 Found 809 hits with Last Name = 'dragovic' and Initial = 'j'
Found 809 hits with Last Name = 'dragovic' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bombesin receptor subtype-3
(RAT) | BDBM50336889
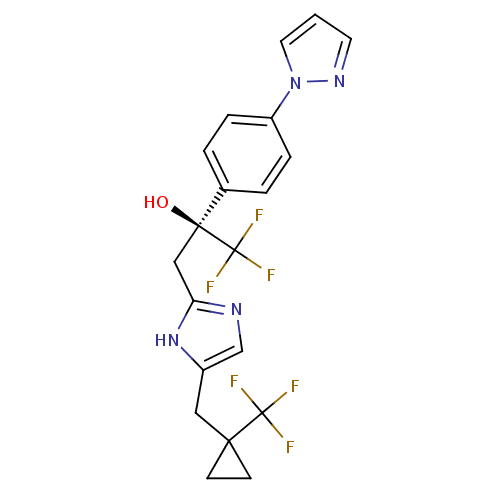
((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]Bag-3 from rat BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(RAT) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]Bag-3 from rat BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Mus musculus) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]Bag-3 from mouse BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin (6-14) from human BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50336886
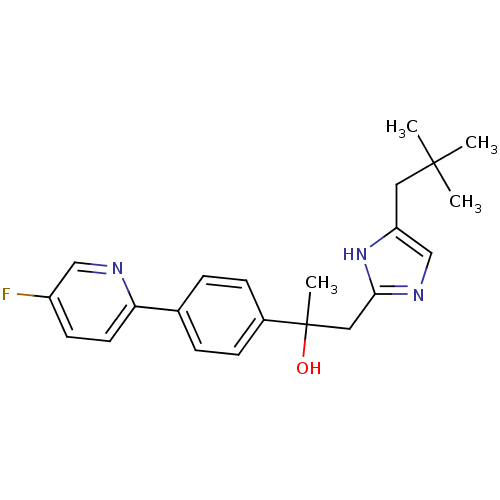
(1-[4-(2,2-dimethylpropyl)-1H-imidazol-2-yl]-2-[4-(...)Show SMILES CC(C)(C)Cc1cnc(CC(C)(O)c2ccc(cc2)-c2ccc(F)cn2)[nH]1 Show InChI InChI=1S/C22H26FN3O/c1-21(2,3)11-18-14-25-20(26-18)12-22(4,27)16-7-5-15(6-8-16)19-10-9-17(23)13-24-19/h5-10,13-14,27H,11-12H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50336885
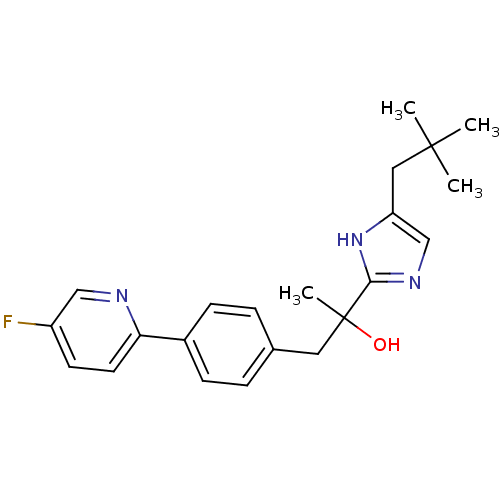
(2-[4-(2,2-dimethylpropyl)-1H-imidazol-2-yl]-1-[4-(...)Show SMILES CC(C)(C)Cc1cnc([nH]1)C(C)(O)Cc1ccc(cc1)-c1ccc(F)cn1 Show InChI InChI=1S/C22H26FN3O/c1-21(2,3)12-18-14-25-20(26-18)22(4,27)11-15-5-7-16(8-6-15)19-10-9-17(23)13-24-19/h5-10,13-14,27H,11-12H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50381918
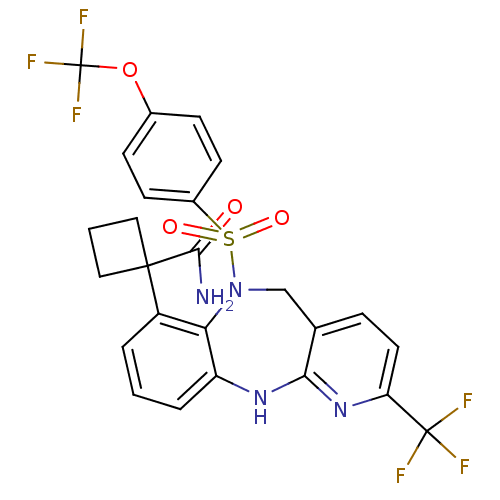
(CHEMBL2023113)Show SMILES NC(=O)C1(CCC1)c1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(F)(F)F Show InChI InChI=1S/C25H20F6N4O4S/c26-24(27,28)19-10-5-14-13-35(40(37,38)16-8-6-15(7-9-16)39-25(29,30)31)20-17(23(22(32)36)11-2-12-23)3-1-4-18(20)33-21(14)34-19/h1,3-10H,2,11-13H2,(H2,32,36)(H,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2845-9 (2012)
Article DOI: 10.1016/j.bmc.2012.03.029
BindingDB Entry DOI: 10.7270/Q2F190R8 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50381917
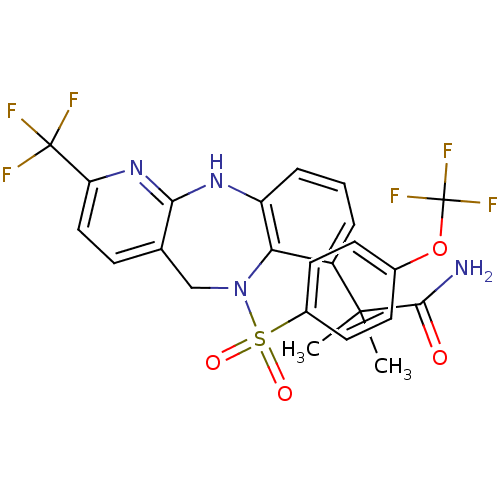
(CHEMBL2023112)Show SMILES CC(C)(C(N)=O)c1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(F)(F)F Show InChI InChI=1S/C24H20F6N4O4S/c1-22(2,21(31)35)16-4-3-5-17-19(16)34(12-13-6-11-18(23(25,26)27)33-20(13)32-17)39(36,37)15-9-7-14(8-10-15)38-24(28,29)30/h3-11H,12H2,1-2H3,(H2,31,35)(H,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2845-9 (2012)
Article DOI: 10.1016/j.bmc.2012.03.029
BindingDB Entry DOI: 10.7270/Q2F190R8 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50381916

