 Found 10238 hits with Last Name = 'im' and Initial = 'i'
Found 10238 hits with Last Name = 'im' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315980
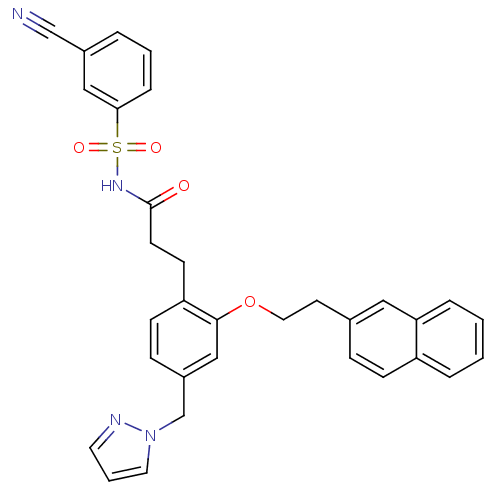
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES O=C(CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1)NS(=O)(=O)c1cccc(c1)C#N Show InChI InChI=1S/C32H28N4O4S/c33-22-25-5-3-8-30(20-25)41(38,39)35-32(37)14-13-28-12-10-26(23-36-17-4-16-34-36)21-31(28)40-18-15-24-9-11-27-6-1-2-7-29(27)19-24/h1-12,16-17,19-21H,13-15,18,23H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50546262
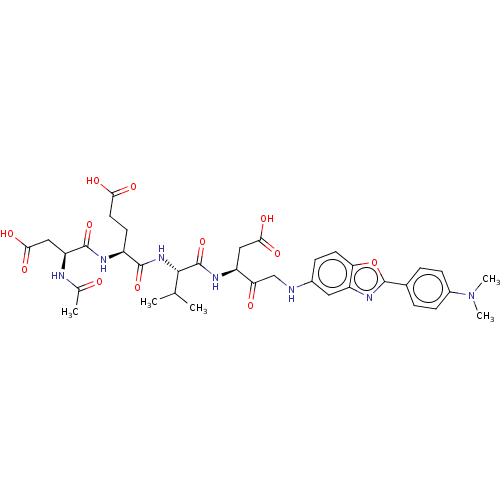
(CHEMBL4751195)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)CNc1ccc2oc(nc2c1)-c1ccc(cc1)N(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315979
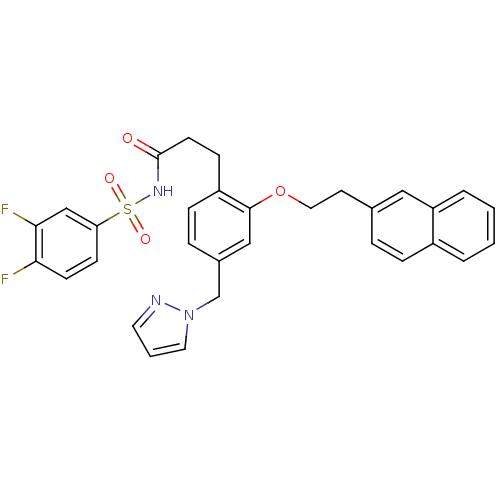
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C31H27F2N3O4S/c32-28-12-11-27(20-29(28)33)41(38,39)35-31(37)13-10-25-9-7-23(21-36-16-3-15-34-36)19-30(25)40-17-14-22-6-8-24-4-1-2-5-26(24)18-22/h1-9,11-12,15-16,18-20H,10,13-14,17,21H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315985

(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(methyl(phenyl...)Show SMILES CN(CCOc1cc(Cn2cccn2)ccc1CCC(=O)NS(=O)(=O)c1ccc(F)c(F)c1)c1ccccc1 Show InChI InChI=1S/C28H28F2N4O4S/c1-33(23-6-3-2-4-7-23)16-17-38-27-18-21(20-34-15-5-14-31-34)8-9-22(27)10-13-28(35)32-39(36,37)24-11-12-25(29)26(30)19-24/h2-9,11-12,14-15,18-19H,10,13,16-17,20H2,1H3,(H,32,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315981
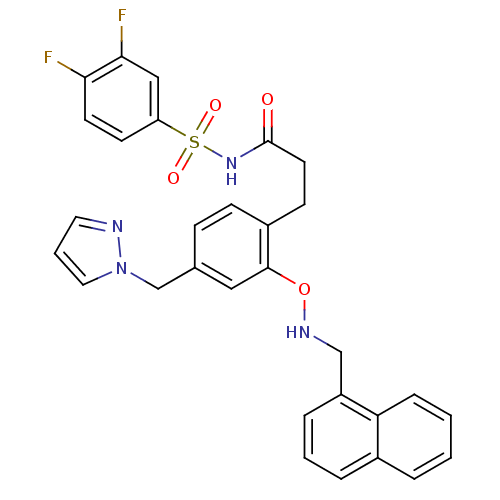
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(naphthalen-1-ylm...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1ONCc1cccc2ccccc12 Show InChI InChI=1S/C30H26F2N4O4S/c31-27-13-12-25(18-28(27)32)41(38,39)35-30(37)14-11-23-10-9-21(20-36-16-4-15-33-36)17-29(23)40-34-19-24-7-3-6-22-5-1-2-8-26(22)24/h1-10,12-13,15-18,34H,11,14,19-20H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315967
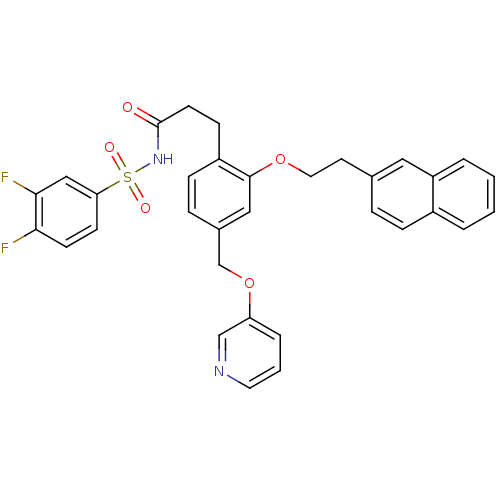
(CHEMBL1092432 | N-(3,4-difluorophenylsulfonyl)-3-(...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(COc2cccnc2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C33H28F2N2O5S/c34-30-13-12-29(20-31(30)35)43(39,40)37-33(38)14-11-26-10-8-24(22-42-28-6-3-16-36-21-28)19-32(26)41-17-15-23-7-9-25-4-1-2-5-27(25)18-23/h1-10,12-13,16,18-21H,11,14-15,17,22H2,(H,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85052
