1-2H3,(H2,38,40,42)(H2,39,41,43)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36649

(3-alkylaminoindazole cyclic urea, (Me))Show SMILES CNc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(NC)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C37H40N8O3/c1-38-35-27-17-25(13-15-29(27)40-42-35)21-44-31(19-23-9-5-3-6-10-23)33(46)34(47)32(20-24-11-7-4-8-12-24)45(37(44)48)22-26-14-16-30-28(18-26)36(39-2)43-41-30/h3-18,31-34,46-47H,19-22H2,1-2H3,(H2,38,40,42)(H2,39,41,43)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100535
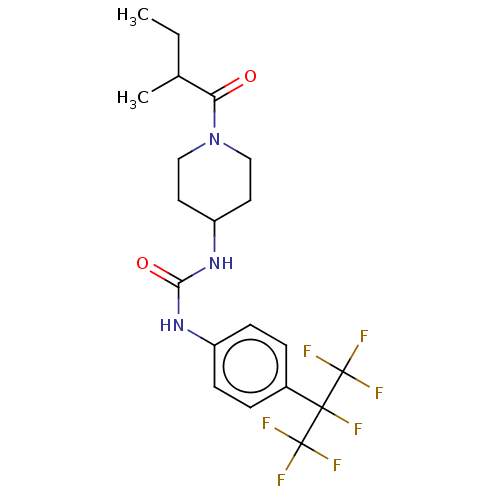
(CHEMBL3327073)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H24F7N3O2/c1-3-12(2)16(31)30-10-8-15(9-11-30)29-17(32)28-14-6-4-13(5-7-14)18(21,19(22,23)24)20(25,26)27/h4-7,12,15H,3,8-11H2,1-2H3,(H2,28,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50448785

(CHEMBL3128069)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1sc(nc1C)[C@](C)(O)CO)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H23FN6O3S/c1-12-19(33-21(28-12)22(3,31)11-30)14-8-18(20(24)25-10-14)32-13(2)16-9-15(23)4-5-17(16)29-26-6-7-27-29/h4-10,13,30-31H,11H2,1-3H3,(H2,24,25)/t13-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay |
J Med Chem 57: 1170-87 (2014)
Article DOI: 10.1021/jm401805h
BindingDB Entry DOI: 10.7270/Q29C6ZX5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100528
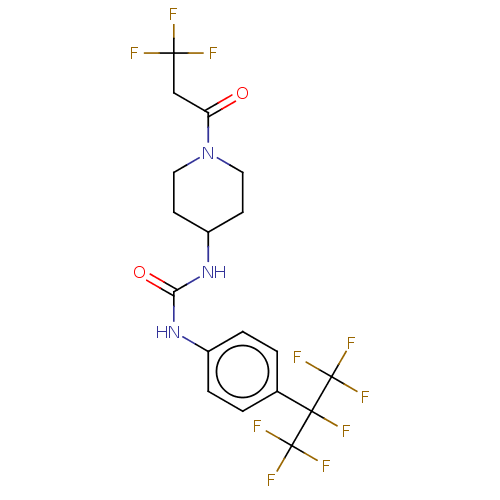
(CHEMBL3327081)Show SMILES FC(F)(F)CC(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H17F10N3O2/c19-15(20,21)9-13(32)31-7-5-12(6-8-31)30-14(33)29-11-3-1-10(2-4-11)16(22,17(23,24)25)18(26,27)28/h1-4,12H,5-9H2,(H2,29,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36655
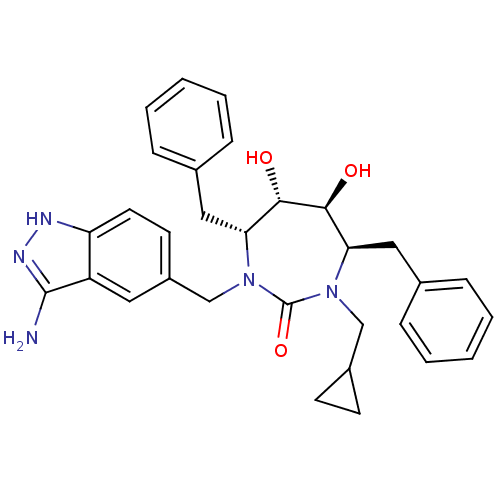
(Cyclopropylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(CC4CC4)C3=O)cc12 |r| Show InChI InChI=1S/C31H35N5O3/c32-30-24-15-23(13-14-25(24)33-34-30)19-36-27(17-21-9-5-2-6-10-21)29(38)28(37)26(16-20-7-3-1-4-8-20)35(31(36)39)18-22-11-12-22/h1-10,13-15,22,26-29,37-38H,11-12,16-19H2,(H3,32,33,34)/t26-,27-,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM50124714
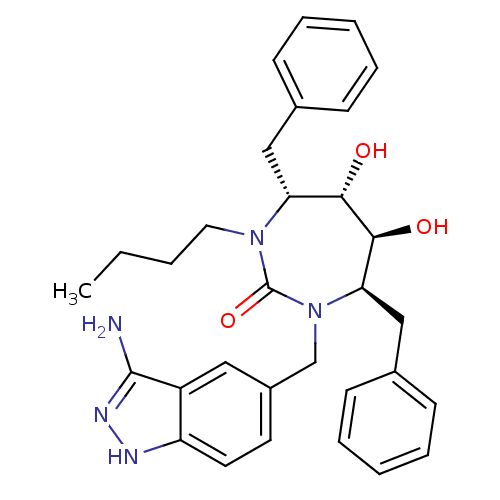
((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...)Show SMILES CCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]nc(N)c3c2)C1=O Show InChI InChI=1S/C31H37N5O3/c1-2-3-16-35-26(18-21-10-6-4-7-11-21)28(37)29(38)27(19-22-12-8-5-9-13-22)36(31(35)39)20-23-14-15-25-24(17-23)30(32)34-33-25/h4-15,17,26-29,37-38H,2-3,16,18-20H2,1H3,(H3,32,33,34)/t26-,27-,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36657

