

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81917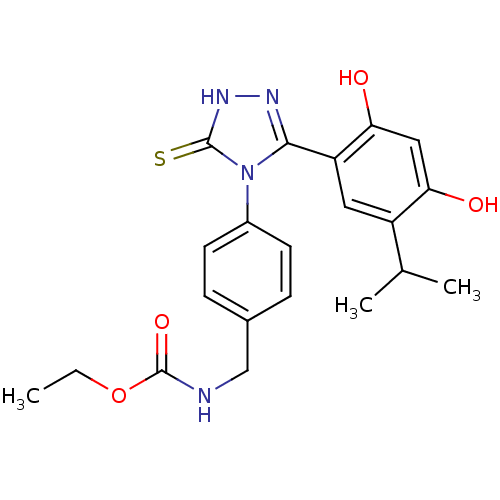 (BX-2819) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077930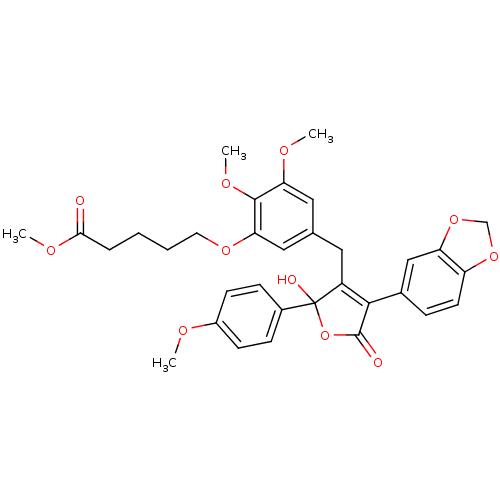 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18012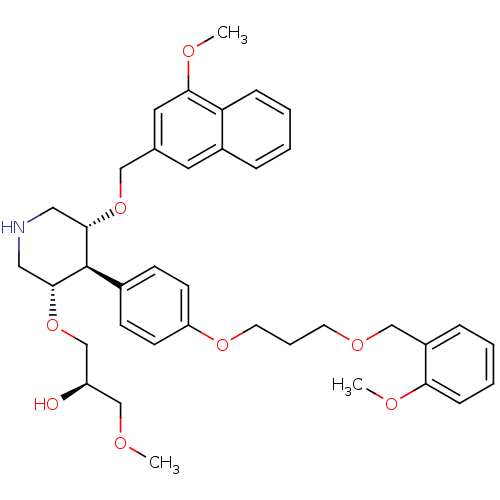 (trans,trans-4-arylpiperidine-based compound, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 17: 3575-80 (2007) Article DOI: 10.1016/j.bmcl.2007.04.052 BindingDB Entry DOI: 10.7270/Q2B56H0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077933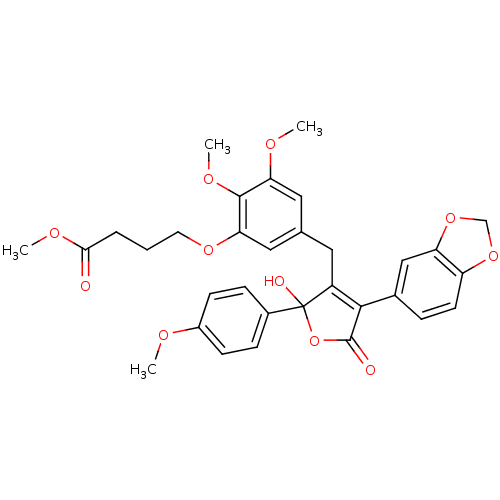 (4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81914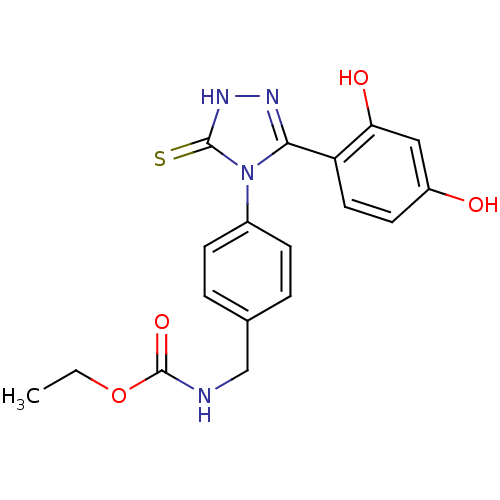 (Ethyl carbamate analog, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81916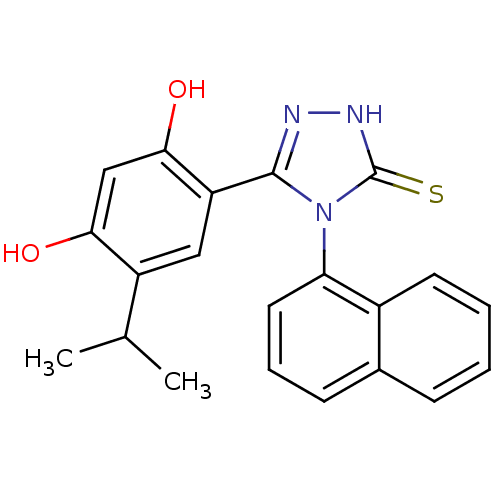 (lspropyl analog, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328726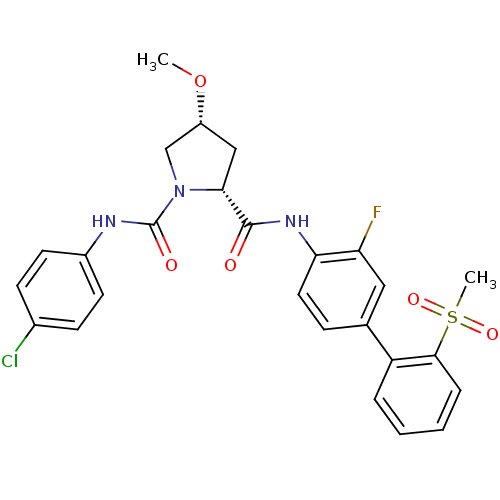 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18033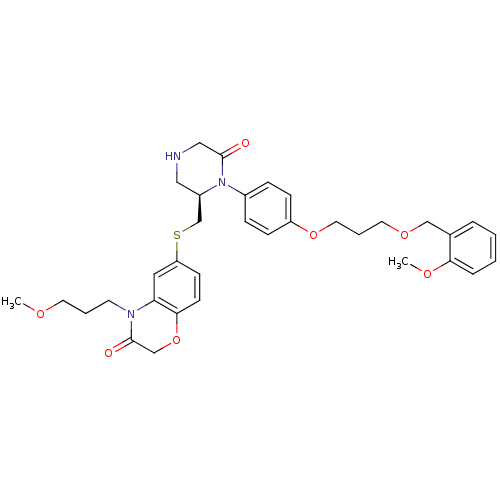 (Ketopiperazine-based