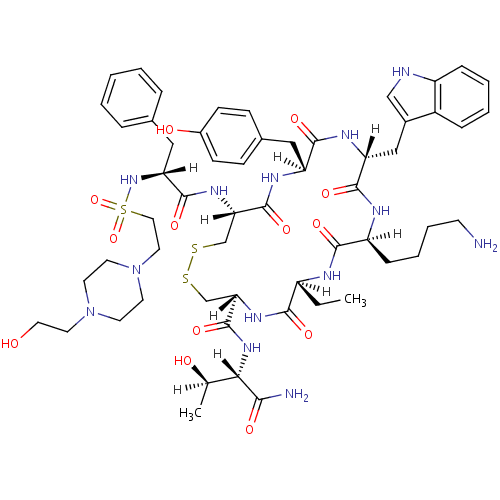
(BIM 23197 | BIM-23197)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccccc1)N[S](=O)(=O)CCN1CCN(CCO)CC1 Show InChI InChI=1S/C57H81N13O13S3/c1-3-41-51(75)65-48(57(81)67-49(35(2)72)50(59)74)34-85-84-33-47(66-55(79)46(30-36-11-5-4-6-12-36)68-86(82,83)28-26-70-23-21-69(22-24-70)25-27-71)56(80)63-44(29-37-16-18-39(73)19-17-37)53(77)64-45(31-38-32-60-42-14-8-7-13-40(38)42)54(78)62-43(52(76)61-41)15-9-10-20-58/h4-8,11-14,16-19,32,35,41,43-49,60,68,71-73H,3,9-10,15,20-31,33-34,58H2,1-2H3,(H2,59,74)(H,61,76)(H,62,78)(H,63,80)(H,64,77)(H,65,75)(H,66,79)(H,67,81)/t35-,41+,43+,44+,45-,46-,47+,48+,49+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50375451

(CHEMBL408579)Show InChI InChI=1S/C13H15N3O/c1-8(14)7-16-13-10(6-15-16)3-4-12-11(13)5-9(2)17-12/h3-6,8H,7,14H2,1-2H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315982
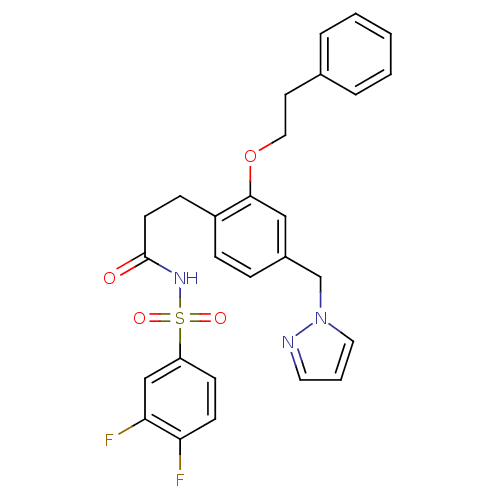
(3-(4-((1H-pyrazol-1-yl)methyl)-2-phenethoxyphenyl)...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccccc1 Show InChI InChI=1S/C27H25F2N3O4S/c28-24-11-10-23(18-25(24)29)37(34,35)31-27(33)12-9-22-8-7-21(19-32-15-4-14-30-32)17-26(22)36-16-13-20-5-2-1-3-6-20/h1-8,10-11,14-15,17-18H,9,12-13,16,19H2,(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50375457
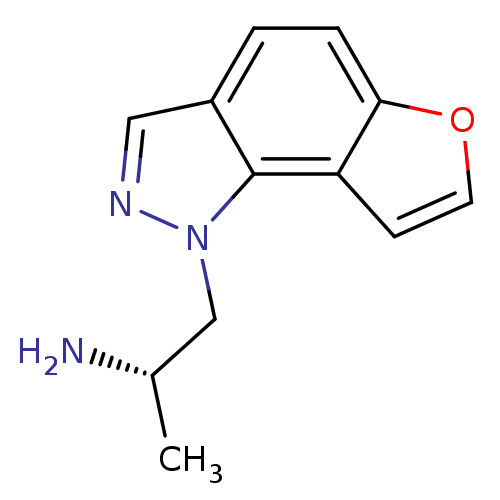
(CHEMBL261476)Show InChI InChI=1S/C12H13N3O/c1-8(13)7-15-12-9(6-14-15)2-3-11-10(12)4-5-16-11/h2-6,8H,7,13H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315978
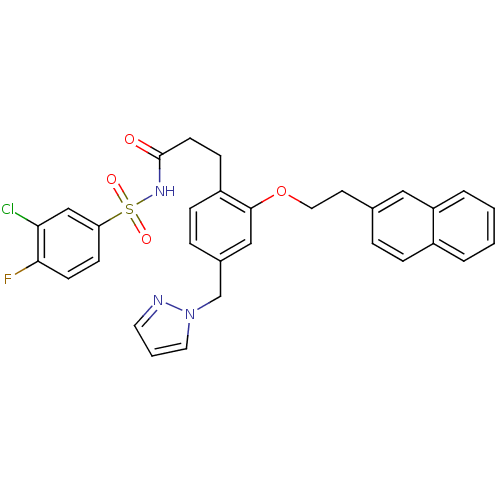
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES Fc1ccc(cc1Cl)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C31H27ClFN3O4S/c32-28-20-27(11-12-29(28)33)41(38,39)35-31(37)13-10-25-9-7-23(21-36-16-3-15-34-36)19-30(25)40-17-14-22-6-8-24-4-1-2-5-26(24)18-22/h1-9,11-12,15-16,18-20H,10,13-14,17,21H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315984
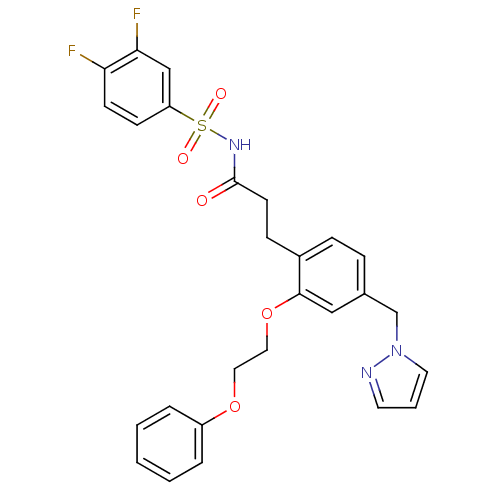
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-phenoxyethoxy)...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCOc1ccccc1 Show InChI InChI=1S/C27H25F2N3O5S/c28-24-11-10-23(18-25(24)29)38(34,35)31-27(33)12-9-21-8-7-20(19-32-14-4-13-30-32)17-26(21)37-16-15-36-22-5-2-1-3-6-22/h1-8,10-11,13-14,17-18H,9,12,15-16,19H2,(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11625