(CHEMBL2023111)Show SMILES CC(C)(O)c1nc(no1)C1(CCC1)c1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(F)(F)F Show InChI InChI=1S/C29H25F6N5O5S/c1-26(2,41)25-38-24(39-45-25)27(13-4-14-27)19-5-3-6-20-22(19)40(15-16-7-12-21(28(30,31)32)37-23(16)36-20)46(42,43)18-10-8-17(9-11-18)44-29(33,34)35/h3,5-12,41H,4,13-15H2,1-2H3,(H,36,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2845-9 (2012)
Article DOI: 10.1016/j.bmc.2012.03.029
BindingDB Entry DOI: 10.7270/Q2F190R8 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50400247

(CHEMBL2179918)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2ccc3ncccc3c12)C(F)(F)F Show InChI InChI=1S/C26H23F3N4O2S/c1-25(2,3)17-7-9-18(10-8-17)36(34,35)33-15-16-6-13-22(26(27,28)29)32-24(16)31-21-12-11-20-19(23(21)33)5-4-14-30-20/h4-14H,15H2,1-3H3,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... |
ACS Med Chem Lett 2: 933-937 (2011)
Article DOI: 10.1021/ml200207w
BindingDB Entry DOI: 10.7270/Q25Q4X77 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394612
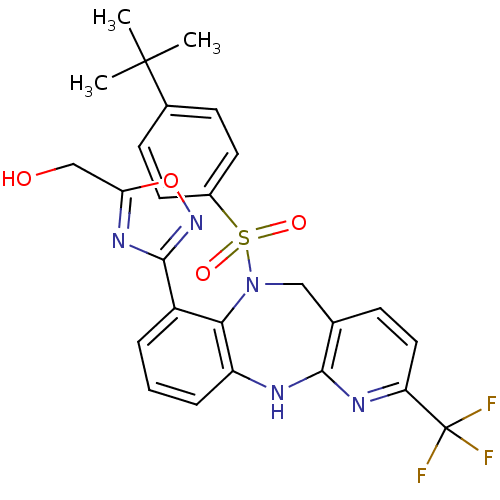
(CHEMBL2164581)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2cccc(-c3noc(CO)n3)c12)C(F)(F)F Show InChI InChI=1S/C26H24F3N5O4S/c1-25(2,3)16-8-10-17(11-9-16)39(36,37)34-13-15-7-12-20(26(27,28)29)31-23(15)30-19-6-4-5-18(22(19)34)24-32-21(14-35)38-33-24/h4-12,35H,13-14H2,1-3H3,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394616
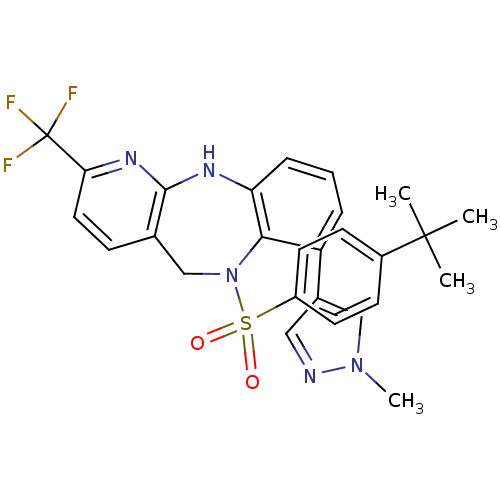
(CHEMBL2164577)Show SMILES Cn1cc(cn1)-c1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(cc1)C(C)(C)C)C(F)(F)F Show InChI InChI=1S/C27H26F3N5O2S/c1-26(2,3)19-9-11-20(12-10-19)38(36,37)35-16-17-8-13-23(27(28,29)30)33-25(17)32-22-7-5-6-21(24(22)35)18-14-31-34(4)15-18/h5-15H,16H2,1-4H3,(H,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50400254
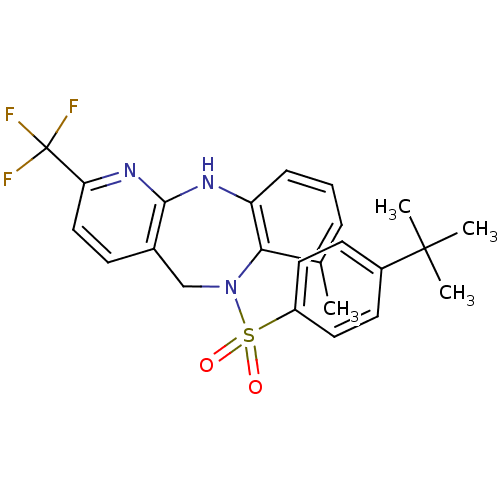
(CHEMBL2179911)Show SMILES Cc1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(cc1)C(C)(C)C)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O2S/c1-15-6-5-7-19-21(15)30(14-16-8-13-20(24(25,26)27)29-22(16)28-19)33(31,32)18-11-9-17(10-12-18)23(2,3)4/h5-13H,14H2,1-4H3,(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... |