inhibitor, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077934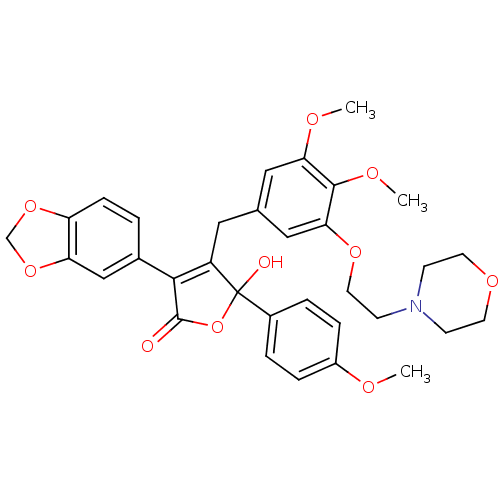 (3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(2-morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077935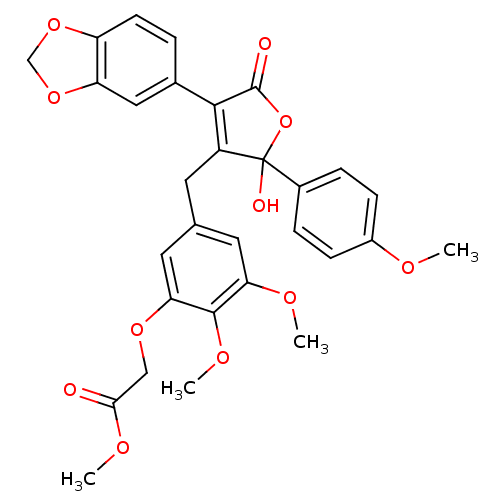 (CHEMBL308646 | {5-[4-Benzo[1,3]dioxol-5-yl-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81915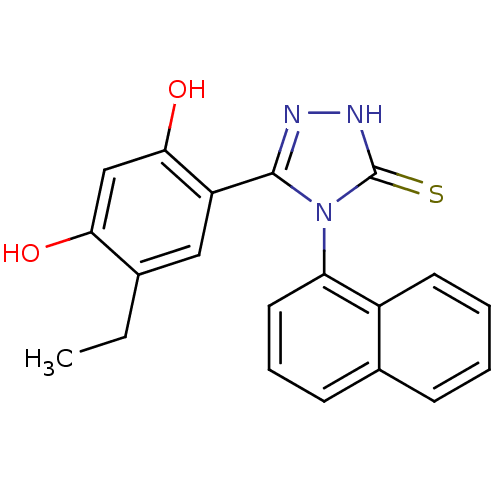 (Ethyl analog, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077936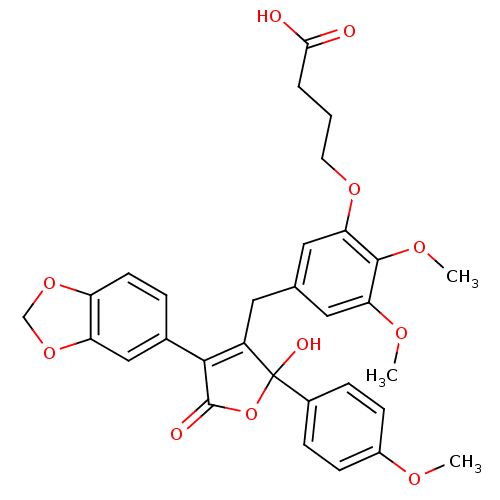 (4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81912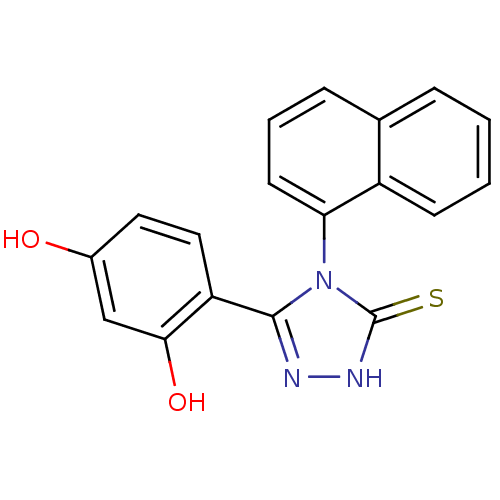 (DC23 | Resorcinol analog, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077946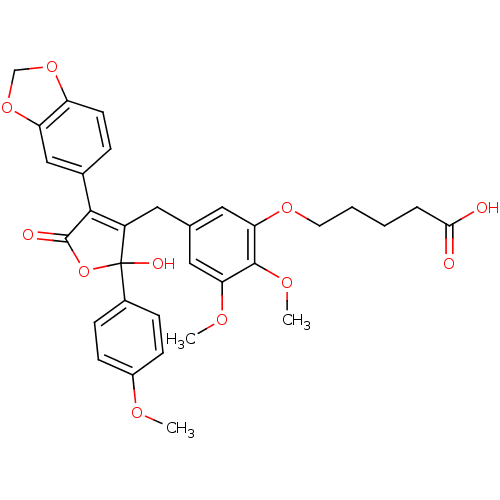 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17967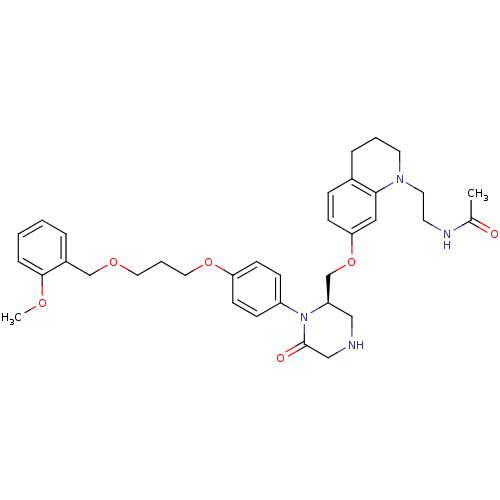 (CHEMBL411885 | Ketopiperazine-based compound, 16 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 13: 2657-64 (2005) Article DOI: 10.1016/j.bmc.2005.01.048 BindingDB Entry DOI: 