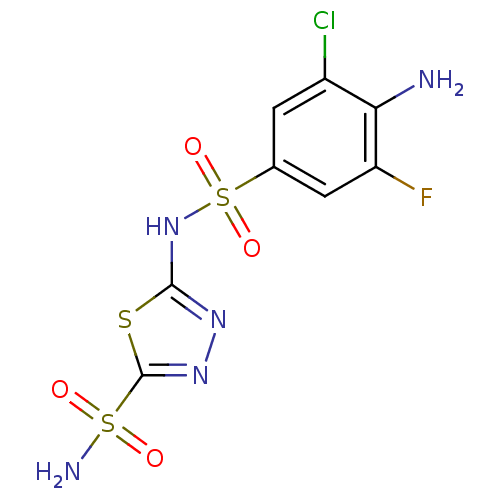
(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Kochi Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 at pH 7.5 by stopped flow CO2 hydration assay |
Bioorg Med Chem 19: 5023-30 (2011)
Article DOI: 10.1016/j.bmc.2011.06.038
BindingDB Entry DOI: 10.7270/Q24T6JRW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860
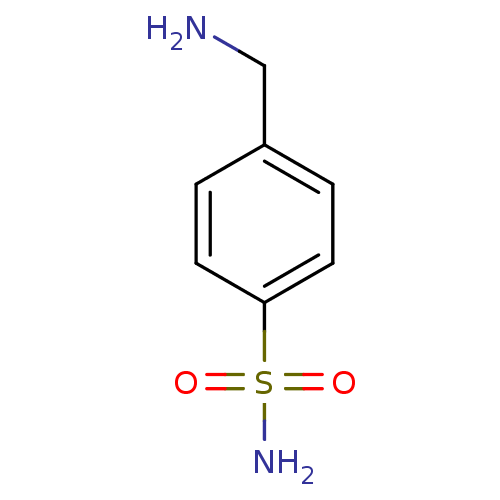
(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3828-33 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.055
BindingDB Entry DOI: 10.7270/Q2C82B2K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Ki value against human carbonic anhydrase XII (hCA XII) |
Bioorg Med Chem Lett 15: 963-9 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.053
BindingDB Entry DOI: 10.7270/Q29887RZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11625

(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50021214
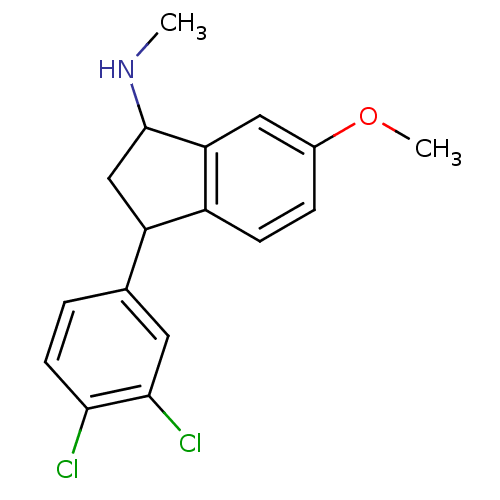
(CHEMBL300019 | CHEMBL537996 | [3-(3,4-Dichloro-phe...)Show InChI InChI=1S/C17H17Cl2NO/c1-20-17-9-13(10-3-6-15(18)16(19)7-10)12-5-4-11(21-2)8-14(12)17/h3-8,13,17,20H,9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes |
J Med Chem 47: 2624-34 (2004)
Article DOI: 10.1021/jm0305873
BindingDB Entry DOI: 10.7270/Q2MK6DF4 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50237869
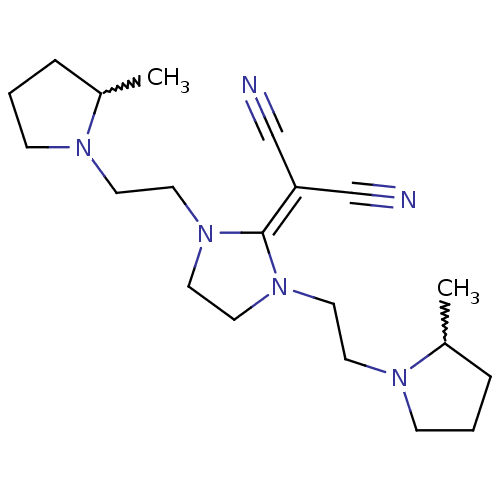
((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6])\[#6]-1=[#6](/C#N)C#N |w:1.0,18.20| Show InChI InChI=1S/C20H32N6/c1-17-5-3-7-23(17)9-11-25-13-14-26(20(25)19(15-21)16-22)12-10-24-8-4-6-18(24)2/h17-18H,3-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85051
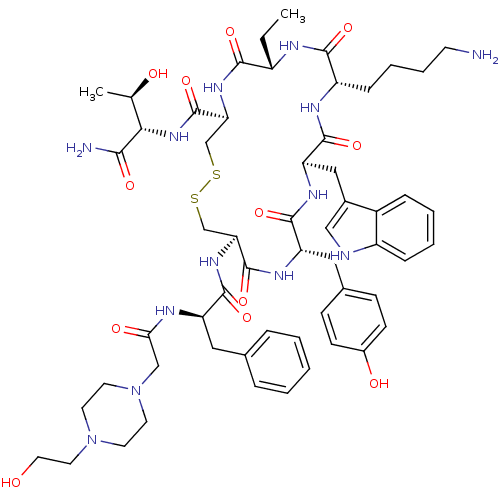
(BIM-23190)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CN1CCN(CCO)CC1 Show InChI InChI=1S/C57H79N13O12S2/c1-3-40-51(76)66-47(57(82)68-49(34(2)72)50(59)75)33-84-83-32-46(67-53(78)43(27-35-11-5-4-6-12-35)61-48(74)31-70-23-21-69(22-24-70)25-26-71)56(81)64-44(28-36-16-18-38(73)19-17-36)54(79)65-45(29-37-30-60-41-14-8-7-13-39(37)41)55(80)63-42(52(77)62-40)15-9-10-20-58/h4-8,11-14,16-19,30,34,40,42-47,49,60,71-73H,3,9-10,15,20-29,31-33,58H2,1-2H3,(H2,59,75)(H,61,74)(H,62,77)(H,63,80)(H,64,81)(H,65,79)(H,66,76)(H,67,78)(H,68,82)/t34-,40+,42+,43-,44+,45-,46+,47+,49+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50237870