ACS Med Chem Lett 2: 933-937 (2011)
Article DOI: 10.1021/ml200207w
BindingDB Entry DOI: 10.7270/Q25Q4X77 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394609
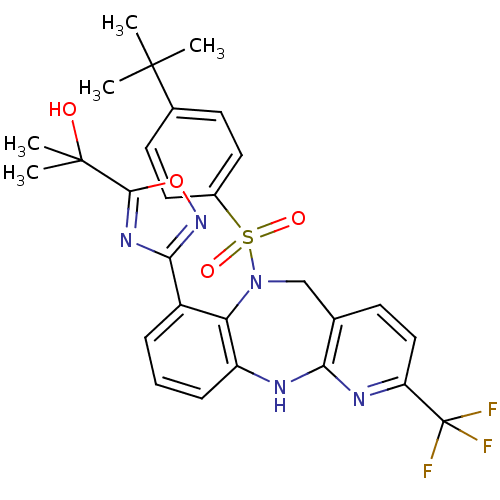
(CHEMBL2164223)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2cccc(-c3noc(n3)C(C)(C)O)c12)C(F)(F)F Show InChI InChI=1S/C28H28F3N5O4S/c1-26(2,3)17-10-12-18(13-11-17)41(38,39)36-15-16-9-14-21(28(29,30)31)33-23(16)32-20-8-6-7-19(22(20)36)24-34-25(40-35-24)27(4,5)37/h6-14,37H,15H2,1-5H3,(H,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239407
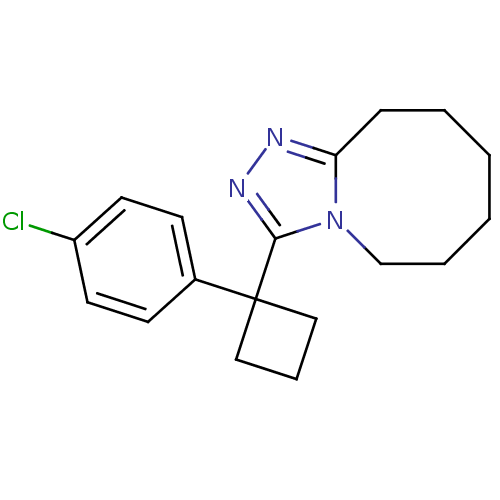
(3-(1-(4-chlorophenyl)cyclobutyl)-5,6,7,8,9,10-hexa...)Show InChI InChI=1S/C18H22ClN3/c19-15-9-7-14(8-10-15)18(11-5-12-18)17-21-20-16-6-3-1-2-4-13-22(16)17/h7-10H,1-6,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 18: 3412-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.014
BindingDB Entry DOI: 10.7270/Q2H99625 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377869
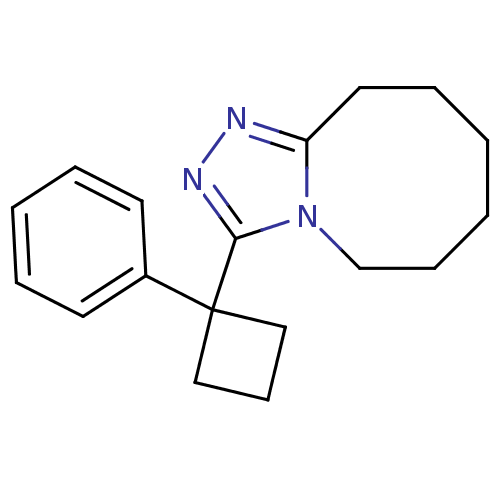
(CHEMBL402552)Show InChI InChI=1S/C18H23N3/c1-2-7-14-21-16(11-6-1)19-20-17(21)18(12-8-13-18)15-9-4-3-5-10-15/h3-5,9-10H,1-2,6-8,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 18: 3412-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.014
BindingDB Entry DOI: 10.7270/Q2H99625 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377880
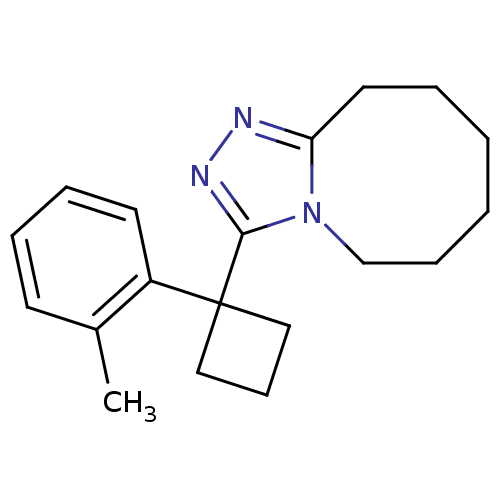
(CHEMBL404436)Show InChI InChI=1S/C19H25N3/c1-15-9-5-6-10-16(15)19(12-8-13-19)18-21-20-17-11-4-2-3-7-14-22(17)18/h5-6,9-10H,2-4,7-8,11-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 18: 3412-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.014
BindingDB Entry DOI: 10.7270/Q2H99625 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377878
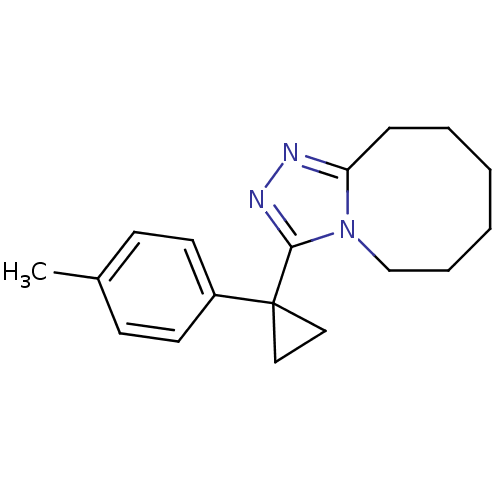
(CHEMBL258447)Show InChI InChI=1S/C18H23N3/c1-14-7-9-15(10-8-14)18(11-12-18)17-20-19-16-6-4-2-3-5-13-21(16)17/h7-10H,2-6,11-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 18: 3412-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.014
BindingDB Entry DOI: 10.7270/Q2H99625 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377883