10.7270/Q2KP80D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267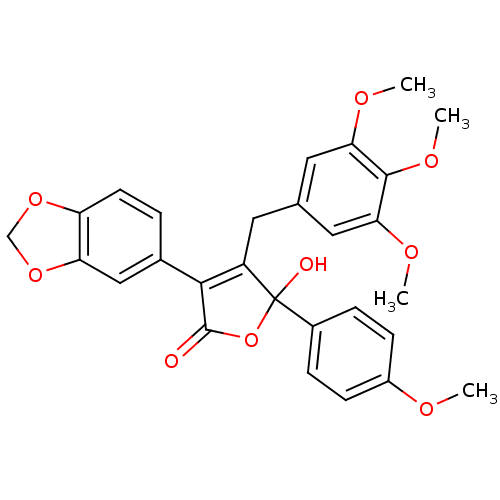 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077944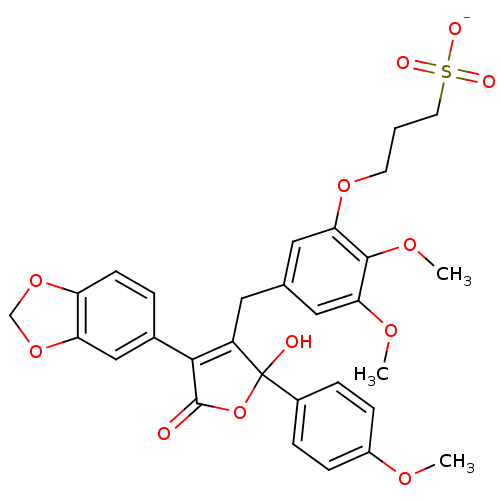 (CHEMBL408055 | Sodium; 3-{5-[4-benzo[1,3]dioxol-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328728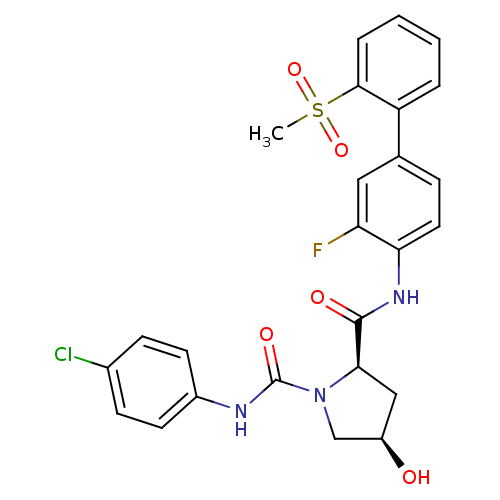 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17967 (CHEMBL411885 | Ketopiperazine-based compound, 16 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against renin in fluorescent tGFP assay | Bioorg Med Chem Lett 15: 4713-6 (2005) Article DOI: 10.1016/j.bmcl.2005.07.063 BindingDB Entry DOI: 10.7270/Q2SF2VP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18025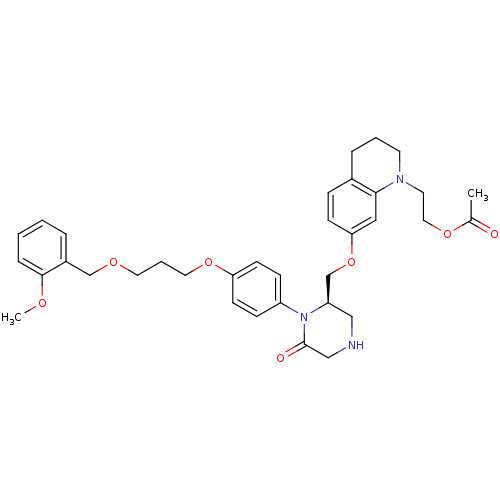 (2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17965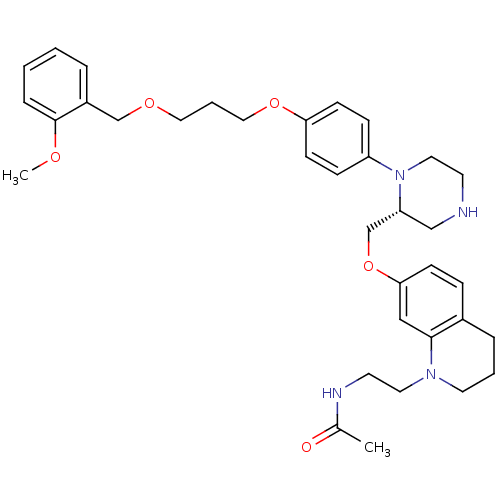 (N-[2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 13: 2657-64 (2005) Article DOI: 10.1016/j.bmc.2005.01.048 BindingDB Entry DOI: 10.7270/Q2KP80D6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81913 (Unsubstituted phenyl ring analog, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18032 (Ketopiperazine-based inhibitor, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077941 (3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(3-morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81692 (4-Substituted Pyrrolidine Ring, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17996 (1,4-benzoxazin-3-one, 33 | N-{2-[(2S)-6-(2,4-diami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 15: 5912-49 (2007) Article DOI: 10.1016/j.bmc.2007.05.069 BindingDB Entry DOI: 10.7270/Q2FX77QX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077943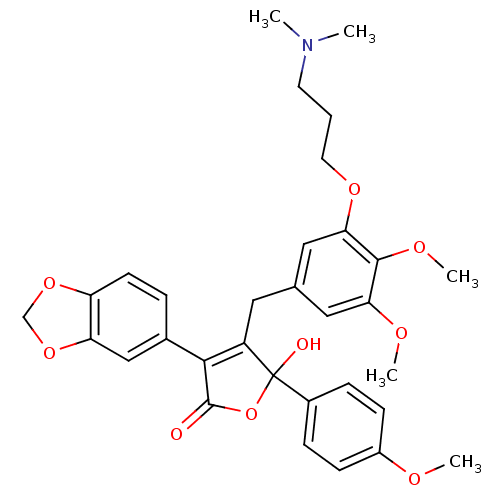 (3-Benzo[1,3]dioxol-5-yl-4-[3-(3-dimethylamino-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81698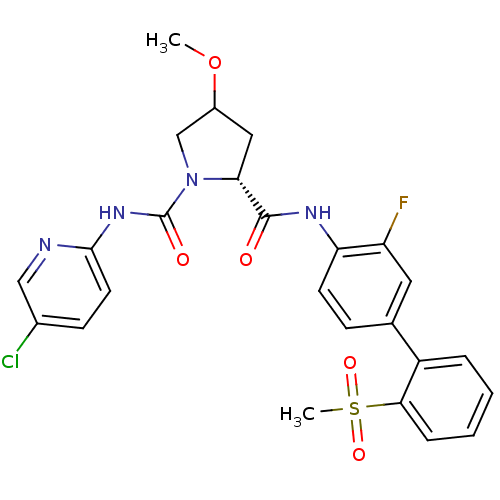 (4-Substituted Pyrrolidine Ring, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17989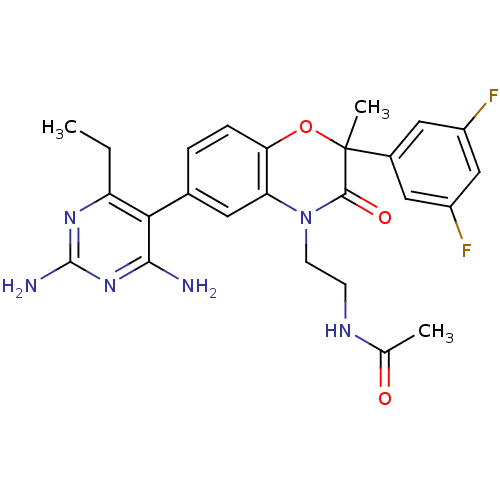 (1,4-benzoxazin-3-one, 26 | N-{2-[6-(2,4-diamino-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 15: 5912-49 (2007) Article DOI: 10.1016/j.bmc.2007.05.069 BindingDB Entry DOI: 10.7270/Q2FX77QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81693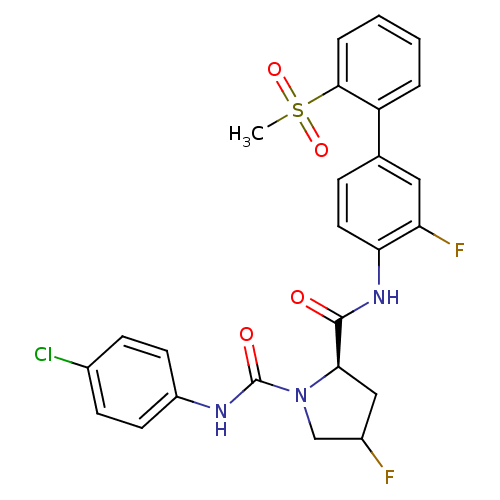 (4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328726 ((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077947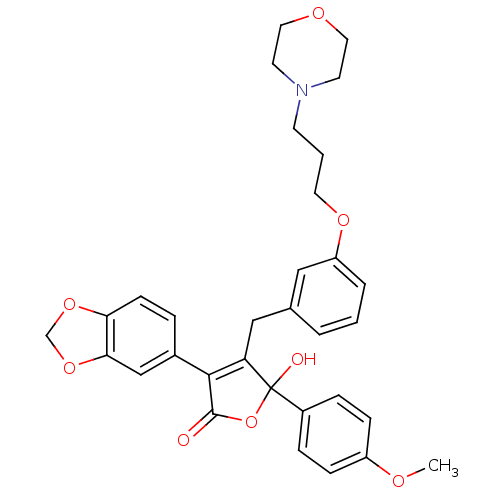 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18005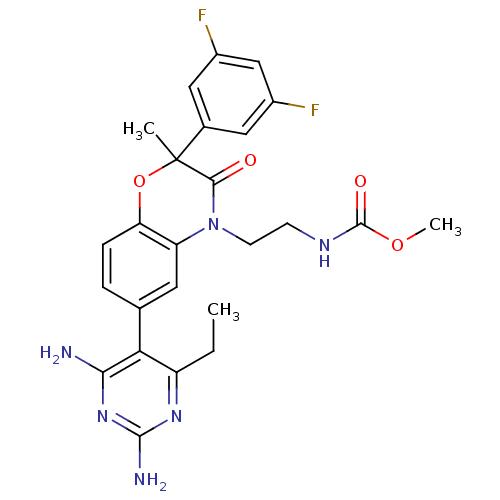 (1,4-benzoxazin-3-one, 42 | methyl N-{2-[6-(2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 15: 5912-49 (2007) Article DOI: 10.1016/j.bmc.2007.05.069 BindingDB Entry DOI: 10.7270/Q2FX77QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase N catalytic chain (Homo sapiens (Human)) | BDBM50201438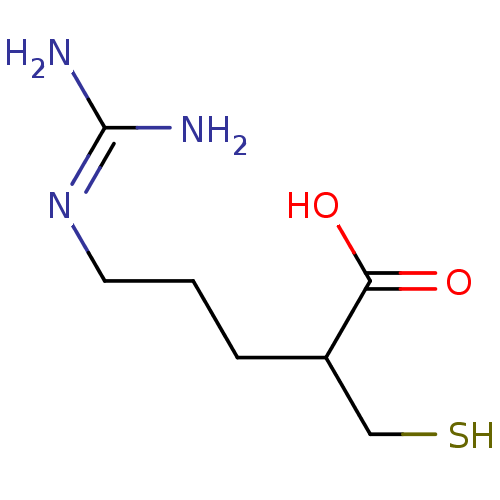 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of human carboxypeptidase N | Bioorg Med Chem Lett 17: 1349-54 (2007) Article DOI: 10.1016/j.bmcl.2006.11.078 BindingDB Entry DOI: 