(2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...)Show SMILES [#6]-[#6@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6])\[#6]-1=[#6](/C#N)C#N Show InChI InChI=1S/C20H32N6/c1-17-5-3-7-23(17)9-11-25-13-14-26(20(25)19(15-21)16-22)12-10-24-8-4-6-18(24)2/h17-18H,3-14H2,1-2H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315975
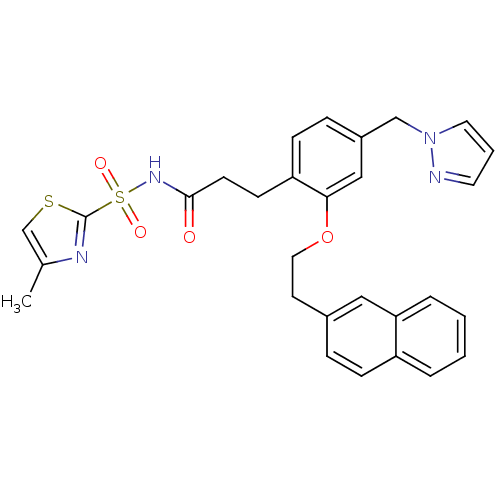
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES Cc1csc(n1)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C29H28N4O4S2/c1-21-20-38-29(31-21)39(35,36)32-28(34)12-11-25-10-8-23(19-33-15-4-14-30-33)18-27(25)37-16-13-22-7-9-24-5-2-3-6-26(24)17-22/h2-10,14-15,17-18,20H,11-13,16,19H2,1H3,(H,32,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50063839
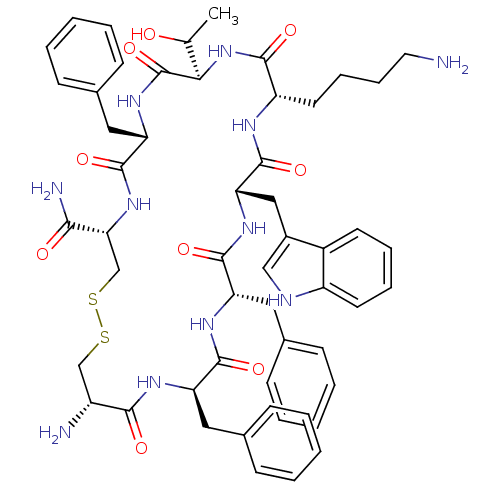
(BIM 23268 | CHEMBL263606 | H-cyclo[DCys-Phe-Phe-DT...)Show SMILES CC(O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O Show InChI InChI=1S/C54H67N11O9S2/c1-32(66)46-54(74)63-43(27-35-19-9-4-10-20-35)52(72)64-45(47(57)67)31-76-75-30-38(56)48(68)60-41(25-33-15-5-2-6-16-33)50(70)61-42(26-34-17-7-3-8-18-34)51(71)62-44(28-36-29-58-39-22-12-11-21-37(36)39)53(73)59-40(49(69)65-46)23-13-14-24-55/h2-12,15-22,29,32,38,40-46,58,66H,13-14,23-28,30-31,55-56H2,1H3,(H2,57,67)(H,59,73)(H,60,68)(H,61,70)(H,62,71)(H,63,74)(H,64,72)(H,65,69)/t32?,38-,40+,41-,42+,43+,44+,45-,46-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50237874
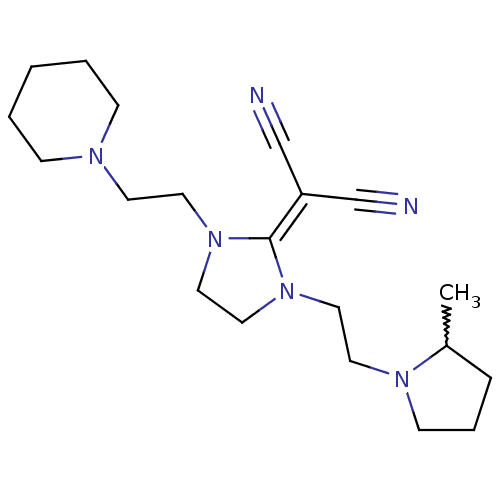
((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)\[#6]-1=[#6](/C#N)C#N |w:1.0| Show InChI InChI=1S/C20H32N6/c1-18-6-5-9-24(18)12-13-26-15-14-25(20(26)19(16-21)17-22)11-10-23-7-3-2-4-8-23/h18H,2-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069294

((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)=NN)NS(=O)(=O)c1ccc2ccccc2c1 |w:19.21| Show InChI InChI=1S/C26H31N5O3S/c1-31(22-8-4-5-9-22)26(32)24(16-18-10-12-20(13-11-18)25(27)29-28)30-35(33,34)23-15-14-19-6-2-3-7-21(19)17-23/h2-3,6-7,10-15,17,22,24,30H,4-5,8-9,16,28H2,1H3,(H2,27,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards human thrombin |
Bioorg Med Chem Lett 8: 2563-8 (1999)