(2-Naphthylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5ccccc5c4)C3=O)cc12 |r| Show InChI InChI=1S/C38H37N5O3/c39-37-31-20-28(16-18-32(31)40-41-37)24-43-34(22-26-11-5-2-6-12-26)36(45)35(44)33(21-25-9-3-1-4-10-25)42(38(43)46)23-27-15-17-29-13-7-8-14-30(29)19-27/h1-20,33-36,44-45H,21-24H2,(H3,39,40,41)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand |
Bioorg Med Chem Lett 14: 1845-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.105
BindingDB Entry DOI: 10.7270/Q2TB17G2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards rat forebrain nicotinic acetylcholine receptor using [3H]EB as radioligand |
Bioorg Med Chem Lett 14: 1845-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.105
BindingDB Entry DOI: 10.7270/Q2TB17G2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand |
Bioorg Med Chem Lett 14: 1845-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.105
BindingDB Entry DOI: 10.7270/Q2TB17G2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand |
Bioorg Med Chem Lett 14: 1845-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.105
BindingDB Entry DOI: 10.7270/Q2TB17G2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36646

(DMP850)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccccc4)C3=O)cc12 |r| Show InChI InChI=1S/C34H35N5O3/c35-33-27-18-26(16-17-28(27)36-37-33)22-39-30(20-24-12-6-2-7-13-24)32(41)31(40)29(19-23-10-4-1-5-11-23)38(34(39)42)21-25-14-8-3-9-15-25/h1-18,29-32,40-41H,19-22H2,(H3,35,36,37)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2/beta-2
(Rattus norvegicus (Rat)) | BDBM50143281
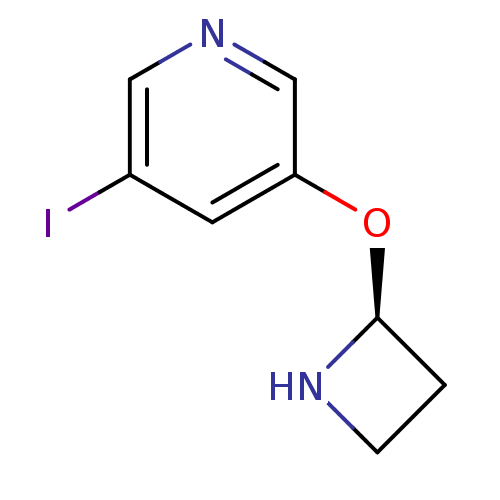