10.7270/Q2RJ4J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077938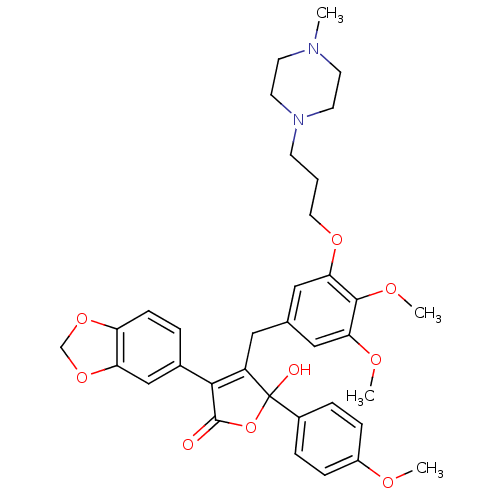 (3-Benzo[1,3]dioxol-5-yl-4-{3,4-dimethoxy-5-[3-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077945 (3-Benzo[1,3]dioxol-5-yl-4-[3-(2-dimethylamino-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM81695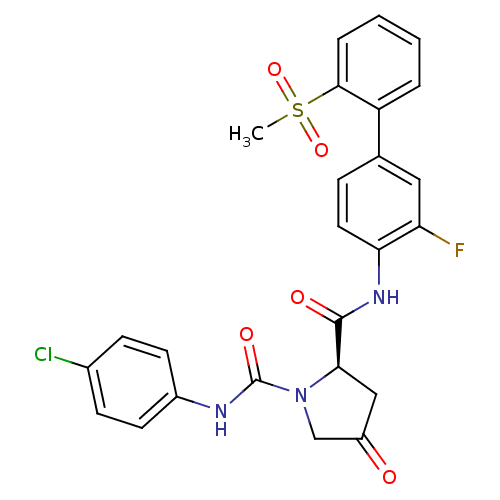 (4-Substituted Pyrrolidine Ring, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development, | Assay Description FXa inhibition were determined by using an inhibition assay. | Chem Biol Drug Des 69: 444-50 (2007) Article DOI: 10.1111/j.1747-0285.2007.00520.x BindingDB Entry DOI: 10.7270/Q2PZ5799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50201429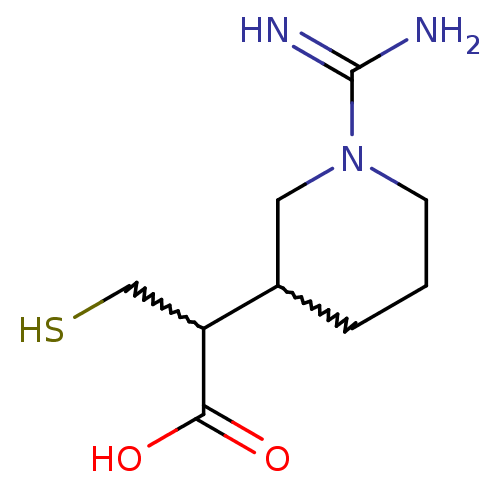 (2-(1-carbamimidoylpiperidin-3-yl)-3-mercaptopropan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic carboxypeptidase B | Bioorg Med Chem Lett 17: 1349-54 (2007) Article DOI: 10.1016/j.bmcl.2006.11.078 BindingDB Entry DOI: 10.7270/Q2RJ4J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18001 (1,4-benzoxazin-3-one, 38 | N-{2-[6-(2,4-diamino-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 15: 5912-49 (2007) Article DOI: 10.1016/j.bmc.2007.05.069 BindingDB Entry DOI: 10.7270/Q2FX77QX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17048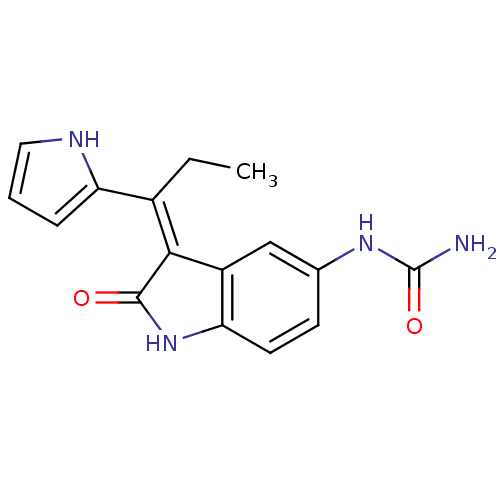 (Indolinone based inhibitor, 4j | [(3Z)-2-oxo-3-[1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3814-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.071 BindingDB Entry DOI: 10.7270/Q25Q4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17014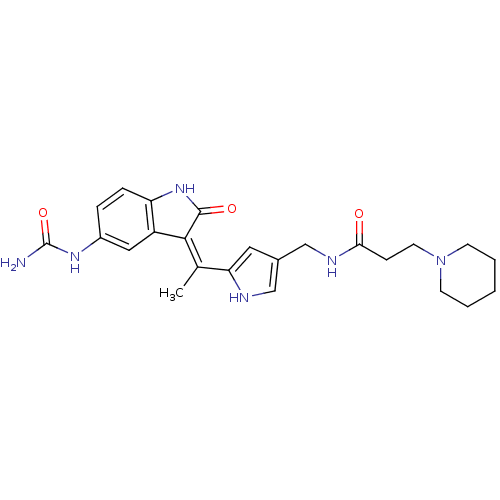 (Indolinone based compound, 22 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17003 (Indolinone based compound, 7l | [(3Z)-3-({4-[3-(am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50201429 (2-(1-carbamimidoylpiperidin-3-yl)-3-mercaptopropan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic carboxypeptidase B | Bioorg Med Chem Lett 17: 1349-54 (2007) Article DOI: 10.1016/j.bmcl.2006.11.078 BindingDB Entry DOI: 