BindingDB Entry DOI: 10.7270/Q20Z72FK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50021226
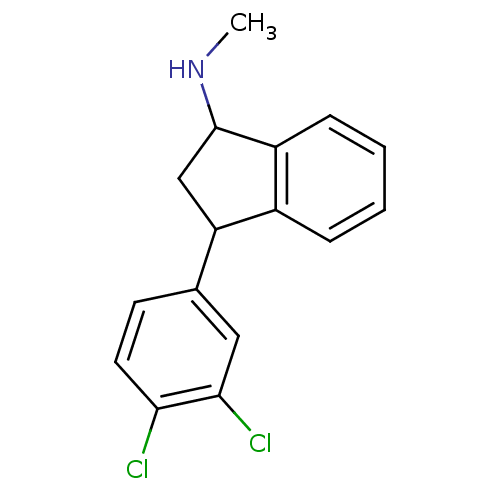
(CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...)Show InChI InChI=1S/C16H15Cl2N/c1-19-16-9-13(11-4-2-3-5-12(11)16)10-6-7-14(17)15(18)8-10/h2-8,13,16,19H,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes |
J Med Chem 47: 2624-34 (2004)
Article DOI: 10.1021/jm0305873
BindingDB Entry DOI: 10.7270/Q2MK6DF4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM82465
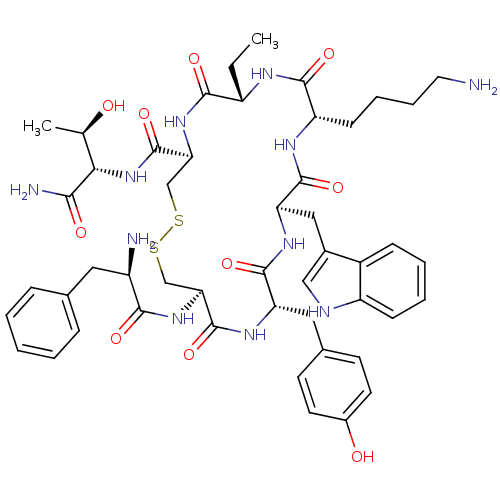
(BIM 23023 | BIM-23023 | D-Phe-L-Cys(1)-L-Tyr-D-Trp...)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C49H65N11O10S2/c1-3-34-44(65)59-40(49(70)60-41(27(2)61)42(52)63)26-72-71-25-39(58-43(64)33(51)21-28-11-5-4-6-12-28)48(69)56-37(22-29-16-18-31(62)19-17-29)46(67)57-38(23-30-24-53-35-14-8-7-13-32(30)35)47(68)55-36(45(66)54-34)15-9-10-20-50/h4-8,11-14,16-19,24,27,33-34,36-41,53,61-62H,3,9-10,15,20-23,25-26,50-51H2,1-2H3,(H2,52,63)(H,54,66)(H,55,68)(H,56,69)(H,57,67)(H,58,64)(H,59,65)(H,60,70)/t27-,33-,34+,36+,37+,38-,39+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315976
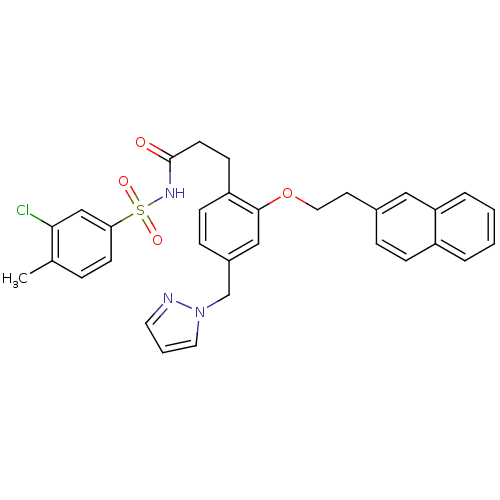
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES Cc1ccc(cc1Cl)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C32H30ClN3O4S/c1-23-7-13-29(21-30(23)33)41(38,39)35-32(37)14-12-27-11-9-25(22-36-17-4-16-34-36)20-31(27)40-18-15-24-8-10-26-5-2-3-6-28(26)19-24/h2-11,13,16-17,19-21H,12,14-15,18,22H2,1H3,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315977

(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C31H27Cl2N3O4S/c32-28-12-11-27(20-29(28)33)41(38,39)35-31(37)13-10-25-9-7-23(21-36-16-3-15-34-36)19-30(25)40-17-14-22-6-8-24-4-1-2-5-26(24)18-22/h1-9,11-12,15-16,18-20H,10,13-14,17,21H2,(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
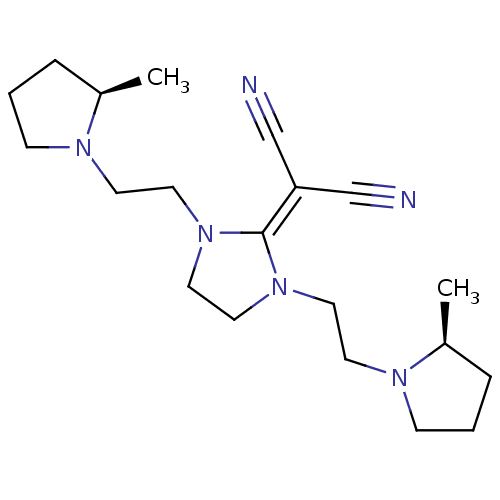
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50237872
(2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...)Show SMILES [#6]-[#6@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6])\[#6]-1=[#6](\C#N)C#N Show InChI InChI=1S/C20H32N6/c1-17-5-3-7-23(17)9-11-25-13-14-26(20(25)19(15-21)16-22)12-10-24-8-4-6-18(24)2/h17-18H,3-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50237870