(CHEMBL256291)Show InChI InChI=1S/C19H24FN3/c20-16-10-5-4-9-15(16)19(12-6-7-13-19)18-22-21-17-11-3-1-2-8-14-23(17)18/h4-5,9-10H,1-3,6-8,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 18: 3412-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.014
BindingDB Entry DOI: 10.7270/Q2H99625 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340399
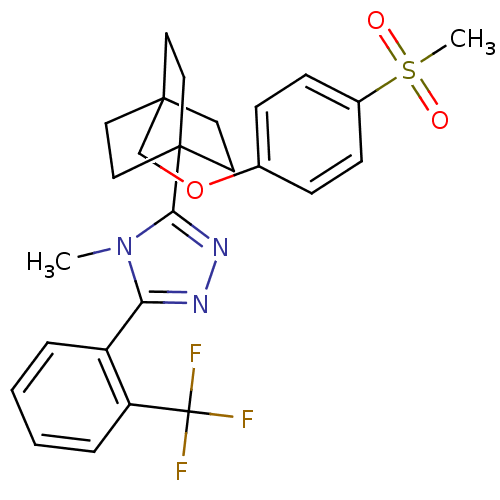
(4-methyl-3-(4-((4-(methylsulfonyl)phenoxy)methyl)b...)Show SMILES Cn1c(nnc1C12CCC(COc3ccc(cc3)S(C)(=O)=O)(CC1)CC2)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H28F3N3O3S/c1-32-22(20-5-3-4-6-21(20)26(27,28)29)30-31-23(32)25-14-11-24(12-15-25,13-16-25)17-35-18-7-9-19(10-8-18)36(2,33)34/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244238
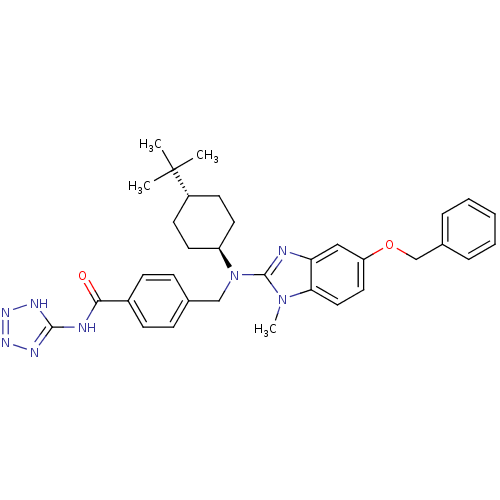
(CHEMBL499372 | trans-4-(((5-(benzyloxy)-1-methyl-1...)Show SMILES Cn1c(nc2cc(OCc3ccccc3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:34.38,wD:37.45,(-7.9,-48.6,;-7.12,-47.26,;-5.7,-46.67,;-5.82,-45.14,;-7.32,-44.78,;-8.06,-43.43,;-9.59,-43.39,;-10.33,-42.04,;-9.53,-40.73,;-10.27,-39.37,;-9.47,-38.06,;-10.21,-36.71,;-11.75,-36.67,;-12.55,-37.99,;-11.81,-39.34,;-10.4,-44.71,;-9.66,-46.06,;-8.13,-46.09,;-4.37,-47.45,;-4.38,-48.99,;-3.05,-49.77,;-1.72,-49,;-.39,-49.78,;-.4,-51.32,;-1.74,-52.08,;-3.07,-51.3,;.93,-52.1,;.92,-53.64,;2.27,-51.33,;3.6,-52.11,;4.12,-53.56,;5.66,-53.52,;6.1,-52.05,;4.83,-51.17,;-3.04,-46.69,;-3.04,-45.15,;-1.69,-44.39,;-.36,-45.17,;-.38,-46.71,;-1.71,-47.47,;.98,-44.41,;2.3,-43.63,;1.75,-45.75,;.21,-43.08,)| Show InChI InChI=1S/C34H40N8O2/c1-34(2,3)26-14-16-27(17-15-26)42(21-23-10-12-25(13-11-23)31(43)36-32-37-39-40-38-32)33-35-29-20-28(18-19-30(29)41(33)4)44-22-24-8-6-5-7-9-24/h5-13,18-20,26-27H,14-17,21-22H2,1-4H3,(H2,36,37,38,39,40,43)/t26-,27- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50377881