10.7270/Q2RJ4J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18028 ((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077942 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50358854 (CHEMBL1923440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of ATPase activity of recombinant Staphylococcus aureus DNA gyrase B hybrid tetramer enzyme reconstituted from Escherichia coli GyrA by am... | Bioorg Med Chem Lett 21: 7416-20 (2011) Article DOI: 10.1016/j.bmcl.2011.10.010 BindingDB Entry DOI: 10.7270/Q2RB7512 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18022 (Ketopiperazine-based inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16996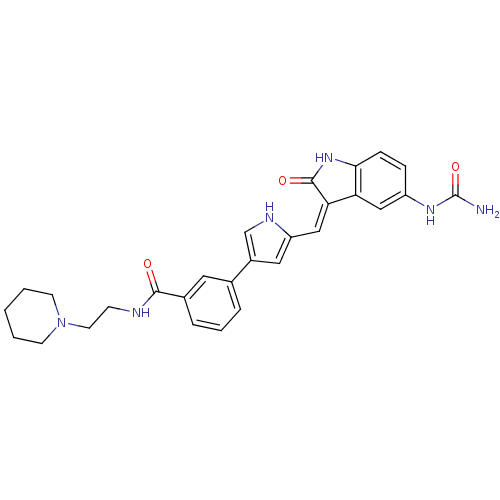 (3-(5-{[(3Z)-5-(carbamoylamino)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16995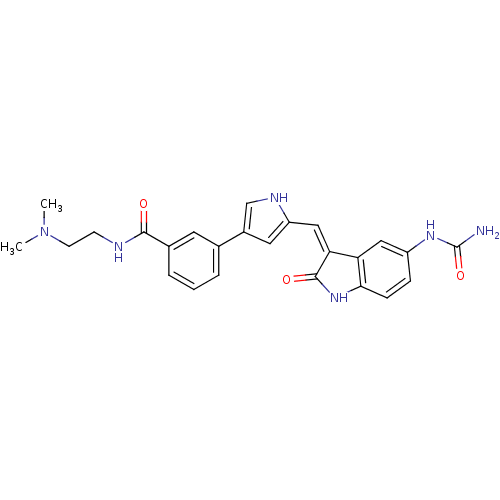 (3-(5-{[(3Z)-5-(carbamoylamino)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17010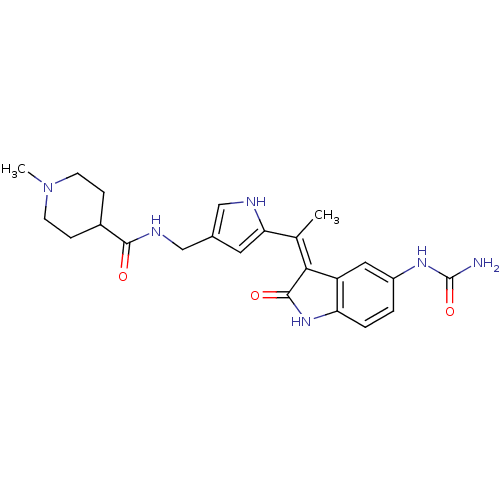 (Indolinone based compound, 18 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 516 total ) | Next | Last >> |