(2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...)Show SMILES [#6]-[#6@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6])\[#6]-1=[#6](/C#N)C#N Show InChI InChI=1S/C20H32N6/c1-17-5-3-7-23(17)9-11-25-13-14-26(20(25)19(15-21)16-22)12-10-24-8-4-6-18(24)2/h17-18H,3-14H2,1-2H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50371874
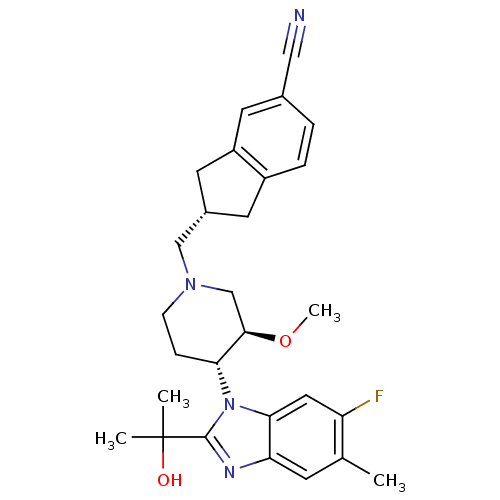
(CHEMBL257733)Show SMILES CO[C@@H]1CN(C[C@H]2Cc3ccc(cc3C2)C#N)CC[C@H]1n1c(nc2cc(C)c(F)cc12)C(C)(C)O Show InChI InChI=1S/C28H33FN4O2/c1-17-9-23-25(13-22(17)29)33(27(31-23)28(2,3)34)24-7-8-32(16-26(24)35-4)15-19-11-20-6-5-18(14-30)10-21(20)12-19/h5-6,9-10,13,19,24,26,34H,7-8,11-12,15-16H2,1-4H3/t19-,24+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 |
Bioorg Med Chem Lett 18: 1402-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.010
BindingDB Entry DOI: 10.7270/Q2M909HR |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50568077
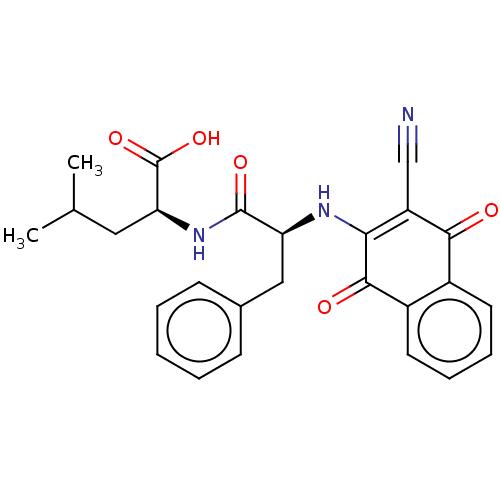
(CHEMBL4865195)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC1=C(C#N)C(=O)c2ccccc2C1=O)C(O)=O |r,c:18| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Trypanosoma brucei rhodesain |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116213
BindingDB Entry DOI: 10.7270/Q2183B90 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50270877
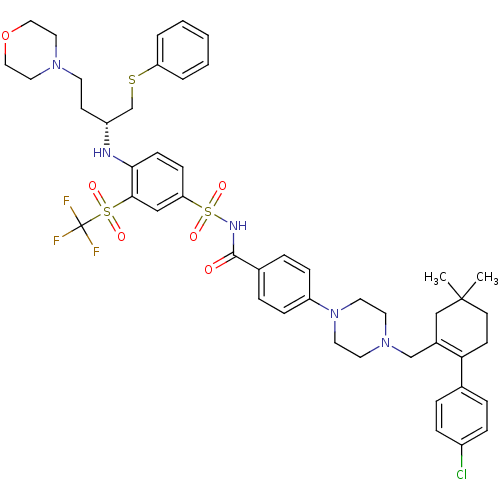
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315969
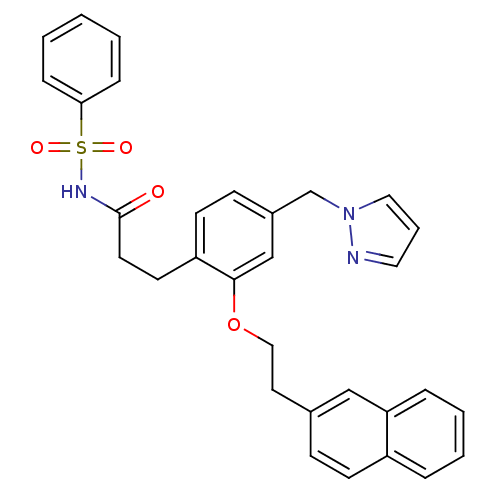
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES O=C(CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H29N3O4S/c35-31(33-39(36,37)29-9-2-1-3-10-29)16-15-27-14-12-25(23-34-19-6-18-32-34)22-30(27)38-20-17-24-11-13-26-7-4-5-8-28(26)21-24/h1-14,18-19,21-22H,15-17,20,23H2,(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
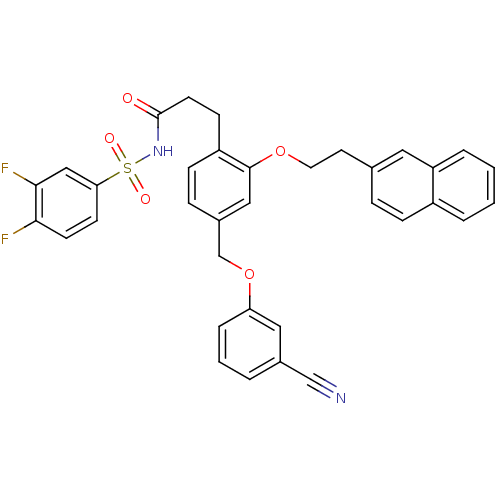
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315966
(3-(4-((3-cyanophenoxy)methyl)-2-(2-(naphthalen-2-y...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(COc2cccc(c2)C#N)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C35H28F2N2O5S/c36-32-14-13-31(21-33(32)37)45(41,42)39-35(40)15-12-28-11-9-26(23-44-30-7-3-4-25(19-30)22-38)20-34(28)43-17-16-24-8-10-27-5-1-2-6-29(27)18-24/h1-11,13-14,18-21H,12,15-17,23H2,(H,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50375452