(CHEMBL256720)Show InChI InChI=1S/C18H22FN3/c19-15-9-5-4-8-14(15)18(11-7-12-18)17-21-20-16-10-3-1-2-6-13-22(16)17/h4-5,8-9H,1-3,6-7,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 by SPA assay |
Bioorg Med Chem Lett 18: 3412-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.014
BindingDB Entry DOI: 10.7270/Q2H99625 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244236
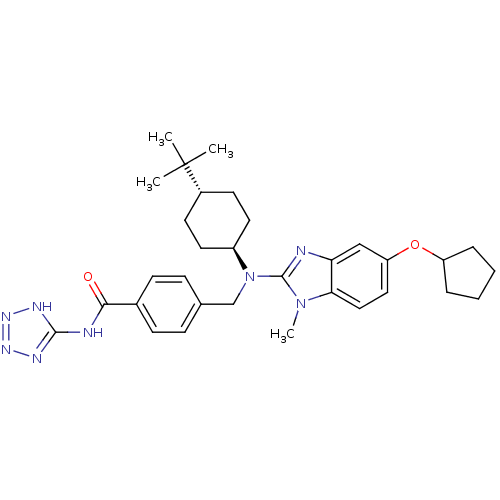
(CHEMBL500315 | trans-4-(((4-tert-butylcyclohexyl)(...)Show SMILES Cn1c(nc2cc(OC3CCCC3)ccc12)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)[C@H]1CC[C@@H](CC1)C(C)(C)C |r,wU:32.36,wD:35.43,(16.18,-49.49,;16.96,-48.15,;18.38,-47.56,;18.26,-46.02,;16.76,-45.67,;16.03,-44.32,;14.49,-44.28,;13.75,-42.93,;14.55,-41.62,;16.09,-41.49,;16.44,-39.99,;15.13,-39.19,;13.96,-40.19,;13.69,-45.6,;14.43,-46.95,;15.96,-46.98,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.69,-50.66,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.98,;25.01,-54.52,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.41,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,)| Show InChI InChI=1S/C32H42N8O2/c1-32(2,3)23-13-15-24(16-14-23)40(20-21-9-11-22(12-10-21)29(41)34-30-35-37-38-36-30)31-33-27-19-26(17-18-28(27)39(31)4)42-25-7-5-6-8-25/h9-12,17-19,23-25H,5-8,13-16,20H2,1-4H3,(H2,34,35,36,37,38,41)/t23-,24- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340380
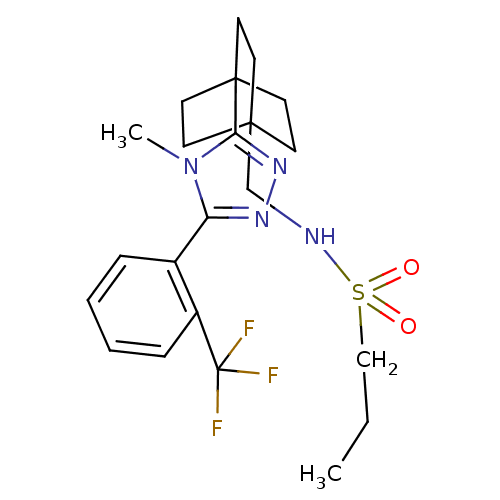
(CHEMBL1761310 | N-((4-(4-methyl-5-(2-(trifluoromet...)Show SMILES CCCS(=O)(=O)NCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C22H29F3N4O2S/c1-3-14-32(30,31)26-15-20-8-11-21(12-9-20,13-10-20)19-28-27-18(29(19)2)16-6-4-5-7-17(16)22(23,24)25/h4-7,26H,3,8-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50244265
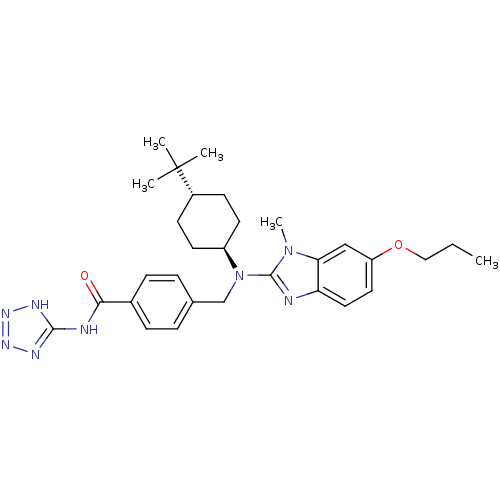
(CHEMBL480113 | trans-4-((4-tert-butylcyclohexyl)(1...)Show SMILES CCCOc1ccc2nc(N(Cc3ccc(cc3)C(=O)Nc3nnn[nH]3)[C@H]3CC[C@@H](CC3)C(C)(C)C)n(C)c2c1 |r,wU:26.27,wD:29.34,(9.13,-42.84,;9.87,-44.19,;11.41,-44.22,;12.15,-45.57,;13.69,-45.6,;14.49,-44.28,;16.03,-44.32,;16.76,-45.67,;18.26,-46.03,;18.38,-47.56,;19.71,-48.34,;19.71,-49.88,;21.04,-50.66,;22.37,-49.89,;23.7,-50.67,;23.69,-52.21,;22.34,-52.97,;21.02,-52.19,;25.02,-52.99,;25.01,-54.53,;26.36,-52.22,;27.68,-53,;28.2,-54.45,;29.74,-54.42,;30.18,-52.94,;28.91,-52.06,;21.04,-47.58,;21.05,-46.04,;22.39,-45.28,;23.72,-46.06,;23.71,-47.6,;22.37,-48.36,;25.06,-45.3,;26.39,-44.52,;25.83,-46.64,;24.3,-43.97,;16.96,-48.15,;16.19,-49.49,;15.96,-46.98,;14.43,-46.95,)| Show InChI InChI=1S/C30H40N8O2/c1-6-17-40-24-15-16-25-26(18-24)37(5)29(31-25)38(23-13-11-22(12-14-23)30(2,3)4)19-20-7-9-21(10-8-20)27(39)32-28-33-35-36-34-28/h7-10,15-16,18,22-23H,6,11-14,17,19H2,1-5H3,(H2,32,33,34,35,36,39)/t22-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 18: 3701-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.072
BindingDB Entry DOI: 10.7270/Q26Q1X25 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340389

(3-(4-((4-chlorophenoxy)methyl)bicyclo[2.2.2]octan-...)Show SMILES Cn1c(nnc1C12CCC(COc3ccc(Cl)cc3)(CC1)CC2)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C25H25ClF3N3O/c1-32-21(19-4-2-3-5-20(19)25(27,28)29)30-31-22(32)24-13-10-23(11-14-24,12-15-24)16-33-18-8-6-17(26)7-9-18/h2-9H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394613
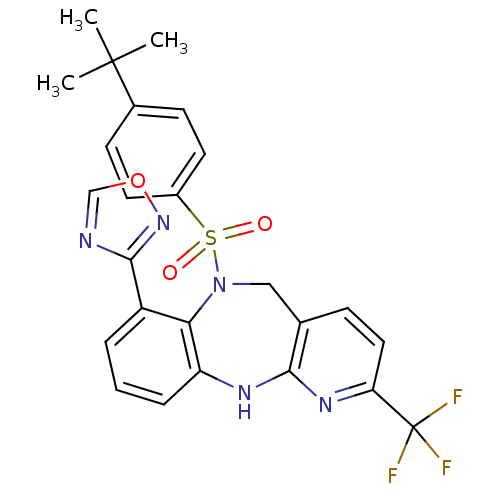
(CHEMBL2164580)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2cccc(-c3ncon3)c12)C(F)(F)F Show InChI InChI=1S/C25H22F3N5O3S/c1-24(2,3)16-8-10-17(11-9-16)37(34,35)33-13-15-7-12-20(25(26,27)28)31-22(15)30-19-6-4-5-18(21(19)33)23-29-14-36-32-23/h4-12,14H,13H2,1-3H3,(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50400257

(CHEMBL2179908)Show SMILES Cc1ccc2Nc3nc(Cl)ccc3CN(c2c1C)S(=O)(=O)c1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C24H26ClN3O2S/c1-15-6-12-20-22(16(15)2)28(14-17-7-13-21(25)27-23(17)26-20)31(29,30)19-10-8-18(9-11-19)24(3,4)5/h6-13H,14H2,1-5H3,(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... |
ACS Med Chem Lett 2: 933-937 (2011)
Article DOI: 10.1021/ml200207w
BindingDB Entry DOI: 10.7270/Q25Q4X77 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340394
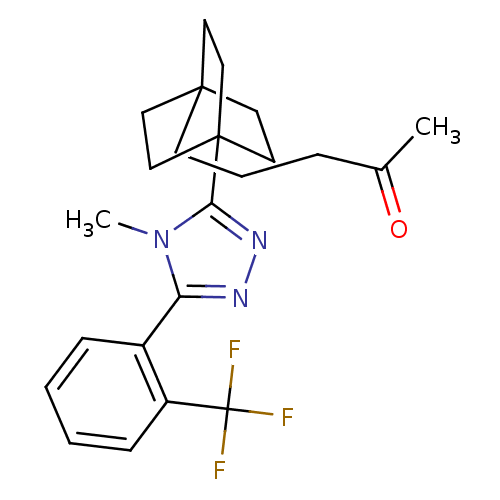
(5-(4-(4-methyl-5-(2-(trifluoromethyl)phenyl)-4H-1,...)Show SMILES CC(=O)CCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H28F3N3O/c1-16(30)6-5-9-21-10-13-22(14-11-21,15-12-21)20-28-27-19(29(20)2)17-7-3-4-8-18(17)23(24,25)26/h3-4,7-8H,5-6,9-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394611