(3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...)Show InChI InChI=1S/C8H9IN2O/c9-6-3-7(5-10-4-6)12-8-1-2-11-8/h3-5,8,11H,1-2H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand |
Bioorg Med Chem Lett 14: 1845-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.09.105
BindingDB Entry DOI: 10.7270/Q2TB17G2 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50069029
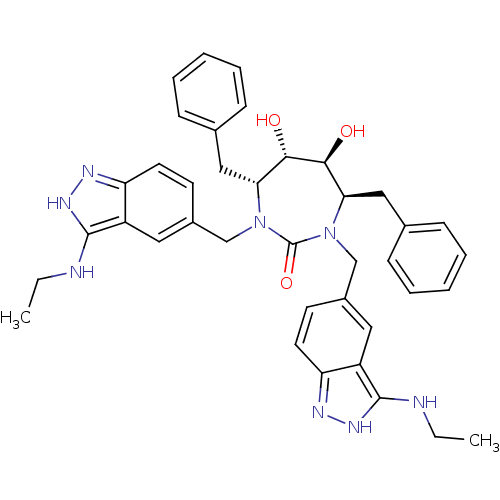
((4R,5S,6S,7R)-4,7-Dibenzyl-1,3-bis-(3-ethylamino-1...)Show SMILES CCNc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(NCC)c5c4)C3=O)cc12 Show InChI InChI=1S/C39H44N8O3/c1-3-40-37-29-19-27(15-17-31(29)42-44-37)23-46-33(21-25-11-7-5-8-12-25)35(48)36(49)34(22-26-13-9-6-10-14-26)47(39(46)50)24-28-16-18-32-30(20-28)38(41-4-2)45-43-32/h5-20,33-36,48-49H,3-4,21-24H2,1-2H3,(H2,40,42,44)(H2,41,43,45)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36658

(n-Pentyl cyclic urea)Show SMILES CCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]nc(N)c3c2)C1=O |r| Show InChI InChI=1S/C32H39N5O3/c1-2-3-10-17-36-27(19-22-11-6-4-7-12-22)29(38)30(39)28(20-23-13-8-5-9-14-23)37(32(36)40)21-24-15-16-26-25(18-24)31(33)35-34-26/h4-9,11-16,18,27-30,38-39H,2-3,10,17,19-21H2,1H3,(H3,33,34,35)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36650
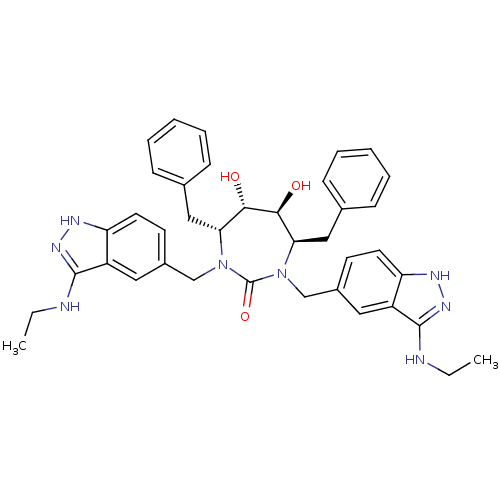
(3-alkylaminoindazole cyclic urea, (Et))Show SMILES CCNc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(NCC)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C39H44N8O3/c1-3-40-37-29-19-27(15-17-31(29)42-44-37)23-46-33(21-25-11-7-5-8-12-25)35(48)36(49)34(22-26-13-9-6-10-14-26)47(39(46)50)24-28-16-18-32-30(20-28)38(41-4-2)45-43-32/h5-20,33-36,48-49H,3-4,21-24H2,1-2H3,(H2,40,42,44)(H2,41,43,45)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data