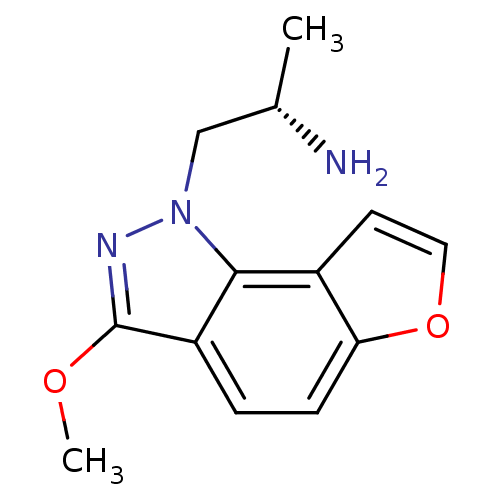
(CHEMBL262092)Show InChI InChI=1S/C13H15N3O2/c1-8(14)7-16-12-9-5-6-18-11(9)4-3-10(12)13(15-16)17-2/h3-6,8H,7,14H2,1-2H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315983
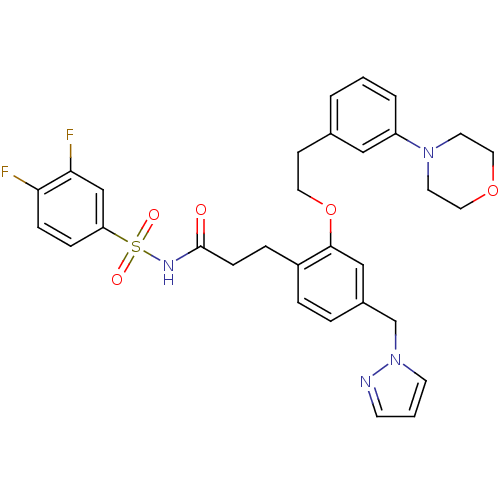
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(3-morpholinophen...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C31H32F2N4O5S/c32-28-9-8-27(21-29(28)33)43(39,40)35-31(38)10-7-25-6-5-24(22-37-13-2-12-34-37)20-30(25)42-16-11-23-3-1-4-26(19-23)36-14-17-41-18-15-36/h1-6,8-9,12-13,19-21H,7,10-11,14-18,22H2,(H,35,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50059090
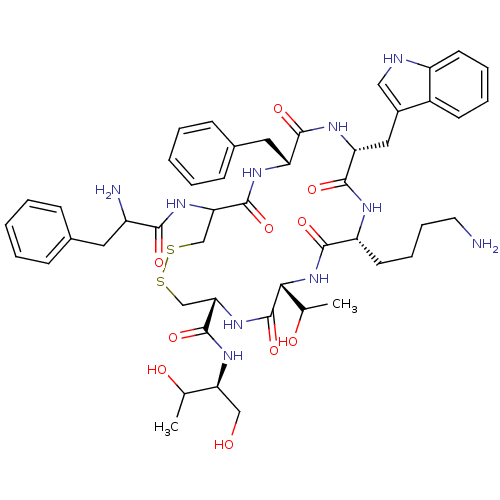
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50315974
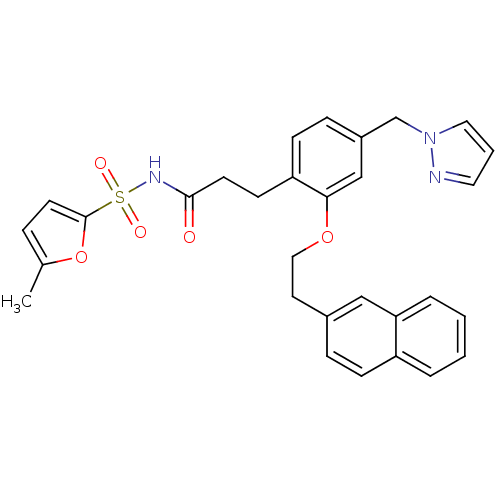
(3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...)Show SMILES Cc1ccc(o1)S(=O)(=O)NC(=O)CCc1ccc(Cn2cccn2)cc1OCCc1ccc2ccccc2c1 Show InChI InChI=1S/C30H29N3O5S/c1-22-7-14-30(38-22)39(35,36)32-29(34)13-12-26-11-9-24(21-33-17-4-16-31-33)20-28(26)37-18-15-23-8-10-25-5-2-3-6-27(25)19-23/h2-11,14,16-17,19-20H,12-13,15,18,21H2,1H3,(H,32,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter |
Bioorg Med Chem Lett 20: 2639-43 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.034
BindingDB Entry DOI: 10.7270/Q23B6099 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM234270
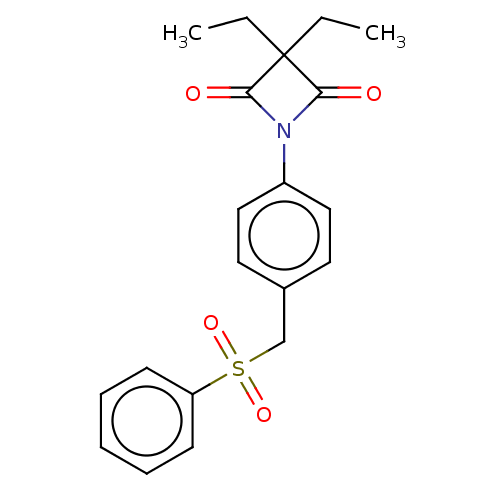
(4-oxo-β-lactam (3))Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C20H21NO4S/c1-3-20(4-2)18(22)21(19(20)23)16-12-10-15(11-13-16)14-26(24,25)17-8-6-5-7-9-17/h5-13H,3-4,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Lisbon
| Assay Description
The inhibition of the HLE was studied at 25°C by continuously monitoring the absorbance at 410 nm for 20 min of a solution prepared by mixing 10 _... |
J Enzyme Inhib Med Chem 26: 169-75 (2011)
Article DOI: 10.3109/14756366.2010.486794
BindingDB Entry DOI: 10.7270/Q2B56HM0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50237869

((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6])\[#6]-1=[#6](/C#N)C#N |w:1.0,18.20| Show InChI InChI=1S/C20H32N6/c1-17-5-3-7-23(17)9-11-25-13-14-26(20(25)19(15-21)16-22)12-10-24-8-4-6-18(24)2/h17-18H,3-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50237874