(CHEMBL2164582)Show SMILES C[C@H](O)c1nc(no1)-c1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(cc1)C(C)(C)C)C(F)(F)F |r| Show InChI InChI=1S/C27H26F3N5O4S/c1-15(36)25-33-24(34-39-25)19-6-5-7-20-22(19)35(14-16-8-13-21(27(28,29)30)32-23(16)31-20)40(37,38)18-11-9-17(10-12-18)26(2,3)4/h5-13,15,36H,14H2,1-4H3,(H,31,32)/t15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340394

(5-(4-(4-methyl-5-(2-(trifluoromethyl)phenyl)-4H-1,...)Show SMILES CC(=O)CCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H28F3N3O/c1-16(30)6-5-9-21-10-13-22(14-11-21,15-12-21)20-28-27-19(29(20)2)17-7-3-4-8-18(17)23(24,25)26/h3-4,7-8H,5-6,9-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394615

(CHEMBL2164578)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2cccc(C3CCNCC3)c12)C(F)(F)F Show InChI InChI=1S/C28H31F3N4O2S/c1-27(2,3)20-8-10-21(11-9-20)38(36,37)35-17-19-7-12-24(28(29,30)31)34-26(19)33-23-6-4-5-22(25(23)35)18-13-15-32-16-14-18/h4-12,18,32H,13-17H2,1-3H3,(H,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50381915

(CHEMBL2023110)Show SMILES CC(C)(O)c1nc(no1)C(C)(C)c1cccc2Nc3nc(ccc3CN(c12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(F)(F)F Show InChI InChI=1S/C28H25F6N5O5S/c1-25(2,23-37-24(44-38-23)26(3,4)40)18-6-5-7-19-21(18)39(14-15-8-13-20(27(29,30)31)36-22(15)35-19)45(41,42)17-11-9-16(10-12-17)43-28(32,33)34/h5-13,40H,14H2,1-4H3,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of 125I-dY-peptide from human BRS3 expressed in NFAT-CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2845-9 (2012)
Article DOI: 10.1016/j.bmc.2012.03.029
BindingDB Entry DOI: 10.7270/Q2F190R8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174299
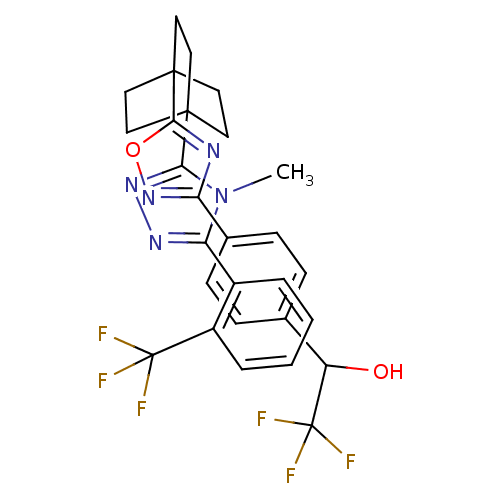
(2,2,2-trifluoro-1-(4-(5-(4-(4-methyl-5-(2-(trifluo...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)C(O)C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C28H25F6N5O2/c1-39-22(18-4-2-3-5-19(18)27(29,30)31)36-37-23(39)25-10-13-26(14-11-25,15-12-25)24-35-21(38-41-24)17-8-6-16(7-9-17)20(40)28(32,33)34/h2-9,20,40H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340389

(3-(4-((4-chlorophenoxy)methyl)bicyclo[2.2.2]octan-...)Show SMILES Cn1c(nnc1C12CCC(COc3ccc(Cl)cc3)(CC1)CC2)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C25H25ClF3N3O/c1-32-21(19-4-2-3-5-20(19)25(27,28)29)30-31-22(32)24-13-10-23(11-14-24,12-15-24)16-33-18-8-6-17(26)7-9-18/h2-9H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340392