((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)\[#6]-1=[#6](/C#N)C#N |w:1.0| Show InChI InChI=1S/C20H32N6/c1-18-6-5-9-24(18)12-13-26-15-14-25(20(26)19(16-21)17-22)11-10-23-7-3-2-4-8-23/h18H,2-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50146042

(1-(3,4-Dichloro-phenyl)-3-diethylamino-indan-5-ol ...)Show InChI InChI=1S/C19H21Cl2NO/c1-3-22(4-2)19-11-15(12-5-8-17(20)18(21)9-12)14-7-6-13(23)10-16(14)19/h5-10,15,19,23H,3-4,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes |
J Med Chem 47: 2624-34 (2004)
Article DOI: 10.1021/jm0305873
BindingDB Entry DOI: 10.7270/Q2MK6DF4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50146043
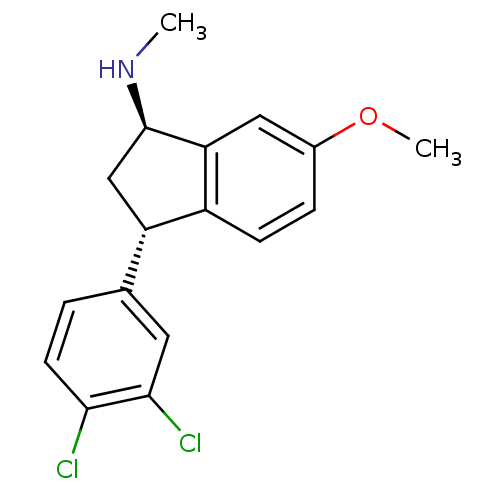
(CHEMBL543372 | [(1R,3S)-3-(3,4-Dichloro-phenyl)-6-...)Show SMILES CN[C@@H]1C[C@H](c2ccc(OC)cc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H17Cl2NO/c1-20-17-9-13(10-3-6-15(18)16(19)7-10)12-5-4-11(21-2)8-14(12)17/h3-8,13,17,20H,9H2,1-2H3/t13-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity for DA transporter using [125I]RTI-55 in frozen rat caudate membranes |
J Med Chem 47: 2624-34 (2004)
Article DOI: 10.1021/jm0305873
BindingDB Entry DOI: 10.7270/Q2MK6DF4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM82470
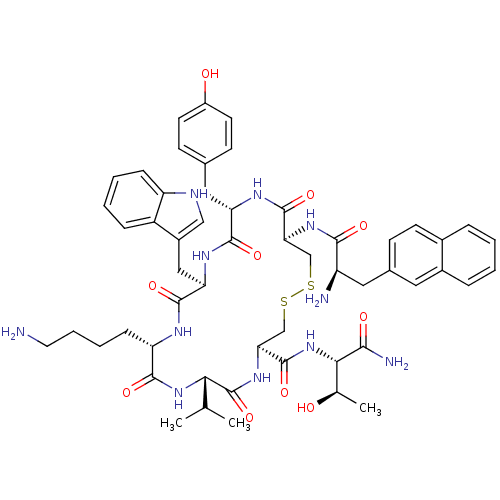
(3-(2-Naphtyl)-D-Ala-L-Cys(1)-L-Tyr-D-Trp-L-Lys-L-V...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccc2ccccc2c1 Show InChI InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)/t30-,38-,40+,41+,42-,43+,44+,45+,46+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50237872

(2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...)Show SMILES [#6]-[#6@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#7]1-[#6]-[#6]-[#7](-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6])\[#6]-1=[#6](\C#N)C#N Show InChI InChI=1S/C20H32N6/c1-17-5-3-7-23(17)9-11-25-13-14-26(20(25)19(15-21)16-22)12-10-24-8-4-6-18(24)2/h17-18H,3-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane |
Bioorg Med Chem Lett 18: 2288-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.006
BindingDB Entry DOI: 10.7270/Q2KW5FSF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50375451

(CHEMBL408579)Show InChI InChI=1S/C13H15N3O/c1-8(14)7-16-13-10(6-15-16)3-4-12-11(13)5-9(2)17-12/h3-6,8H,7,14H2,1-2H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data