(4-methyl-3-(4-(2-(2,2,2-trifluoroethylsulfonyl)eth...)Show SMILES Cn1c(nnc1C12CCC(CCS(=O)(=O)CC(F)(F)F)(CC1)CC2)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C22H25F6N3O2S/c1-31-17(15-4-2-3-5-16(15)22(26,27)28)29-30-18(31)20-9-6-19(7-10-20,8-11-20)12-13-34(32,33)14-21(23,24)25/h2-5H,6-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
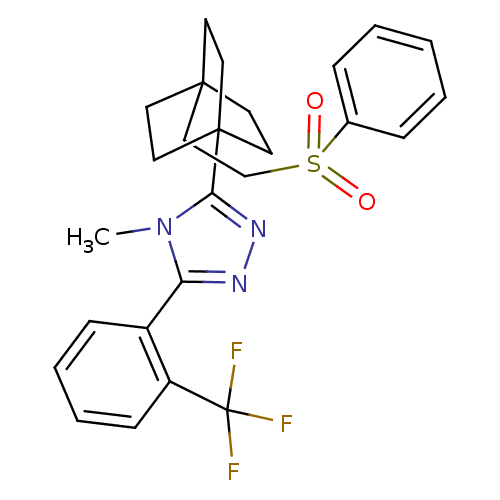
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340373
(4-methyl-3-(4-(2-(phenylsulfonyl)ethyl)bicyclo[2.2...)Show SMILES Cn1c(nnc1C12CCC(CCS(=O)(=O)c3ccccc3)(CC1)CC2)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H28F3N3O2S/c1-32-22(20-9-5-6-10-21(20)26(27,28)29)30-31-23(32)25-14-11-24(12-15-25,13-16-25)17-18-35(33,34)19-7-3-2-4-8-19/h2-10H,11-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174288
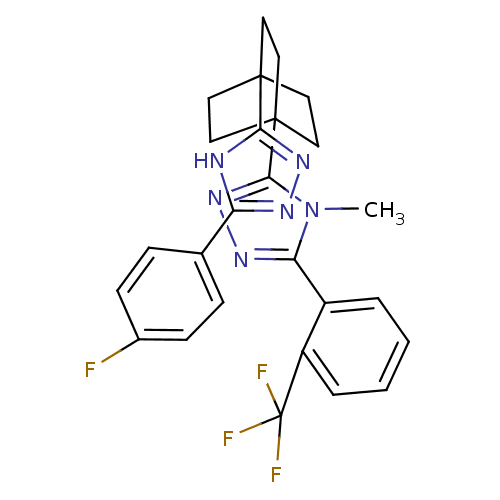
(3-(4-(5-(4-fluorophenyl)-4H-1,2,4-triazol-3-yl)bic...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nnc([nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H24F4N6/c1-36-21(18-4-2-3-5-19(18)26(28,29)30)33-35-23(36)25-13-10-24(11-14-25,12-15-25)22-31-20(32-34-22)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340402
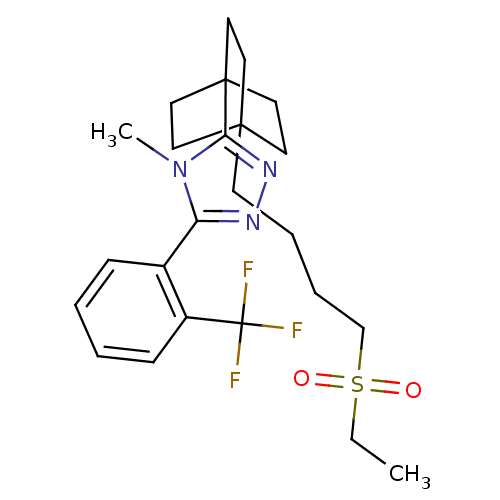
(3-(4-(4-(ethylsulfonyl)butyl)bicyclo[2.2.2]octan-1...)Show SMILES CCS(=O)(=O)CCCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C24H32F3N3O2S/c1-3-33(31,32)17-7-6-10-22-11-14-23(15-12-22,16-13-22)21-29-28-20(30(21)2)18-8-4-5-9-19(18)24(25,26)27/h4-5,8-9H,3,6-7,10-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50400246
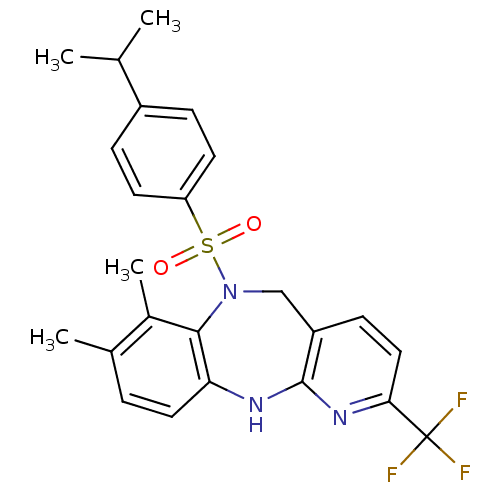
(CHEMBL2179473)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2ccc(C)c(C)c12)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O2S/c1-14(2)17-6-9-19(10-7-17)33(31,32)30-13-18-8-12-21(24(25,26)27)29-23(18)28-20-11-5-15(3)16(4)22(20)30/h5-12,14H,13H2,1-4H3,(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin(6-14) from human BRS3 expressed in CHO cells expressing NFAT after 2 hrs by liquid sc... |
ACS Med Chem Lett 2: 933-937 (2011)
Article DOI: 10.1021/ml200207w
BindingDB Entry DOI: 10.7270/Q25Q4X77 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340396
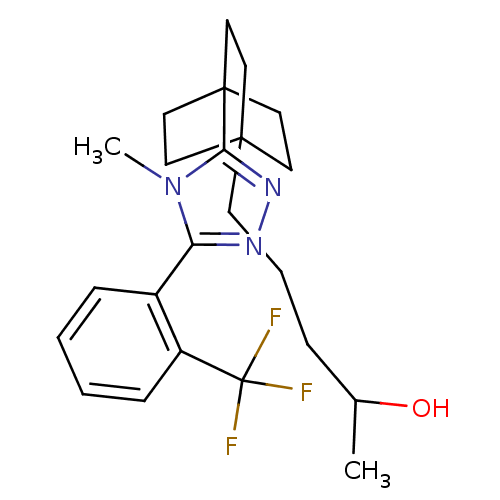
(5-(4-(4-methyl-5-(2-(trifluoromethyl)phenyl)-4H-1,...)Show SMILES CC(O)CCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H30F3N3O/c1-16(30)6-5-9-21-10-13-22(14-11-21,15-12-21)20-28-27-19(29(20)2)17-7-3-4-8-18(17)23(24,25)26/h3-4,7-8,16,30H,5-6,9-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394608

(CHEMBL2164224)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2cccc(-c3noc(n3)C(C)(C)O)c12)C(F)(F)F Show InChI InChI=1S/C27H26F3N5O4S/c1-15(2)16-8-11-18(12-9-16)40(37,38)35-14-17-10-13-21(27(28,29)30)32-23(17)31-20-7-5-6-19(22(20)35)24-33-25(39-34-24)26(3,4)36/h5-13,15,36H,14H2,1-4H3,(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50394617

(CHEMBL2164576)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1Cc2ccc(nc2Nc2cccc(-c3cccnc3)c12)C(F)(F)F Show InChI InChI=1S/C28H25F3N4O2S/c1-27(2,3)20-10-12-21(13-11-20)38(36,37)35-17-19-9-14-24(28(29,30)31)34-26(19)33-23-8-4-7-22(25(23)35)18-6-5-15-32-16-18/h4-16H,17H2,1-3H3,(H,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-dY-peptide from human BRS-3 expressed in NFAT-CHO cells after 2 hrs by by liquid scintillation counting |
ACS Med Chem Lett 3: 252-256 (2012)
Article DOI: 10.1021/ml200304j
BindingDB Entry DOI: 10.7270/Q2PG1ST9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340402

(3-(4-(4-(ethylsulfonyl)butyl)bicyclo[2.2.2]octan-1...)Show SMILES CCS(=O)(=O)CCCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C24H32F3N3O2S/c1-3-33(31,32)17-7-6-10-22-11-14-23(15-12-22,16-13-22)21-29-28-20(30(21)2)18-8-4-5-9-19(18)24(25,26)27/h4-5,8-9H,3,6-7,10-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174281
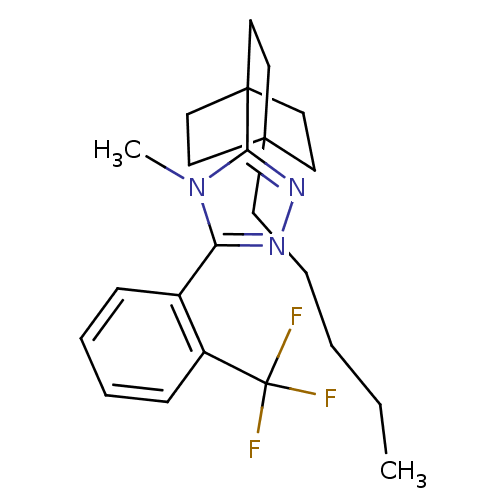
(4-methyl-3-(4-pentylbicyclo[2.2.2]octan-1-yl)-5-(2...)Show SMILES CCCCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H30F3N3/c1-3-4-7-10-21-11-14-22(15-12-21,16-13-21)20-28-27-19(29(20)2)17-8-5-6-9-18(17)23(24,25)26/h5-6,8-9H,3-4,7,10-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340376
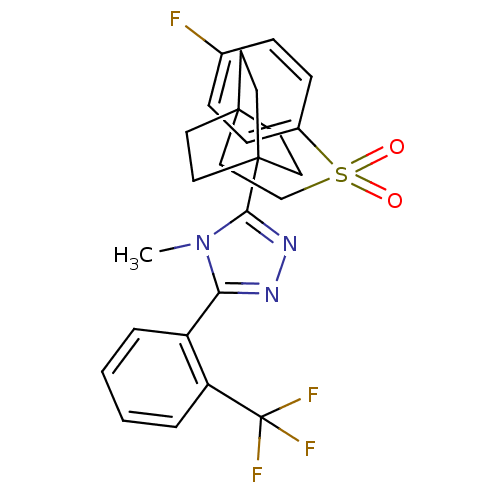
(3-(4-(2-(4-fluorophenylsulfonyl)ethyl)bicyclo[2.2....)Show SMILES Cn1c(nnc1C12CCC(CCS(=O)(=O)c3ccc(F)cc3)(CC1)CC2)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H27F4N3O2S/c1-33-22(20-4-2-3-5-21(20)26(28,29)30)31-32-23(33)25-13-10-24(11-14-25,12-15-25)16-17-36(34,35)19-8-6-18(27)7-9-19/h2-9H,10-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO-K1 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2568-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.018
BindingDB Entry DOI: 10.7270/Q2QJ7HMK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174284
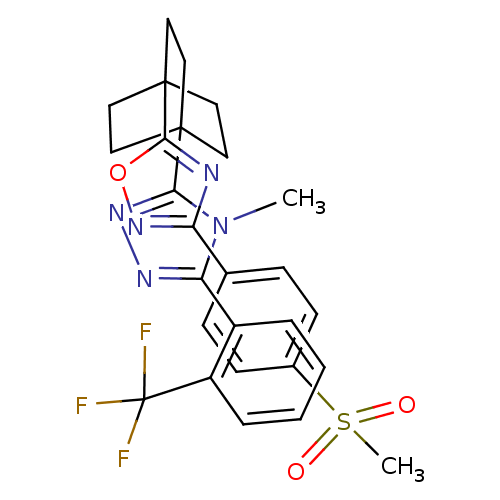
(4-methyl-3-(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(C)(=O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H26F3N5O3S/c1-35-22(19-5-3-4-6-20(19)27(28,29)30)32-33-23(35)25-11-14-26(15-12-25,16-13-25)24-31-21(34-38-24)17-7-9-18(10-8-17)39(2,36)37/h3-10H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174298
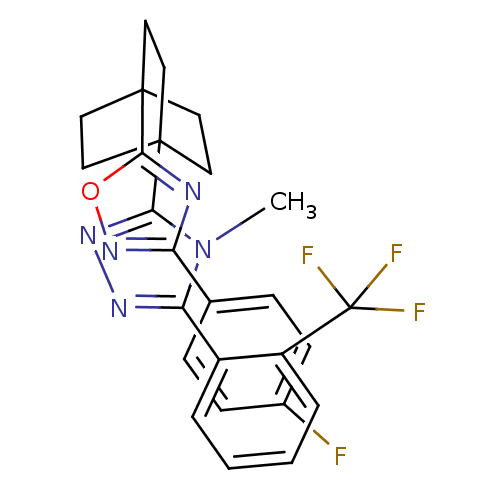
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174295
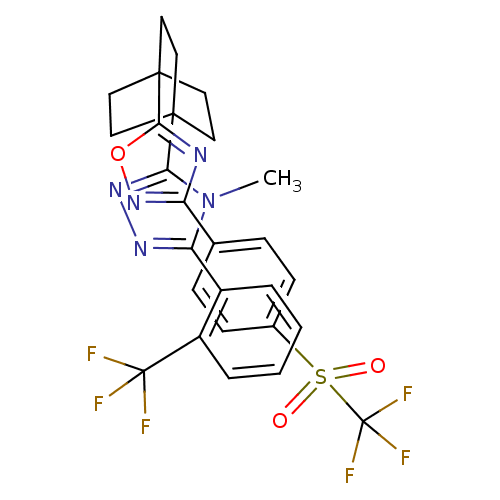
(4-methyl-3-(2-(trifluoromethyl)phenyl)-5-(4-(3-(4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(=O)(=O)C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H23F6N5O3S/c1-38-21(18-4-2-3-5-19(18)26(28,29)30)35-36-22(38)24-10-13-25(14-11-24,15-12-24)23-34-20(37-41-23)16-6-8-17(9-7-16)42(39,40)27(31,32)33/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data